Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study
Abstract
1. Introduction
2. Experimental Section
2.1. In Vitro Study
2.1.1. Isolation and Expansion of Human Articular Chondrocytes
2.1.2. IL-1β Treatment Protocol
2.1.3. CG Treatment
2.1.4. Chondrogenic Differentiation in Pellet Culture
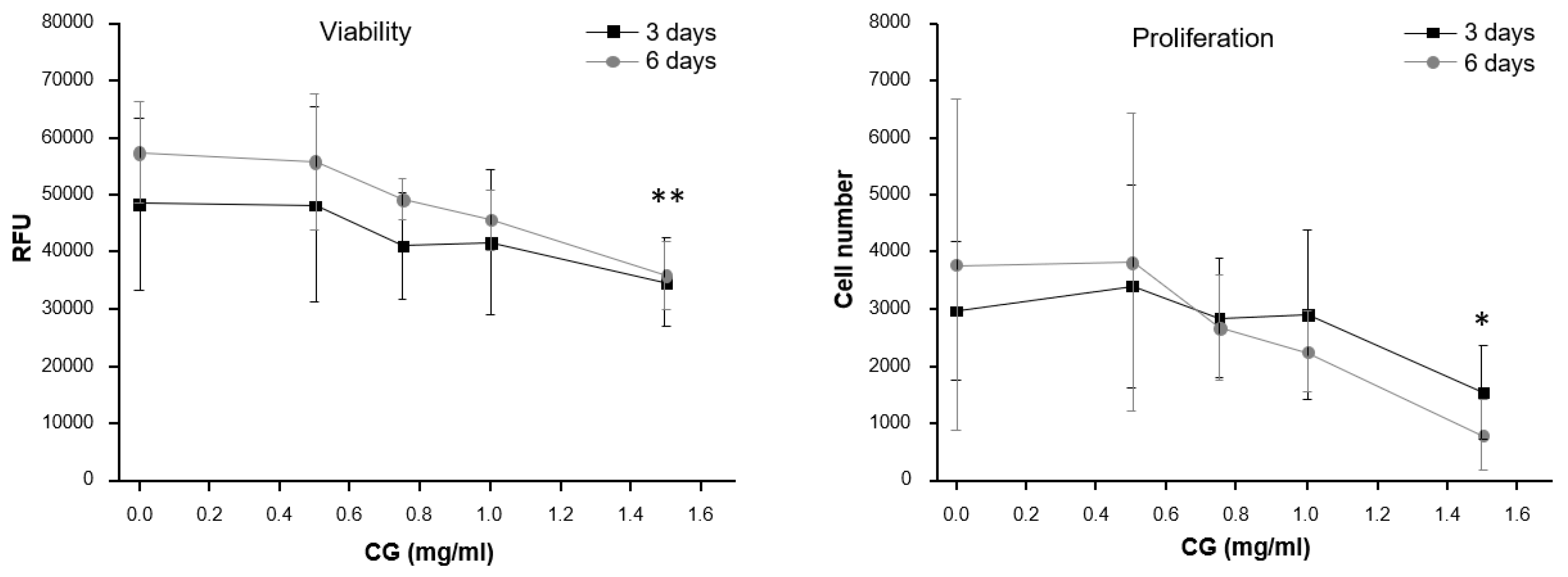
2.1.5. Viability Assay (Alamar Blue)
2.1.6. Proliferation Assay (CyQuant)
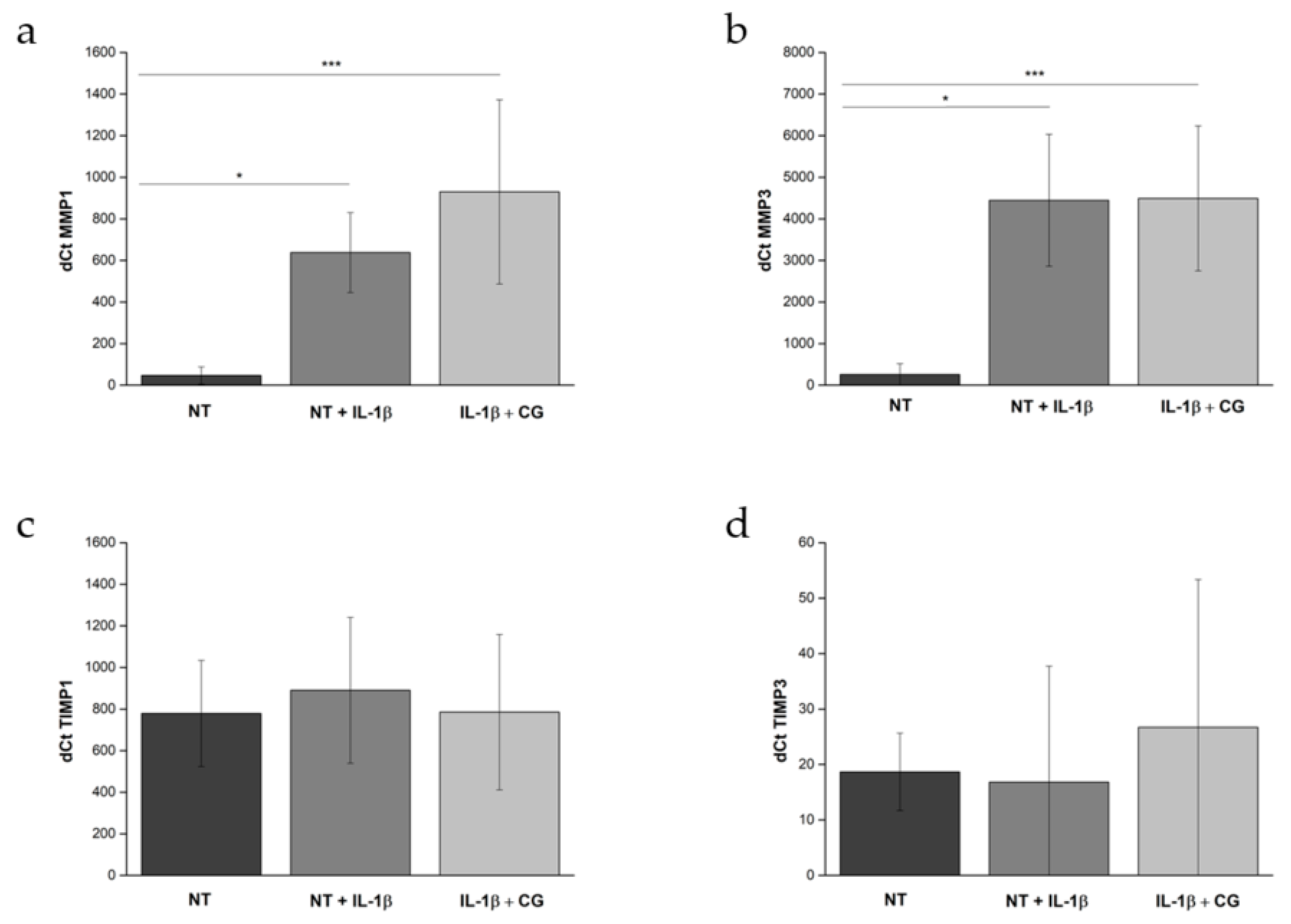
2.1.7. Gene Expression Analysis
2.1.8. Determination of TGFβ1, IGF-1, and VEGF
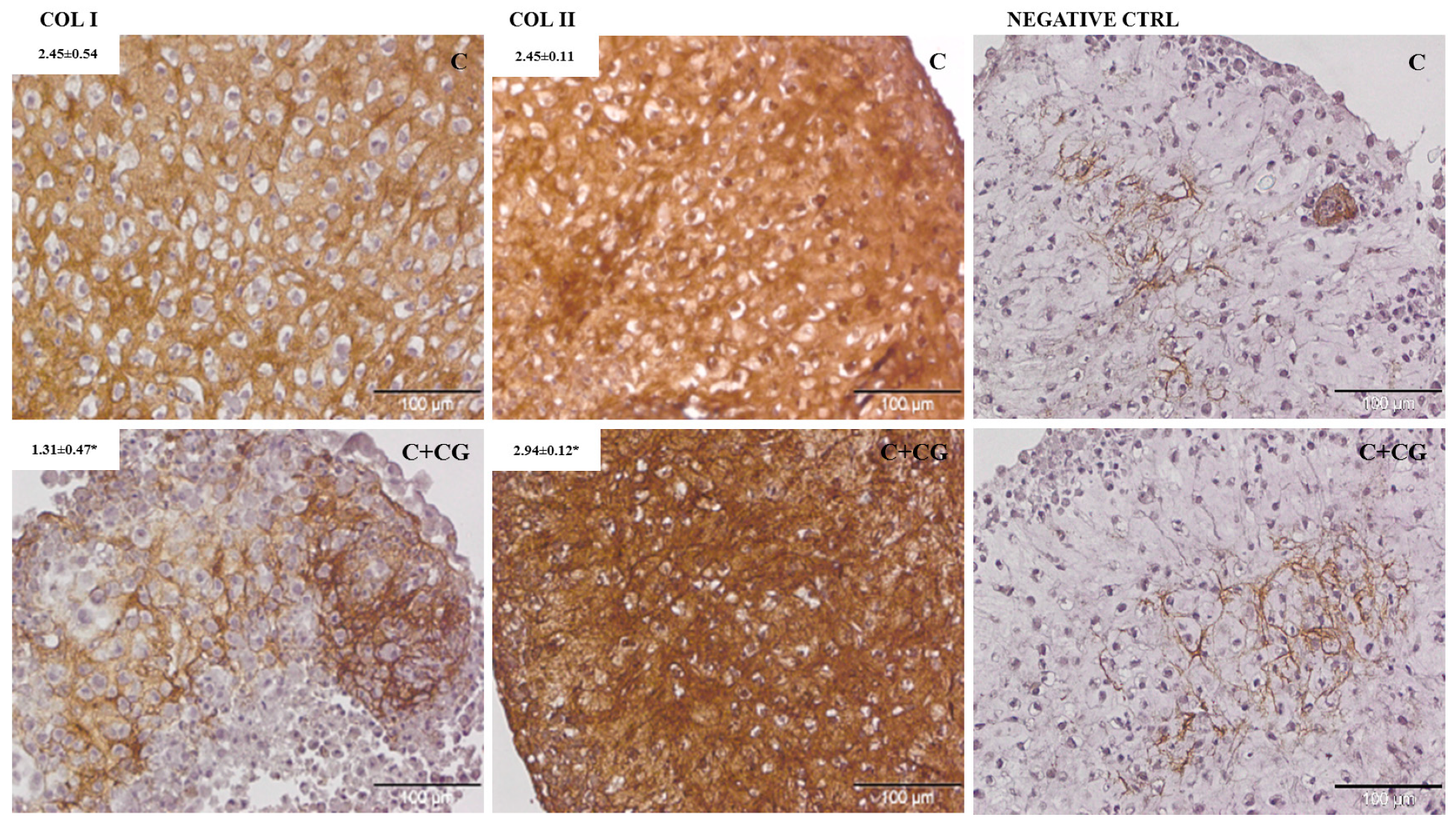
2.1.9. Histological Analysis
2.2. Retrospective Clinical Study
2.3. Treatment
2.4. Statistical Analysis
3. Results
3.1. In Vitro Assessment
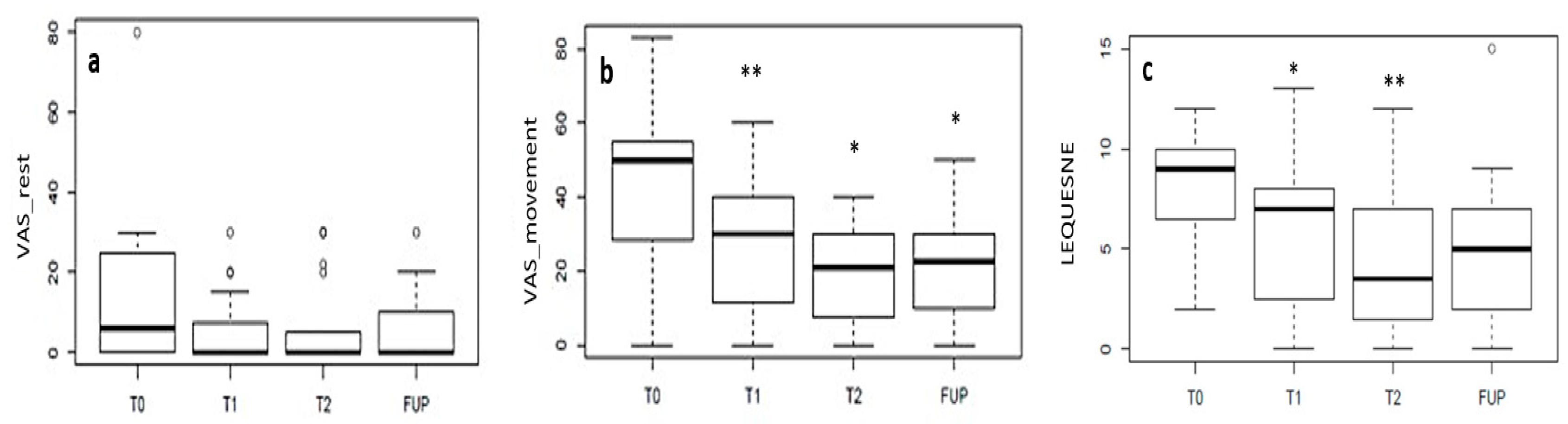
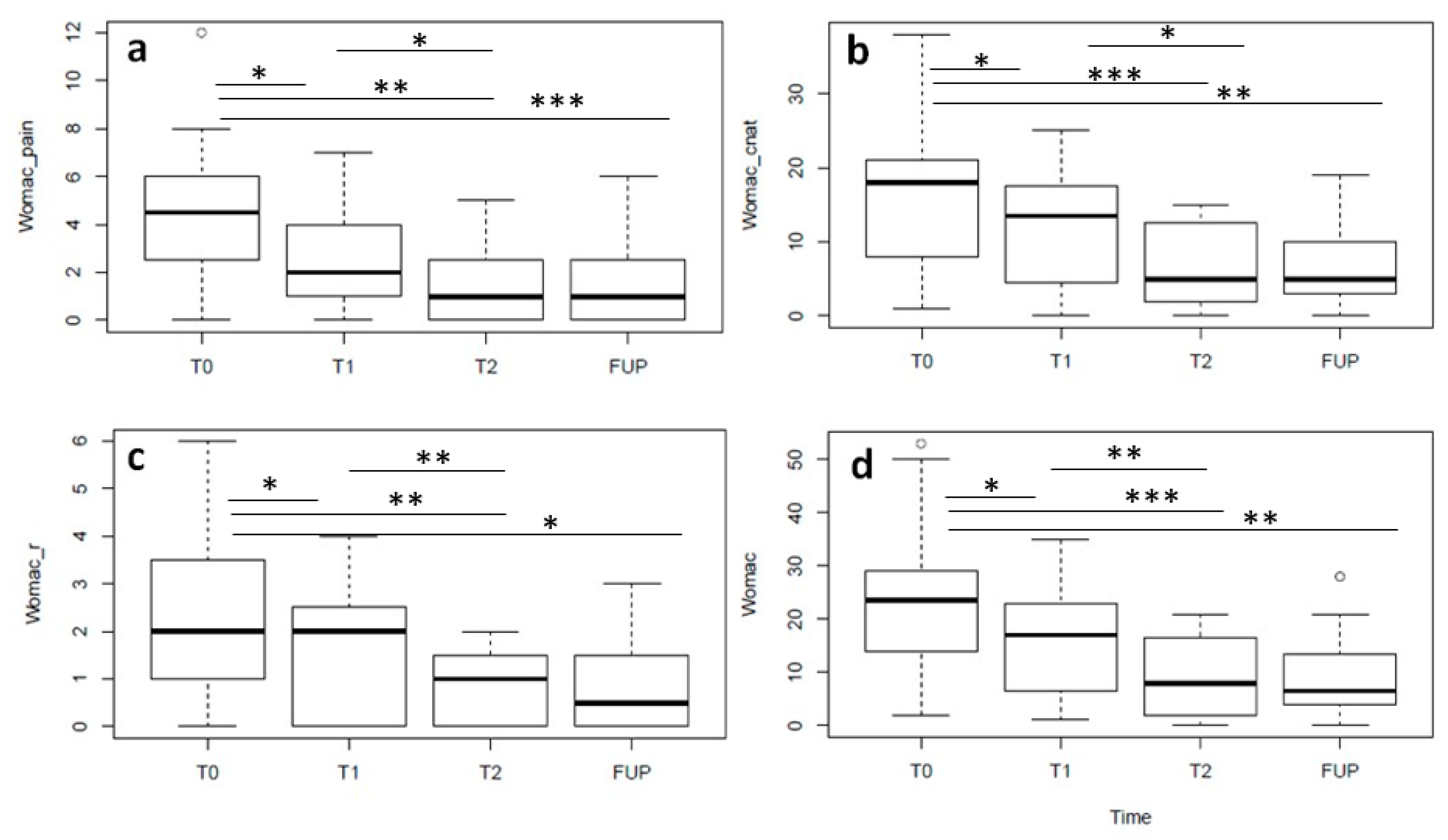
3.2. Retrospective Clinical Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, D.; Cho, Y.; Kim, J.H. Epigenetic regulation of chondrocyte catabolism and anabolism in osteoarthritis. Mol. Cells 2015, 38, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Kovic, B.; Jin, X.; He, X.; Wang, M.; Silvestre, C. Economic and Humanistic Burden of Osteoarthritis: A Systematic Review of Large Sample Studies. Pharmacoeconomics 2016, 34, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Latourte, A.; Frazier, A. Safety