Dispelling Myths about Antenatal TAPS: A Call for Action for Routine MCA-PSV Doppler Screening in the United States
Abstract
:1. Introduction
1.1. MYTH 1: The Natural History of TAPS is Unknown and so MCA-PSV Screening Cannot be Recommended
1.2. MYTH 2: MCA-PSV Doppler is not a Reliable Test for TAPS
1.3. MYTH 3: TAPS Presents with Other Symptoms that Will be Visible via Other Tests
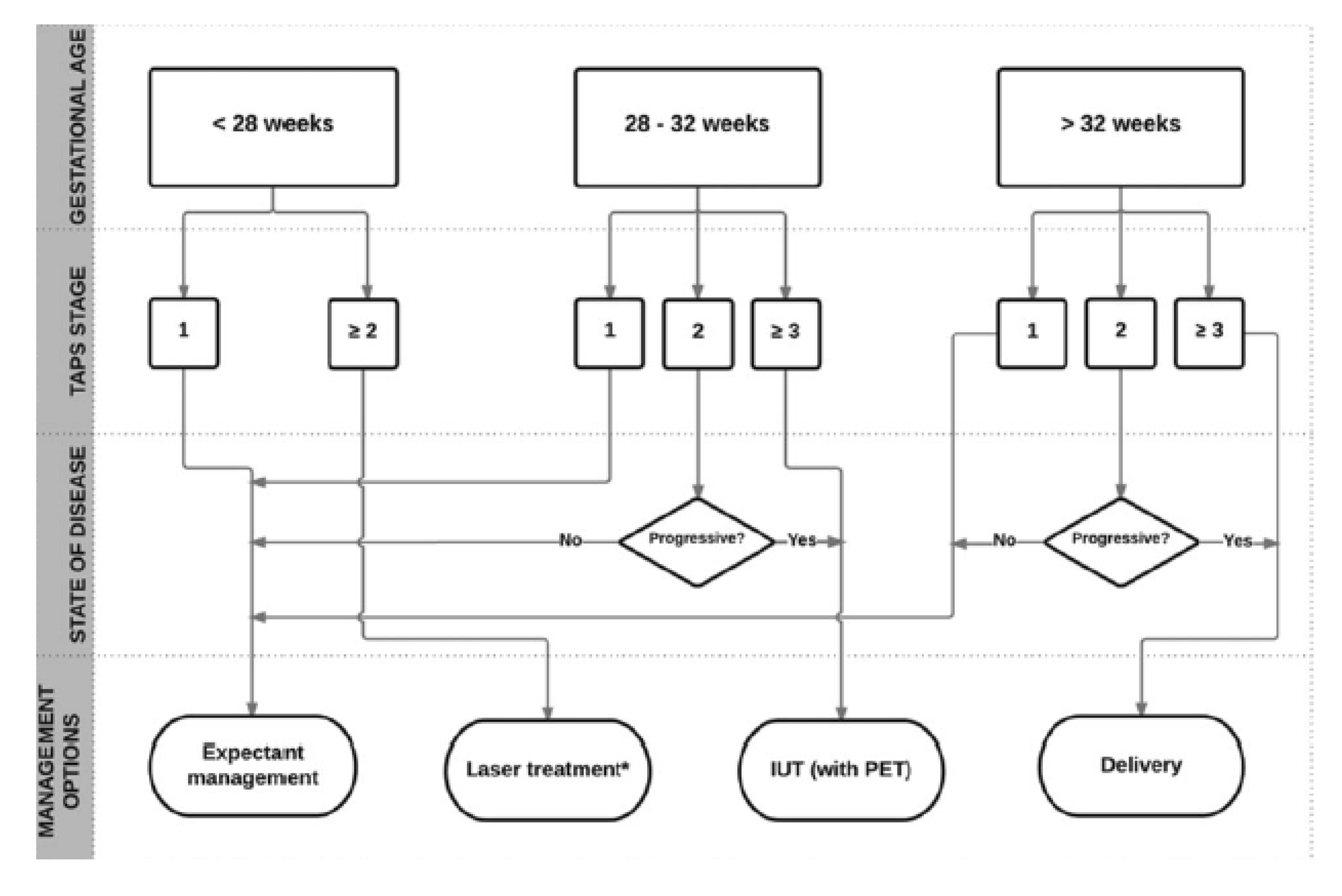
1.4. MYTH 4: There is an Unclear Treatment Protocol for TAPS
1.5. MYTH 5: If You Go Looking for Something to Be Wrong, You’re Going to Find It
1.6. MYTH 6: MCA-PSV Doppler Screening Results will Just Give Pregnant Women Undue Stress
1.7. MYTH 7: TAPS is Incredibly Rare and So Routine Screening is Not Necessary Unless the Patient is Post-Laser or Having Other Complications
1.8. MYTH 8: Routine MCA-PSV Doppler Screening Results in Too Many Unnecessary Premature Births
2. Current U.S. Clinical Recommendations for TAPS Screening
3. Ethics of TAPS Screening and Management
3.1. Preserving the Fiduciary Relationship
3.2. Justice and the Rights of the Pregnant Patient
3.3. Beneficent Acts and Avoidance of Harm
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sperling, L.; Kiil, C.; Larsen, L.U.; Brocks, V.; Wojdemann, K.R.; Qvist, I.; Schwartz, M.; Jørgensen, C.; Espersen, G.; Skajaa, K.; et al. Detection of chromosomal abnormalities, congenital abnormalities and transfusion syndrome in twins. Ultrasound Obstet. Gynecol. 2007, 29, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.J.; Wang, C.J.; Yu, M.W.; Lee, T.K. Perinatal Mortality and Prevalence of Major Congenital Malformations of Twins in Taipei City. Acta Genet. Med. Gemellol. Twin Res. 1992, 41, 197–203. [Google Scholar] [CrossRef]
- Bahtiyar, M.O.; Dulay, A.T.; Weeks, B.P.; Friedman, A.H.; Copel, J.A. Prevalence of congenital heart defects in monochorionic/diamniotic twin gestations: A systematic literature review. J. Ultrasound Med. 2007, 26, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Mackie, F.L.; Morris, R.K.; Kilby, M.D. The prediction, diagnosis and management of complications in monochorionic twin pregnancies: The OMMIT (Optimal Management of Monochorionic Twins) study. BMC Pregnancy Childbirth 2017, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Glennon, C.L.; Shemer, S.A.; Palma-Dias, R.; Umstad, M.P. The History of Treatment of Twin-to-Twin Transfusion Syndrome. Twin Res. Hum. Genet. 2016, 19, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robyr, R.; Lewi, L.; Salomon, L.J.; Yamamoto, M.; Bernard, J.-P.; Deprest, J.; Ville, Y. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2006, 194, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E.; Middeldorp, J.M.; Oepkes, D.; Kanhai, H.H.; Walther, F.J.; Vandenbussche, F.P.H.A. Twin Anemia–Polycythemia Sequence in Two Monochorionic Twin Pairs Without Oligo-Polyhydramnios Sequence. Placenta 2007, 28, 47–51. [Google Scholar] [CrossRef]
