Tadalafil 5 mg Alone or in Combination with Tamsulosin 0.4 mg for the Management of Men with Lower Urinary Tract Symptoms and Erectile Dysfunction: Results of a Prospective Observational Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Statistical Analysis
3. Results
Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gacci, M.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Kaplan, S.A.; Maggi, M.; McVary, K.T.; Mirone, V.; Porst, H.; Roehrborn, C.G. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur. Urol. 2011, 60, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.; Muracca, E.; Nakano, É.; Assalin, A.R.; Cordeiro, P.; Paranhos, M.; Cury, J.; Srougi, M.; Antunes, A.A. Interactions between lower urinary tract symptoms and cardiovascular risk factors determine distinct patterns of erectile dysfunction: A latent class analysis. J. Urol. 2013, 190, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur. Urol. 2005, 47, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Gravas, S.; Bach, T.; Drake, M.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Madersbacher, S.; Mamoulakis, C.; Tikkinen, K.A.O. Treatment of Non-Neurogenic Male LUTS. 2017. Available online: http://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ (accessed on 7 May 2019).
- Hedlund, H.; Andersson, K.E.; Larsson, B. Alpha-adrenoceptors and muscarinic receptors in the isolated human prostate. J. Urol. 1985, 134, 1291–1298. [Google Scholar] [CrossRef]
- Abrams, P.; Schulman, C.C.; Vaage, S. The European Tamsulosin Study Group. Tamsulosin, a selective alpha 1c-adrenoceptor antagonist: A randomized, controlled trial in patients with benign prostatic ‘obstruction’ (symptomatic BPH). Br. J. Urol. 1995, 76, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Ficarra, V.; Sebastianelli, A.; Corona, G.; Serni, S.; Shariat, S.F.; Maggi, M.; Zattoni, F.; Carini, M.; Novara, G. Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: A systematic review and meta-analysis. J. Sex. Med. 2014, 11, 1554–1566. [Google Scholar] [CrossRef]
- Corona, G.; Tirabassi, G.; Santi, D.; Maseroli, E.; Gacci, M.; Dicuio, M.; Sforza, A.; Mannucci, E.; Maggi, M. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: A comprehensive review and meta-analysis. Andrology 2017, 5, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Mirone, V.; Sessa, A.; Giuliano, F.; Berges, R.; Kirby, M.; Moncada, I. Current benign prostatic hyperplasia treatment: Impact on sexual function and management of related sexual adverse events. Int. J. Clin. Pract. 2011, 65, 1005–1013. [Google Scholar] [CrossRef]