and efficacy of paracetamol and NSAIDs in osteoarthritis: Which drug to recommend? Expert Opin. Drug Saf. 2015, 14, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Freire, V.; Bureau, N.J. Injectable Corticosteroids: Take Precautions and Use Caution. Semin. Musculoskelet Radiol. 2016, 20, 401–408. [Google Scholar] [CrossRef]
- Da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, E21–E33. [Google Scholar] [CrossRef]
- Taylor, N. Nonsurgical Management of Osteoarthritis Knee Pain in the Older Adult. Clin. Geriatr. Med. 2017, 33, 41–51. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, B.; Wang, Y.; Wang, X.; Han, D.; Ding, D.; Zheng, Y.; Cao, Y.; Zhan, H.; Zhou, Y. The Effectiveness of Manual Therapy for Relieving Pain, Stiffness, and Dysfunction in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Pain Physician 2017, 20, 229–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 19, CD005321. [Google Scholar] [CrossRef] [PubMed]
- Trojian, T.H.; Concoff, A.L.; Joy, S.M.; Hatzenbuehler, J.R.; Saulsberry, W.J.; Coleman, C.I. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes. Clin. J. Sport Med. 2016, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Bay-Jensen, A.C.; van Spil, W.E.; Larkin, J.; Levesque, M.C. Osteoarthritis Year in Review 2016: Biomarkers (biochemical markers). Osteoarthr. Cartil. 2017, 25, 199–208. [Google Scholar] [CrossRef]