- Slaghekke, F.; Tollenaar, L.; Middeldorp, A.; Haak, M.; Klumper, F.; Lopriore, E.; Oepkes, D. 460: Additional value of delta MCA-PSV to predict TAPS. Am. J. Obstet. Gynecol. 2018, 218, S277–S278. [Google Scholar] [CrossRef] [Green Version]
- Slaghekke, F.; van Klink, J.M.M.; Koopman, H.M.; Middeldorp, J.M.; Oepkes, D.; Lopriore, E. Neurodevelopmental outcome in twin anemia-polycythemia sequence after laser surgery for twin-twin transfusion syndrome: Long-term outcome in post-laser TAPS. Ultrasound Obstet. Gynecol. 2014, 44, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Van Winden, K.R.; Quintero, R.A.; Kontopoulos, E.V.; Korst, L.M.; Llanes, A.; Chmait, R.H. Perinatal survival in cases of twin–twin transfusion syndrome complicated by selective intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2015, 28, 1549–1553. [Google Scholar] [CrossRef]
- Simpson, L.L. Twin-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2013, 208, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, L.D.; Fischbein, R.L.; Bhamidipalli, S.S. Twin anemia-polycythemia sequence and routine monitoring practices amongst maternal-fetal medicine specialists in the United States: An initial investigation. J. Perinat. Med. 2019, 47, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Lewi, L.; Gucciardo, L.; Huber, A.; Jani, J.; Van Mieghem, T.; Doné, E.; Cannie, M.; Gratacós, E.; Diemert, A.; Hecher, K.; et al. Clinical outcome and placental characteristics of monochorionic diamniotic twin pairs with early—And late-onset discordant growth. Am. J. Obstet. Gynecol. 2008, 199, 511. [Google Scholar] [CrossRef] [PubMed]
- Slaghekke, F.; Kist, W.J.; Oepkes, D.; Pasman, S.A.; Middeldorp, J.M.; Klumper, F.J.; Walther, F.J.; Vandenbussche, F.P.H.A.; Lopriore, E. Twin Anemia-Polycythemia Sequence: Diagnostic Criteria, Classification, Perinatal Management and Outcome. Fetal Diagn. Ther. 2010, 27, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.A.; Slaghekke, F.; Middeldorp, J.M.; Klumper, F.J.; Haak, M.C.; Oepkes, D.; Lopriore, E. Twin Anemia Polycythemia Sequence: Current Views on Pathogenesis, Diagnostic Criteria, Perinatal Management, and Outcome. Twin Res. Hum. Genet. 2016, 19, 222–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopriore, E.; Van den Wijngaard, J.P.H.M.; Pasman, S.A.; Oepkes, D.; Walther, F.J.; Van Gemert, M.J.C.; Vandenbussche, F.P.H.A. Quantification of Feto-fetal Transfusion Rate through a Single Placental Arterio-venous Anastomosis in a Monochorionic Twin Pregnancy. Placenta 2009, 30, 223–225. [Google Scholar] [CrossRef]