- McVary, K.T.; Roehrborn, C.G.; Kaminetsky, J.C.; Auerbach, S.; Wachs, B.; Young, J.; Esler, A.; Sides, G.; Denes, B. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J. Urol. 2007, 177, 1401–1407. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; McVary, K.T.; Elion-Mboussa, A.; Viktrup, L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A dose finding study. J. Urol. 2008, 180, 1228–1234. [Google Scholar] [CrossRef]
- Broderick, G.A.; Brock, G.B.; Roehrborn, C.G.; Watts, S.D.; Elion-Mboussa, A.; Viktrup, L. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia in men with or without erectile dysfunction. Urology 2010, 75, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Porst, H.; Kim, E.D.; Casabé, A.R.; Mirone, V.; Secrest, R.J.; Xu, L.; Sundin, D.P.; Viktrup, L. LVHJ Study Team. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Results of an international randomized, double-blind, placebo-controlled trial. Eur. Urol. 2011, 60, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Andersson, K.E.; Chapple, C.; Maggi, M.; Mirone, V.; Oelke, M.; Porst, H.; Roehrborn, C.; Stief, C.; Giuliano, F. Latest Evidence on the Use of Phosphodiesterase Type 5 Inhibitors for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Eur. Urol. 2016, 70, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Filippi, S.; Morelli, A.; Sandner, P.; Fibbi, B.; Mancina, R.; Marini, M.; Gacci, M.; Vignozzi, L.; Vannelli, G.B.; Carini, M.; et al. Characterization and functional role of androgendependent PDE5 activity in the bladder. Endocrinology 2007, 148, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Vignozzi, L.; Gacci, M.; Cellai, I.; Morelli, A.; Maneschi, E.; Comeglio, P.; Santi, R.; Filippi, S.; Sebastianelli, A.; Nesi, G.; et al. PDE5 inhibitors blunt inflammation in human BPH: A potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 2013, 73, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Comeglio, P.; Filippi, S.; Sarchielli, E.; Vignozzi, L.; Maneschi, E.; Cellai, I.; Gacci, M.; Lenzi, A.; Vannelli, G.B.; et al. Mechanism of action of phosphodiesterase type 5 inhibition in metabolic syndrome-associated prostate alterations: An experimental study in the rabbit. Prostate 2013, 73, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Corona, G.; Salvi, M.; Vignozzi, L.; McVary, K.T.; Kaplan, S.A.; Roehrborn, C.G.; Serni, S.; Mirone, V.; Carini, M.; et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur. Urol. 2012, 61, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Fowler, F.J.