- Ohara, H.; Iida, H.; Ito, K.; Takeuchi, Y.; Nomura, Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci. Biotechnol. Biochem. 2010, 74, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa-Carballeda, J.; Muñoz-Chablé, O.A.; Barrios-Payán, J.; Hernández-Pando, R. Effect of polymerized-type I collagen in knee osteoarthritis. I. In vitro study. Eur. J. Clin. Investig. 2009, 39, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Comblain, F.; Dubuc, J.E.; Lambert, C.; Sanchez, C.; Lesponne, I.; Serisier, S.; Henrotin, Y. Identification of Targets of a New Nutritional Mixture for Osteoarthritis Management Composed by Curcuminoids Extract, Hydrolyzed Collagen and Green Tea Extract. PLoS ONE 2016, 11, e0156902. [Google Scholar] [CrossRef] [PubMed]
- Naraoka, T.; Ishibashi, Y.; Tsuda, E.; Yamamoto, Y.; Kusumi, T.; Toh, S. Periodic knee injections of collagen tripeptide delay cartilage degeneration in rabbit experimental osteoarthritis. Arthritis Res. Ther. 2013, 15, R32. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Muñoz-Chablé, O.A.; Macías-Hernández, S.I.; Agualimpia-Janning, A. Effect of polymerized-type I collagen in knee osteoarthritis. II. In vivo study. Eur. J. Clin. Investig. 