- Derks, J.B.; Vandenbussche, F.P.H.A. Nederlandse Vereniging voor Obstetrie en Gynaecologie Guidelines for Multiple Pregnancy; NVOG Press: Utrecht, The Netherlands, 2011. [Google Scholar]
- Fishel-Bartal, M.; Weisz, B.; Mazaki-Tovi, S.; Ashwal, E.; Chayen, B.; Lipitz, S.; Yinon, Y. Can middle cerebral artery peak systolic velocity predict polycythemia in monochorionic-diamniotic twins? Evidence from a prospective cohort study: MCA and polycythemia in MCDA twins. Ultrasound Obstet. Gynecol. 2016, 48, 470–475. [Google Scholar] [CrossRef]
- Slaghekke, F.; Zhao, D.P.; Middeldorp, J.M.; Klumper, F.J.; Haak, M.C.; Oepkes, D.; Lopriore, E. Antenatal management of twin-twin transfusion syndrome and twin anemia-polycythemia sequence. Expert Rev. Hematol. 2016, 9, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Slaghekke, F.; Lewi, L.; Middeldorp, J.M.; Weingertner, A.S.; Klumper, F.J.; Dekoninck, P.; Devlieger, R.; Lanna, M.M.; Deprest, J.; Favre, R.; et al. Residual anastomoses in twin-twin transfusion syndrome after laser: The Solomon randomized trial. Am. J. Obstet. Gynecol. 2014, 211, 285. [Google Scholar] [CrossRef]
- Weingertner, A.S.; Kohler, A.; Kohler, M.; Bouffet, N.; Hunsinger, M.C.; Mager, C.; Hornecker, F.; Neumann, M.; Schmerber, E.; Tanghe, M.; et al. Clinical and placental characteristics in four new cases of twin anemia-polycythemia sequence. Ultrasound Obstet. Gynecol. 2010, 35, 490–494. [Google Scholar] [CrossRef]
- Khalil, A.; Rodgers, M.; Baschat, A.; Bhide, A.; Gratacos, E.; Hecher, K.; Kilby, M.D.; Lewi, L.; Nicolaides, K.H.; Oepkes, D.; et al. ISUOG Practice Guidelines: Role of ultrasound in twin pregnancy: ISUOG Guidelines. Ultrasound Obstet. Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.A.; Lopriore, E.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.J.; Oepkes, D.; Slaghekke, F. Improved prediction of twin anemia–polycythemia sequence by delta middle cerebral artery peak systolic velocity: New antenatal classification system. Ultrasound Obstet. Gynecol. 2019, 53, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Movva, V.C.; Rijhsinghani, A. Discrepancy in placental echogenicity: A sign of twin anemia polycythemia sequence: Discrepant placental echodensity in TAPS. Prenat. Diagn. 2014, 34, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, L.P.; Howe, D.T. Starry sky liver in twin anemia-polycythemia sequence: Letter to the Editor. Ultrasound Obstet. Gynecol. 2014, 43, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Slaghekke, F.; Klumper, F.; Peeters, S.; Favre, R.; Middeldorp, J.; Oepkes, D.; Lopriore, E. 227: Antenatal twin anemia polycythemia sequence (TAPS): Management and outcome. Am. J. Obstet. Gynecol. 2014, 210, S120–S121. [Google Scholar] [CrossRef]