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. Measurement Committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Gacci, M.; Corona, G.; Vignozzi, L.; Salvi, M.; Serni, S.; De Nunzio, C.; Tubaro, A.; Oelke, M.; Carini, M.; Maggi, M. Metabolic syndrome and benign prostatic enlargement: A systematic review and meta-analysis. BJU Int. 2015, 115, 24–31. [Google Scholar] [CrossRef]
- Andersson, K.E. Storage and voiding symptoms: Pathophysiologic aspects. Urology 2003, 62 (Suppl. 2), 3–10. [Google Scholar] [CrossRef]
- Gacci, M.; Sebastianelli, A.; Spatafora, P.; Corona, G.; Serni, S.; De Ridder, D.; Gravas, S.; Abrams, P. Best practice in the management of storage symptoms in male lower urinary tract symptoms: A review of the evidence base. Ther. Adv. Urol. 2017, 10, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Egerdie, R.B.; Auerbach, S.; Roehrborn, C.G.; Costa, P.; Garza, M.S.; Esler, A.L.; Wong, D.G.; Secrest, R.J. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: Results of a randomized, placebo-controlled, double-blind study. J. Sex. Med. 2012, 9, 271. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Chapple, C.; Oelke, M.; Cox, D.; Esler, A.; Viktrup, L. Effects of tadalafil once daily on maximum urinary flow rate in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. J. Urol. 2014, 191, 1045. [Google Scholar] [CrossRef]
- Yokoyama, O.; Yoshida, M.; Kim, S.C.; Wang, C.J.; Imaoka, T.; Morisaki, Y.; Viktrup, L. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int. J. Urol. 2013, 20, 193. [Google Scholar] [CrossRef] [PubMed]
- Alan, W.; Shindel, M.D. 2009 update on phosphodiesterase type 5 inhibitor therapy part 1: Recent studies on routine dosing for penile rehabilitation, lower urinary tract symptoms, and other indications. J. Sex. Med. 2009, 6, 1794–1808. [Google Scholar]
- Laydner, H.K.; Oliveira, P.; Oliveira, C.R.; Makarawo, T.P.; Andrade, W.S.; Tannus, M.; Araújo, J.L. Phosphodiesterase 5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A systematic review. BJU Int. 2011, 107, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Giuliano, F.; Mirone, V.; Xu, L.; Cox, D.; Viktrup, L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur. Urol. 2012, 61, 917–925. [Google Scholar] [CrossRef]