2009, 39, 598–606. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Lima, G.; Llorente, L.; Nuñez-Álvarez, C.; Ruiz-Ordaz, B.H.; Echevarría-Zuno, S.; Hernández-Cuevas, V. Polymerized-type I collagen downregulates inflammation and improves clinical outcomes in patients with symptomatic knee osteoarthritis following arthroscopic lavage: A randomized, double-blind, and placebo-controlled clinical trial. Sci. World J. 2012, 2012, 342854. [Google Scholar] [CrossRef] [PubMed]
- Martin Martin, L.S.; Massafra, U.; Bizzi, E.; Migliore, A. A double blind randomized active-controlled clinical trial on the intra-articular use of Md-Knee versus sodium hyaluronate in patients with knee osteoarthritis (Joint). BMC Musculoskelet Disord. 2016, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Kouroupis, D.; Viganò, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human Diseased Articular Cartilage Contains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Daheshia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Ceriani, C.; Cecchinato, R.; Zagra, L.; Moretti, M.; Colombini, A. Stability of housekeeping genes in human intervertebral disc, endplate and articular cartilage cells in multiple conditions for reliable transcriptional analysis. Eur. Cell. Mater. 2016, 31, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Barbero, A.; Winkelmann, V.; Rieser, F.; Fitzsimmons, J.S.; O’Driscoll, S.; Martin, I.; Mainil-Varlet, P. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006, 12, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Lanteri, P.; Lombardi, G.; Grasso, D.; Recordati, C.; Lovi, A.; Banfi, G.; Bassani, R.; Brayda-Bruno, M. Metabolic effects of vitamin D active metabolites in monolayer and micromass cultures of nucleus pulposus and annulus fibrosus cells isolated from human intervertebral disc. Int. J. Biochem. Cell. Biol. 2012, 44, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.; Lawrence, J. Atlas of Standard Radiographs: The Epidemiology of Chronic Rheumatism; Blackwell Scientific Publications: Oxford, UK, 1963; Volume 2. [Google Scholar]
- Lequesne, M.G.; Mery, C.; Samson, M.; Gerard, P. Indexes of severity for osteoarthritis of the hip and knee. Validation--value in comparison with other assessment tests. Scand. J. Rheumatol. Suppl. 1987, 65, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N. WOMAC Osteoarthritis Index User Guide IX; Brisbane Queensland, Australia, 2008. [Google Scholar]