- Genova, L.; Slaghekke, F.; Klumper, F.J.; Middeldorp, J.M.; Steggerda, S.J.; Oepkes, D.; Lopriore, E. Management of Twin Anemia-Polycythemia Sequence Using Intrauterine Blood Transfusion for the Donor and Partial Exchange Transfusion for the Recipient. Fetal Diagn. Ther. 2013, 34, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E.; Slaghekke, F.; Kersbergen, K.J.; de Vries, L.S.; Drogtrop, A.P.; Middeldorp, J.M.; Oepkes, D.; Benders, M.J. Severe cerebral injury in a recipient with twin anemia-polycythemia sequence: Cerebral injury in TAPS. Ultrasound Obstet. Gynecol. 2013, 41, 702–706. [Google Scholar] [CrossRef]
- Leiden University Medical Center Long-term Outcomes: Childhood. Available online: https://www.thetapstrial.com/longterm-outcome (accessed on 19 May 2019).
- Papanna, R.; Johnson, A.; Wilkins-Haug, L. Twin-Twin Transfusion Syndrome and Twin Anemia Polycythemia Sequence: Pathogenesis and Diagnosis. Available online: https://www.uptodate.com/contents/twin-twin-transfusion-syndrome-and-twin-anemia-polycythemia-sequence-pathogenesis-and-diagnosis (accessed on 15 April 2019).
- Oepkes, D.; Seaward, P.G.; Vandenbussche, F.P.H.A.; Windrim, R.; Kingdom, J.; Beyene, J.; Kanhai, H.H.H.; Ohlsson, A.; Ryan, G. Doppler Ultrasonography versus Amniocentesis to Predict Fetal Anemia. N. Engl. J. Med. 2006, 355, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Portilla, R.J.; Lopez-Felix, J.; Hawkins-Villareal, A.; Villafan-Bernal, J.R.; Paz y Miño, F.; Figueras, F.; Borrell, A. Performance of middle cerebral artery peak systolic velocity for the prediction of fetal anemia in untransfused and transfused fetuses: A diagnostic test accuracy meta-analysis. Ultrasound Obstet. Gynecol. 2019. [Google Scholar] [CrossRef]
- Abbasi, N.; Johnson, J.-A.; Ryan, G. Fetal anemia. Ultrasound Obstet. Gynecol. 2017, 50, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Non Invasive Diagnosis of Fetal Anemia. Available online: https://www.youtube.com/watch?v=BEveWKtPFnI (accessed on 15 May 2019).
- National Guideline Alliance hosted by the Royal College of Obstetricians and Gynaecologists. Twin and Triplet Pregnancy Evidence Review for Ultrasound Screening for Twin Anaemia Polycythaemia Sequences. Available online: https://www.nice.org.uk/guidance/gid-ng10063/documents/evidence-review-3 (accessed on 15 April 2019).