- Oger, S.; Behr-Roussel, D.; Gorny, D.; Lecoz, O.; Lebret, T.; Denoux, Y.; Faix, A.; Leriche, A.; Wayman, C.; Alexandre, L.; et al. Combination of doxazosin and sildenafil exerts an additive relaxing effect compared to each compound alone on human cavernosal and prostatic tissue. J. Sex. Med. 2009, 6, 836–847. [Google Scholar] [CrossRef]
- Liguori, G.; Trombetta, C.; De Giorgi, G.; Pomara, G.; Maio, G.; Vecchio, D.; Ocello, G.; Ollandini, G.; Bucci, S.; Belgrano, E. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: An integrated approach to the preliminary report. J. Sex. Med. 2009, 6, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zong, H.; Cui, Y.; Li, N.; Zhang, Y. The efficacy of PDE5 inhibitors alone or in combination with alpha-blockers for the treatment of erectile dysfunction and lower urinary tract symptoms due to benign prostatic hyperplasia: A systematic review and meta-analysis. J. Sex. Med. 2014, 11, 1539–1545. [Google Scholar] [CrossRef]
- McVary, K.T.; Monnig, W.; Camps, J.L.; Young, J.M.; Tseng, L.J.; van den Ende, G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: A randomized double-blind trial. J. Urol. 2007, 177, 1071–1077. [Google Scholar] [CrossRef]
- Tuncel, A.; Nalcacioglu, V.; Ener, K.; Aslan, Y.; Aydin, O.; Atan, A. Sildenafil citrate and tamsulosin combination is not superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. World J. Urol. 2010, 28, 17–22. [Google Scholar] [CrossRef]
- Kaplan, S.; Gonzalez, R.; Te, A. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur. Urol. 2007, 51, 1717–1723. [Google Scholar] [CrossRef]
- Bechara, A.; Romano, S.; Casabé, A.; Haime, S.; Dedola, P.; Hernández, C.; Rey, H. Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J. Sex. Med. 2008, 5, 2170–2178. [Google Scholar] [CrossRef]
- Chung, B.H.; Lee, J.Y.; Lee, S.H.; Yoo, S.J.; Lee, S.W.; Oh, C.Y. Safety and efficacy of the simultaneous administration of udenafil and an alpha-blocker in men with erectile dysfunction concomitant with BPH/LUTS. Int. J. Impot. Res. 2009, 21, 122–128. [Google Scholar] [CrossRef][Green Version]
- Kloner, R.; Jackson, G.; Emmick, J.; Mitchell, M.; Bedding, A.; Warner, L.; Pereira, A. Interaction between the phosphodiesterase 5 inhibitor. Tadalafil and 2 alfa-blockers, doxazosin and tamsulosin in healthy normotensive men. J. Urol. 2004, 172, 1935–1940. [Google Scholar] [CrossRef]