- Lopa, S.; Colombini, A.; Sansone, V.; Preis, F.W.; Moretti, M. Influence on chondrogenesis of human osteoarthritic chondrocytes in co-culture with donor-matched mesenchymal stem cells from infrapatellar fat pad and subcutaneous adipose tissue. Int. J. Immunopathol. Pharmacol. 2013, 26 (Suppl. 1), 23–31. [Google Scholar] [CrossRef]
- Lovati, A.B.; Colombini, A.; Recordati, C.; Ceriani, C.; Zagra, L.; Berzero, G.; Moretti, M. Chondrogenic capability of osteoarthritic chondrocytes from the trapeziometacarpal and hip joints. Cell Tissue Bank. 2016, 17, 171–177. [Google Scholar] [CrossRef]
- Heilmann, H.H.; Lindenhayn, K.; Walther, H.U. Synovial volume of healthy and arthrotic human knee joints. Z. Orthop. Ihre Grenzgeb. 1996, 134, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Stabler, T.V.; Kong, S.Y.; Varju, G.; McDaniel, G. Measurement of synovial fluid volume using urea. Osteoarthr. Cartil. 2007, 15, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Mano, H.; Sampei, C.; Shimizu, J.; Wada, M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartil. 2009, 17, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Boonmaleerat, K.; Wanachewin, O.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Fish Collagen Hydrolysates Modulate Cartilage Metabolism. Cell Biochem. Biophys. 2017, 76, 279–392. [Google Scholar] [CrossRef] [PubMed]
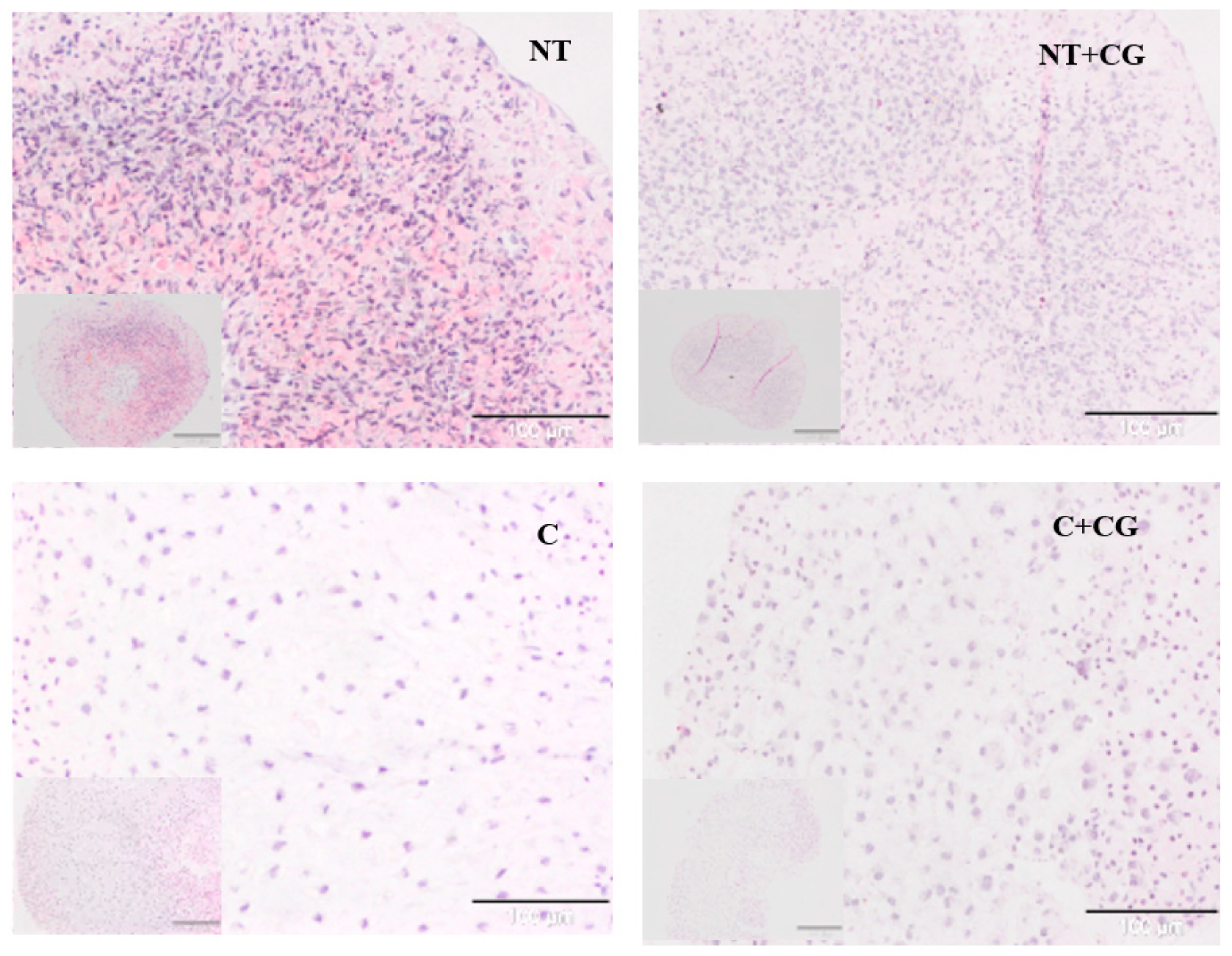