- Slaghekke, F.; Pasman, S.; Veujoz, M.; Middeldorp, J.M.; Lewi, L.; Devlieger, R.; Favre, R.; Lopriore, E.; Oepkes, D. Middle cerebral artery peak systolic velocity to predict fetal hemoglobin levels in twin anemia-polycythemia sequence. Ultrasound Obstet. Gynecol. 2015, 46, 432–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veujoz, M.; Sananes, N.; Severac, F.; Meyer, N.; Weingertner, A.S.; Kohler, M.; Favre, R. Evaluation of prenatal and postnatal diagnostic criteria for twin anemiapolycythemia sequence. Prenat. Diagn. 2015, 35, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Fairley, C.K.; Cohen, B.J.; Seng, C. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br. J. Obs. Gynaecol 1998, 105, 174–178. [Google Scholar] [CrossRef]
- Dembinski, J.; Haverkamp, F.; Maara, H.; Hansmann, M.; Eis-Hubinger, A.M.; Bartmann, P. Neurodevelopmental outcome after intrauterine red cell transfusion for parvovirus. BJOG 2002, 109, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.P.; Lindenburg, I.T.; van Klink, J.M.; Oepkes, D.; van Kamp, I.L.; Walther, F.J.; Lopriore, E. Intrauterine transfusion for parvovirus B19 infection: Long-term neurodevelopmental outcome. Am. J. Obstet. Gynecol. 2012, 206, e1–e204. [Google Scholar] [CrossRef] [PubMed]
- Prefumo, F.; Fichera, A.; Fratelli, N.; Sartori, E. Fetal anemia: Diagnosis and management. Best Pract. Res. Clin. Obstet. Gynaecol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.; Carpenter, R.J.J.; Durig, P.; Mari, G. Longitudinal measurement of peak systolic velocity in the fetal middle cerebral artery for monitoring pregnancies complicated by red cell alloimmunisation: A prospective multicentre trial with intention-to-treat. BJOG 2002, 109, 746–752. [Google Scholar] [CrossRef]
- Lopriore, E.; Oepkes, D. Fetal and neonatal haematological complications in monochorionic twins. Semin. Fetal Neonatal Med. 2008, 13, 231–238. [Google Scholar] [CrossRef]
- Kusanovic, J.P.; Romero, R.; Gotsch, F.; Mittal, P.; Erez, O.; Kim, C.J.; Hassan, S.S.; Espinoza, J.; Yeo, L. Discordant placental echogenicity: A novel sign of impaired placental perfusion in twin-twin transfusion syndrome? J. Matern. Fetal Neonatal Med. 2010, 23, 103–106. [Google Scholar] [CrossRef]
- Moaddab, A.; Nassr, A.A.; Espinoza, J.; Ruano, R.; Bateni, Z.H.; Shamshirsaz, A.A.; Mandy, G.T.; Welty, S.E.; Erfani, H.; Popek, E.J.; et al. Twin anemia polycythemia sequence: A single center experience and literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 158–164. [Google Scholar] [CrossRef]
- Ashwal, E.; Yinon, Y.; Fishel-Bartal, M.; Tsur, A.; Chayen, B.; Weisz, B.; Lipitz, S. Twin Anemia-Polycythemia Sequence: Perinatal Management and Outcome. Fetal Diagn. Ther. 2016, 40, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Moise, K.J.; Johnson, A. There is NO diagnosis of twins. Am. J. Obstet. Gynecol. 2010, 203, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Slaghekke, F.; Favre, R.; Peeters, S.H.P.; Middeldorp, J.M.; Weingertner, A.S.; van Zwet, E.W.; Klumper, F.J.; Oepkes, D.; Lopriore, E. Laser surgery as a management option for twin anemia-polycythemia sequence: Laser surgery in TAPS. Ultrasound Obstet. Gynecol. 2014, 44, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.M.; Masoudian, P.; Fung-Kee-Fung, K.; El Demellawy, D. Intrauterine Interventions for the Treatment of Twin Anemia-Polycythemia Sequence: A Systematic Review. J. Obstet. Gynaecol. Can. 2018, 41, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Herway, C.; Johnson, A.; Moise, K.; Moise, K.J. Fetal intraperitoneal transfusion for iatrogenic twin anemia-polycythemia sequence after laser therapy. Ultrasound Obstet. Gynecol. 2009, 33, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Gourounti, C.; Karpathiotaki, N.; Vaslamatzis, G. Psychosocial stress in high risk pregnancy. Int. Arch. Med. 2015, 8. [Google Scholar] [CrossRef]