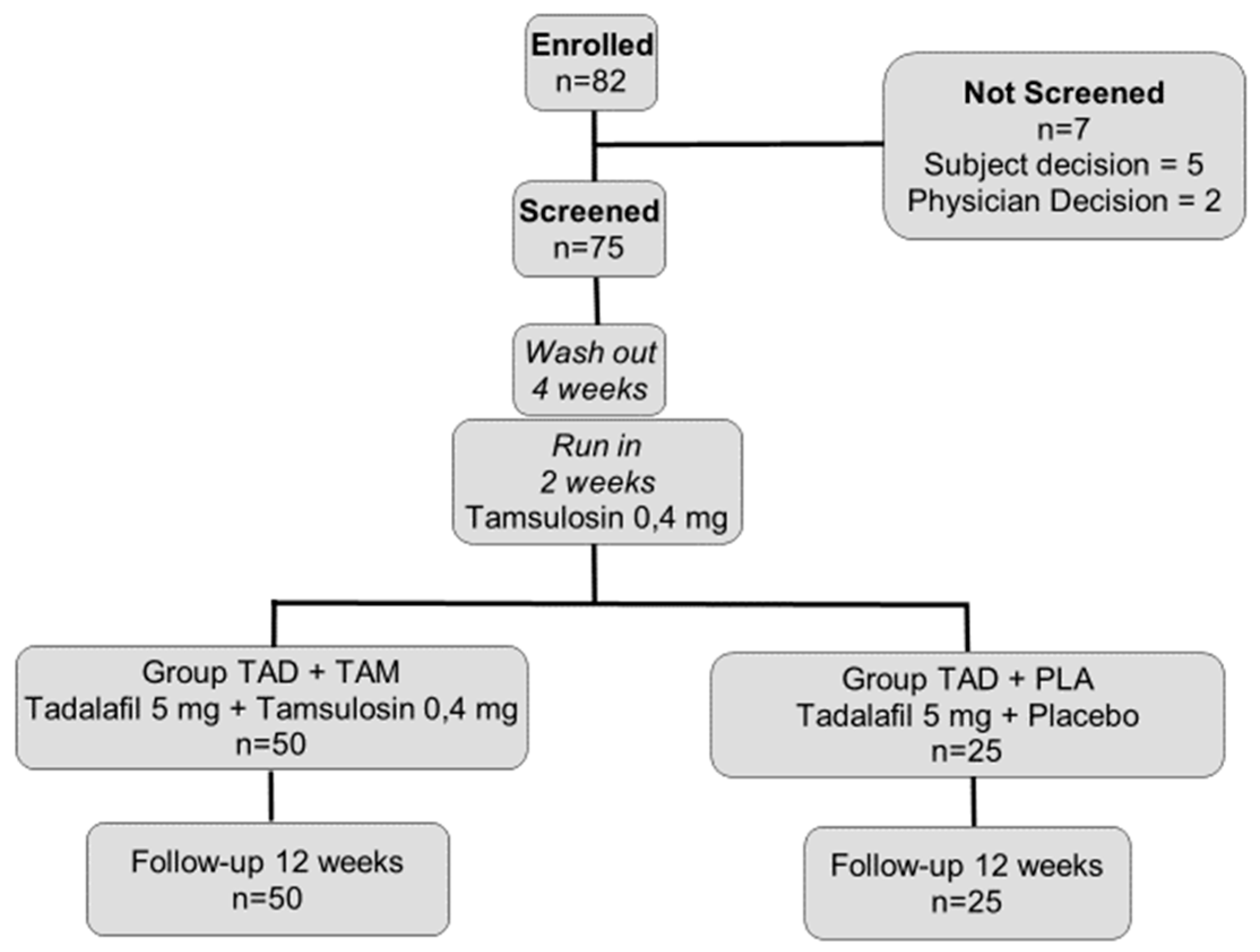
| All Patients | TAD + TAM Group n = 50 | TAD + PLA Group n = 25 | p | ||
|---|---|---|---|---|---|
| Baseline Characteristics | Mean ± SD Deviation | (Minimum-Maximum) | Mean ± SD Deviation | (Minimum-Maximum) | |
| Age (years) | 65.7 ± 9.1 | 47–78 | 65.5 ± 6.3 | 51–74 | 0.238 |
| Weight (kg) | 78.2 ± 9.2 | 69–86 | 75.8 ± 10.4 | 67–83 | 0.216 |
| Body mass index (kg/m2) | 27 ± 3.1 | 25–31 | 26.7 ± 3.6 | 24–31 | 0.174 |
| Abdominal obesity: waist circumference (cm) | 108.8 ± 4.1 | 92–135 | 102.3 ± 5.4 | 76–120 | 0.136 |
| Triglycerides (mg/dL) | 156.7 ± 8.4 | 76–247 | 129.2 ± 7.2 | 83–185 | 0.117 |
| HDL cholesterol (mg/dL) | 49.4 ± 2.8 | 31–76 | 49.8 ± 2.3 | 32–60 | 0.259 |
| Glycemia (mg/dL) | 111.3 ± 3.5 | 76–211 | 102.7 ± 5.7 | 72–188 | 0.113 |
| IPSS base | 18.8 ± 5.9 | 8–32 | 17 ± 6.1 | 8–29 | 0.224 |
| IPSS voiding base | 8.6 ± 3.8 | 1–20 | 10 ± 4.1 | 3–18 | 0.146 |
| IPSS storage base | 8.3 ± 3.2 | 0–14 | 6.9 ± 4.2 | 1–15 | 0.118 |
| IPSS QoL base | 3.9 ± 1 | 2–6 | 3.5 ± 1.4 | 1–6 | 0.145 |
| IIEF-5 base | 12 ± 3.5 | 6–21 | 13.8 ± 5.2 | 1–21 | 0.09 |
| Q max base (mL/s) | 10.3 ± 3.5 | 3.4–17.8 | 9.6 ± 2.8 | 6–17 | 0.369 |
| Variables Assesed | TAD + TAM Group n = 50 | TAD + PLA Group n = 25 | p Value (Anova Analysis) |
|---|---|---|---|
| IPSS 12 week (Mean ± SD) | 11.5 ± 5.4 | 11.8 ± 6.3 | |
| Delta3 M (baseline - 12wks) | −7 | −5.2 | |
| p value (paired samples T-test) | <0.001 | <0.001 | 0.084 |
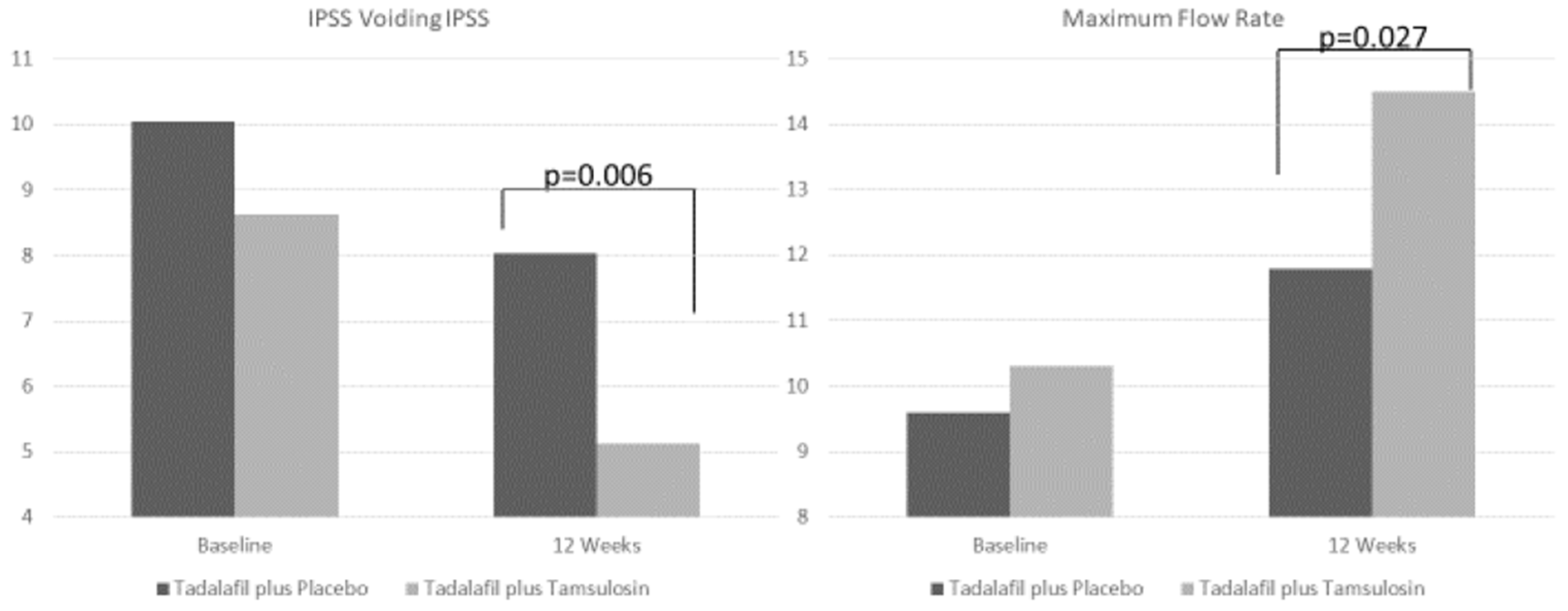
| IPSS voiding 12 week (Mean ± SD) | 5.1 ± 2.7 | 8 ± 4.7 | |
| Delta3 M (baseline - 12wks) | −3.5 | −2 | |
| p value (paired samples T-test) | <0.001 | 0.074 | 0.006 |
| IPSS storage 12 week (Mean ± SD) | 5.3 ± 2.7 | 3.8 ± 3.4 | |
| Delta3 M (baseline - 12wks) | −3 | −3.1 | |
| p value (paired samples T-test) | <0.001 | <0.001 | 0.08 |
| IPSS QoL 12 week (Mean ± SD) | 2.1 ± 1 | 2.1 ± 1.7 | |
| Delta3 M (baseline - 12wks) | −1.8 | −1.3 | |
| p value (paired samples T-test) | <0.001 | 0.009 | 0.321 |
| IIEF-5 12 week (Mean ± SD) | 17.7 ± 3.3 | 19.9 ± 5.1 | |
| Delta3 M (baseline - 12wks) | 5.7 | 6.1 | |
| p value (paired samples T-test) | <0.001 | <0.001 | 0.255 |
| Q max 12 week (Mean ± SD) | 14.5 ± 3.7 | 11.8 ± 4 | |
| Delta3 M (baseline - 12wks) | 4.2 | 2.2 | |
| p value (paired samples T-test) | <0.001 | <0.001 | 0.027 |