| Treatment | A Cell Density/Matrix Amount (Score 0–3) | B Cell Morphology (score 0–3) | A+B (Score 0–6) |
|---|---|---|---|
| NT a | 0.85 ± 0.76 | 0.4 ± 0.38 | 1.25 ± 1 |
| NT + CG b | 0.6 ± 0.57 | 0.75 ± 0.83 | 1.35 ± 1.28 |
| C c | 2.5 ± 0.31 ***§§§ | 2.10 ± 0.42 | 4.6 ± 0.63 ***§§§ |
| C+CG d | 2.05 ± 0.41 *§§ | 2.20 ± 0.45 * | 4.25 ± 0.77 ***§§§ |
| Treatment | Collagen I | Collagen II |
|---|---|---|
| C a | 2.45 ± 0.54 | 2.45 ± 0.11 |
| C + CG b | 1.31 ± 0.47 * | 2.94 ± 0.12 * |
| Parameter | Mean ± SD or Y/N (%/%) |
|---|---|
| Age (years) | 58.1 ± 11.1 |
| Weight (kg) | 76.5 ± 14.1 |
| Height (cm) | 173.7 ± 9 |
| BMI (Kg/m2) | 25.2 ± 3.3 |
| KL score 1,2,3,4 (%) | 4 (20); 11 (55); 3 (15); 3 (10) |
| M/F (%) | 14/6 (70/30) |
| Diabetes Y/N (%) | 2/18 (10/90) |
| Cardiovascular disorders Y/N (%) | 7/13 (35/65) |
| Metabolic disorders Y/N (%) | 4/16 (20/80) |
| Concomitant treatment Y/N (%) | 11/9 (55/45) |
| Baseline (Before First Injection) | T1 (15 Days after First Injection) | T2 (30 Days after First Injection) | FUP (About 6 Months after First Injection) | FUP vs. Baseline (%) | |
|---|---|---|---|---|---|
| Time (days) | N/A | 14.5.1 ± 2 | 22.9 ± 11.2 | 172.1 ± 22.7 | N/A |
| VAS at rest | 6 (22.5) | 0 (6.25) | 0 (5) | 0 (10) | −100% |
| VAS when moving | 50 (23.25) | 30 (27.75) | 21 (21.25) | 22.5 (20) | −55% |