- Fairbrother, N.; Young, A.H.; Zhang, A.; Janssen, P.; Antony, M.M. The prevalence and incidence of perinatal anxiety disorders among women experiencing a medically complicated pregnancy. Arch. Womens Ment. Health 2017, 20, 311–319. [Google Scholar] [CrossRef]
- Aite, L.; Zaccara, A.; Trucchi, A.; Brizzi, C.; Nahom, A.; Iacobelli, B.; Capolupo, I.; Bagolan, P. When uncertainty generates more anxiety than severity: The prenatal experience with cystic adenomatoid malformation of the lung. J. Perinat. Med. 2009, 37, 539–542. [Google Scholar] [CrossRef]
- Brisch, K.H.; Munz, D.; Bemmerer-Mayer, K.; Kächele, H.; Terinde, R.; Kreienberg, R. Ultrasound scanning for diagnosis of foetal abnormality and maternal anxieties in a longitudinal perspective. J. Reprod. Infant Psychol. 2002, 20, 223–235. [Google Scholar] [CrossRef]
- Kaasen, A.; Helbig, A.; Malt, U.F.; Næs, T.; Skari, H.; Haugen, G. Maternal psychological responses during pregnancy after ultrasonographic detection of structural fetal anomalies: A prospective longitudinal observational study. PLoS ONE 2017, 12, e0174412. [Google Scholar] [CrossRef]
- McCarthy, F.P.; Khashan, A.S.; North, R.A.; Moss-Morris, R.; Baker, P.N.; Dekker, G.; Poston, L.; Kenny, L.C.; On behalf of the SCOPE consortium. A Prospective Cohort Study Investigating Associations between Hyperemesis Gravidarum and Cognitive, Behavioural and Emotional Well-Being in Pregnancy. PLoS ONE 2011, 6, e27678. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.; Alder, J.; Gaab, J.; Berger, T.; Ihde, K.; Urech, C. Patient satisfaction and psychological well-being after internet-based cognitive behavioral stress management (IB-CBSM) for women with preterm labor: A randomized controlled trial. J. Psychosom. Res. 2016, 80, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Beauquier-Maccotta, B.; Chalouhi, G.E.; Picquet, A.-L.; Carrier, A.; Bussières, L.; Golse, B.; Ville, Y. Impact of Monochorionicity and Twin to Twin Transfusion Syndrome on Prenatal Attachment, Post Traumatic Stress Disorder, Anxiety and Depressive Symptoms. PLoS ONE 2016, 11, e0145649. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.; Davenport, M.; Pernet, A. Antenatally diagnosed surgical anomalies: The psychological effect of parental antenatal counseling. J. Pediatr. Surg 1998, 33, 1376–1379. [Google Scholar] [CrossRef]
- Benirschke, K. The biology of the twinning process: How placentation influences outcome. Semin. Perinatol. 1995, 19, 342–350. [Google Scholar] [CrossRef]
- Lewi, L.; Lewi, P.; Diemert, A.; Jani, J.; Gucciardo, L.; Van Mieghem, T.; Doné, E.; Gratacós, E.; Huber, A.; Hecher, K.; et al. The role of ultrasound examination in the first trimester and at 16 weeks’ gestation to predict fetal complications in monochorionic diamniotic twin pregnancies. Am. J. Obstet. Gynecol. 2008, 199, 493. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.C.; Prefumo, F. Perinatal Outcomes of Twin Anemia–Polycythemia Sequence: A Systematic Review. J. Obstet. Gynaecol. Can. 2014, 36, 701–707. [Google Scholar] [CrossRef]
- Chervenak, F.A.; McCullough, L.B. An Ethically Justified Framework for Clinical Investigation to Benefit Pregnant and Fetal Patients. Am. J. Bioeth. 2011, 11, 39–49. [Google Scholar] [CrossRef]
- Antiel, R.M. Ethical challenges in the new world of maternal–fetal surgery. Semin. Perinatol. 2016, 40, 227–233. [Google Scholar] [CrossRef]
- Dondorp, W.J.; Page-Christiaens, G.C.M.L.; de Wert, G.M.W.R. Genomic futures of prenatal screening: Ethical reflection: Genomic futures of prenatal screening. Clin. Genet. 2016, 89, 531–538. [Google Scholar] [CrossRef]
- McCullough, L.B.; Chervenak, F.A. Ethics in Obstetrics and Gynecology; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Feinberg, J. The Child’s Right to an Open Future. In Whose Child? Children’s Rights, Parental Authority, and State Power; Aiken, W., LaFollette, H., Eds.; Rowman and Littlefield: Totowa, NJ, USA, 1980; pp. 124–153. [Google Scholar]