| Adverse Events | TAD + TAM Group n = 50, (n %) | TAD + PLA Group n = 25, (n %) | p Value |
|---|---|---|---|
| Any TEAEs | 11 (22%) | 4 (16%) | 0.075 |
| Serious AEs | 0 (0%) | 0 (0%) | 0.267 |
| Intensity | |||
| mild | 7 (14%) | 3 (12%) | 0.114 |
| moderate | 4 (8%) | 1 (4%) | 0.098 |
| severe | 0 (0%) | 0 (0%) | 0.286 |
| Headache | 4 (8%) | 2 (8%) | 0.163 |
| Nasopharyngitis | 0 (0%) | 1 (4%) | 0.196 |
| Back pain | 3 (6%) | 1(4%) | 0.087 |
| Dizziness | 1 (2%) | 0 (0%) | 0.173 |
| Dyspepsia | 1 (2%) | 0 (0%) | 0.185 |
| Ejaculatory dysfunction | 2 (4%) | 0 (0%) | 0.072 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastianelli, A.; Spatafora, P.; Frizzi, J.; Saleh, O.; Sessa, M.; De Nunzio, C.; Tubaro, A.; Vignozzi, L.; Maggi, M.; Serni, S.; et al. Tadalafil 5 mg Alone or in Combination with Tamsulosin 0.4 mg for the Management of Men with Lower Urinary Tract Symptoms and Erectile Dysfunction: Results of a Prospective Observational Trial. J. Clin. Med. 2019, 8, 1126. https://doi.org/10.3390/jcm8081126
Sebastianelli A, Spatafora P, Frizzi J, Saleh O, Sessa M, De Nunzio C, Tubaro A, Vignozzi L, Maggi M, Serni S, et al. Tadalafil 5 mg Alone or in Combination with Tamsulosin 0.4 mg for the Management of Men with Lower Urinary Tract Symptoms and Erectile Dysfunction: Results of a Prospective Observational Trial. Journal of Clinical Medicine. 2019; 8(8):1126. https://doi.org/10.3390/jcm8081126
Chicago/Turabian StyleSebastianelli, Arcangelo, Pietro Spatafora, Jacopo Frizzi, Omar Saleh, Maurizio Sessa, Cosimo De Nunzio, Andrea Tubaro, Linda Vignozzi, Mario Maggi, Sergio Serni, and et al. 2019. "Tadalafil 5 mg Alone or in Combination with Tamsulosin 0.4 mg for the Management of Men with Lower Urinary Tract Symptoms and Erectile Dysfunction: Results of a Prospective Observational Trial" Journal of Clinical Medicine 8, no. 8: 1126. https://doi.org/10.3390/jcm8081126
APA StyleSebastianelli, A., Spatafora, P., Frizzi, J., Saleh, O., Sessa, M., De Nunzio, C., Tubaro, A., Vignozzi, L., Maggi, M., Serni, S., McVary, K. T., Kaplan, S. A., Gravas, S., Chapple, C., & Gacci, M. (2019). Tadalafil 5 mg Alone or in Combination with Tamsulosin 0.4 mg for the Management of Men with Lower Urinary Tract Symptoms and Erectile Dysfunction: Results of a Prospective Observational Trial. Journal of Clinical Medicine, 8(8), 1126. https://doi.org/10.3390/jcm8081126