| Lequesne Index | 9 (3.25) | 7 (5.25) | 3.5 (5.25) | 5 (5) | −44% |
| WOMAC (pain) | 4.5 (3.25) | 2 (3) | 1 (2.25) | 1 (2.25) | −77.8% |
| WOMAC (stiffness) | 2 (2.25) | 2 (2.25) | 1 (2.25) | 0.5 (1.25) | −75% |
| WOMAC (physical function) | 18 (13) | 13.5 (12.5) | 5 (10.25) | 5 (7) | −72.2% |
| WOMAC (Total) | 23.5 (14.5) | 17 (15.75) | 8 (13.75) | 6.5 (9.25) | −72.3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; de Girolamo, L.; Volpi, P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study. J. Clin. Med. 2019, 8, 975. https://doi.org/10.3390/jcm8070975
De Luca P, Colombini A, Carimati G, Beggio M, de Girolamo L, Volpi P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study. Journal of Clinical Medicine. 2019; 8(7):975. https://doi.org/10.3390/jcm8070975
Chicago/Turabian StyleDe Luca, Paola, Alessandra Colombini, Giulia Carimati, Michelangelo Beggio, Laura de Girolamo, and Piero Volpi. 2019. "Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study" Journal of Clinical Medicine 8, no. 7: 975. https://doi.org/10.3390/jcm8070975
APA StyleDe Luca, P., Colombini, A., Carimati, G., Beggio, M., de Girolamo, L., & Volpi, P. (2019). Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study. Journal of Clinical Medicine, 8(7), 975. https://doi.org/10.3390/jcm8070975