- Millum, J. The Foundation of the Child’s Right to an Open Future: Foundation of Child’s Right to Open Future. J. Soc. Philos. 2014, 45, 522–538. [Google Scholar] [CrossRef]
- McGillivray, G.; Rosenfeld, J.A.; McKinlay Gardner, R.J.; Gillam, L.H. Genetic counselling and ethical issues with chromosome microarray analysis in prenatal testing: Genetic counselling and ethical Issues with prenatal CMA. Prenat. Diagn. 2012, 32, 389–395. [Google Scholar] [CrossRef]
- Strong, C. Fetal anomalies: Ethical and legal considerations in screening, detection, and management. Clin. Perinatol. 2003, 30, 113–126. [Google Scholar] [CrossRef]
- Wilson, J.M.; Jungner, Y.G. [Principles and practice of mass screening for disease]. Bol. Oficina Sanit Panam 1968, 65, 281–393. [Google Scholar]
- Laventhal, N.T.; Treadwell, M.C. Ethical considerations in the care of complicated twin pregnancies. Semin. Fetal Neonatal Med. 2018, 23, 7–12. [Google Scholar] [CrossRef]
| Predictive Values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Sample | Interval between MCA-PSV Measure and Delivery/Reference Measurement | MCA-PSV Measurement | Outcome and Reference Standard | Sensitivity (95% CI) | Specificity (95% CI) | Positive (95% CI) | Negative (95% CI) |
| Slaghekke et al. 2015 [36] | 43 TAPS pregnancies | Pre-natal MCA-PSV within 24 h of prenatal HB assessment or 24 hours of delivery | MCA-PSV ≥ 1.5 MoM | Severe anemia in TAPS donors as measured by prenatal or postnatal Hb levels | 94% (85–98%) | 74% (62–83%) | 76% (65–85%) | 94% (83–98%) |
| MCA-PSV ≤ 1.0 MoM | Polycythemia in TAPS recipients as measured by prenatal or postnatal HB levels | 97% (87–99%) | 96% (89–99%) | 93% (81–97) | 99% (93–100%) | |||
| Tollenaar et al. 2019 [23] | 35 TAPS pregnancies and 45 uncomplicated MC pregnancies | Pre-natal MCA-PSV within 1 week preceding delivery | MCA-PSV > 1.5 MoM in the donor; <1.0 MoM in the recipient | Postnatal TAPS | 46% (30–62%) | 100% (92–100%) | 100% (81–100%) | 70% (58–80%) |
| Delta MCA-PSV > 0.5 | Postnatal TAPS | 83% (67–92%) | 100% (92–100%) | 100% (88–100%) | 88% (77–94%) | |||
| Veujoz et al. 2015 [37] | 40 TAPS pregnancies; 20 spontaneous; 20 post-laser | Pre-natal MCA-PSV within 48 h preceding delivery, or preceding in-utero transfusion | MCA-PSV > 1.5 MoM in the donor; <1.0 MoM in the recipient | Postnatal TAPS | 71% (29–96%) * | 50% (1–99%) * | 83% | 33% |
| Area Under the Curve (AUC) (95% CI) | ||||||||
| Fishel-Bartel et al. 2016 [18] | 69 MCDA pregnancies | Pre-natal MCA-PSV within 1 week preceding delivery | MCA-PSV >1.5 MoM in donor; <1.0 in MoM in the recipient | Postnatal TAPS | AUC = 0.87 (0.76–0.99) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholas, L.; Fischbein, R.; Aultman, J.; Ernst-Milner, S. Dispelling Myths about Antenatal TAPS: A Call for Action for Routine MCA-PSV Doppler Screening in the United States. J. Clin. Med. 2019, 8, 977. https://doi.org/10.3390/jcm8070977
Nicholas L, Fischbein R, Aultman J, Ernst-Milner S. Dispelling Myths about Antenatal TAPS: A Call for Action for Routine MCA-PSV Doppler Screening in the United States. Journal of Clinical Medicine. 2019; 8(7):977. https://doi.org/10.3390/jcm8070977
Chicago/Turabian StyleNicholas, Lauren, Rebecca Fischbein, Julie Aultman, and Stephanie Ernst-Milner. 2019. "Dispelling Myths about Antenatal TAPS: A Call for Action for Routine MCA-PSV Doppler Screening in the United States" Journal of Clinical Medicine 8, no. 7: 977. https://doi.org/10.3390/jcm8070977
APA StyleNicholas, L., Fischbein, R., Aultman, J., & Ernst-Milner, S. (2019). Dispelling Myths about Antenatal TAPS: A Call for Action for Routine MCA-PSV Doppler Screening in the United States. Journal of Clinical Medicine, 8(7), 977. https://doi.org/10.3390/jcm8070977





