Twin-Twin Transfusion Syndrome with and without Selective Fetal Growth Restriction Prior to Fetoscopic Laser Surgery: Short and Long-Term Outcome
Abstract
:1. Introduction
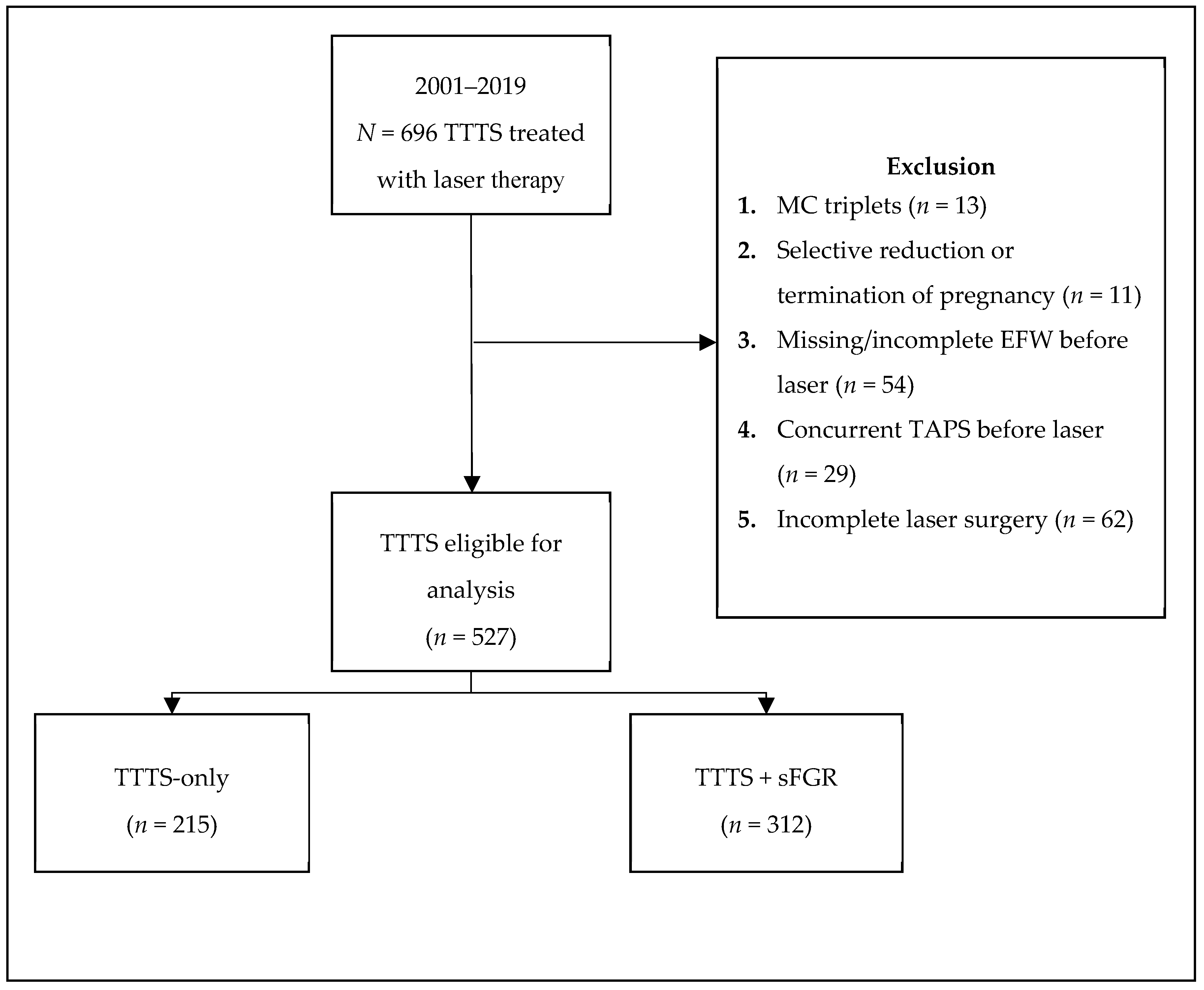
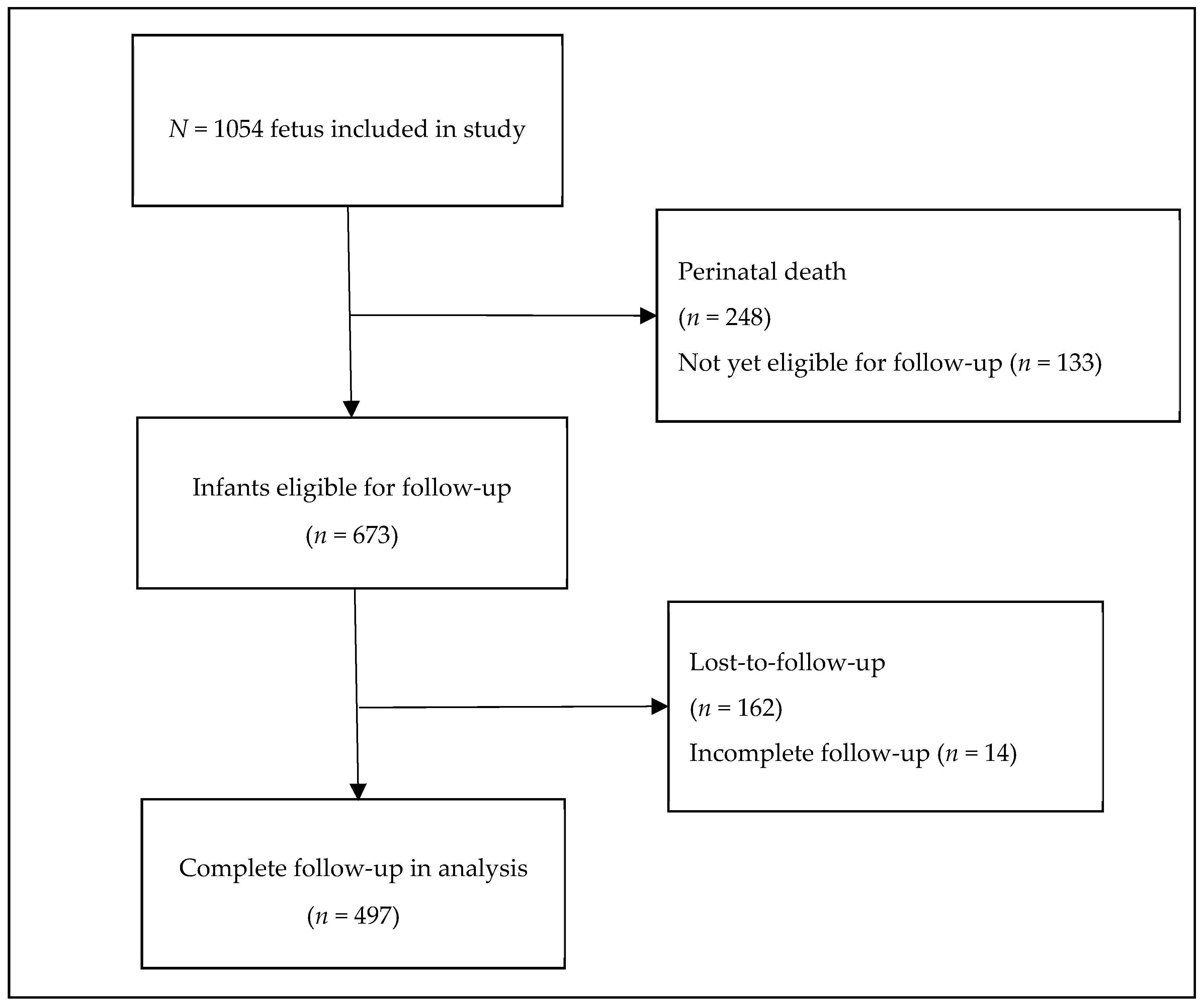
2. Materials and Methods
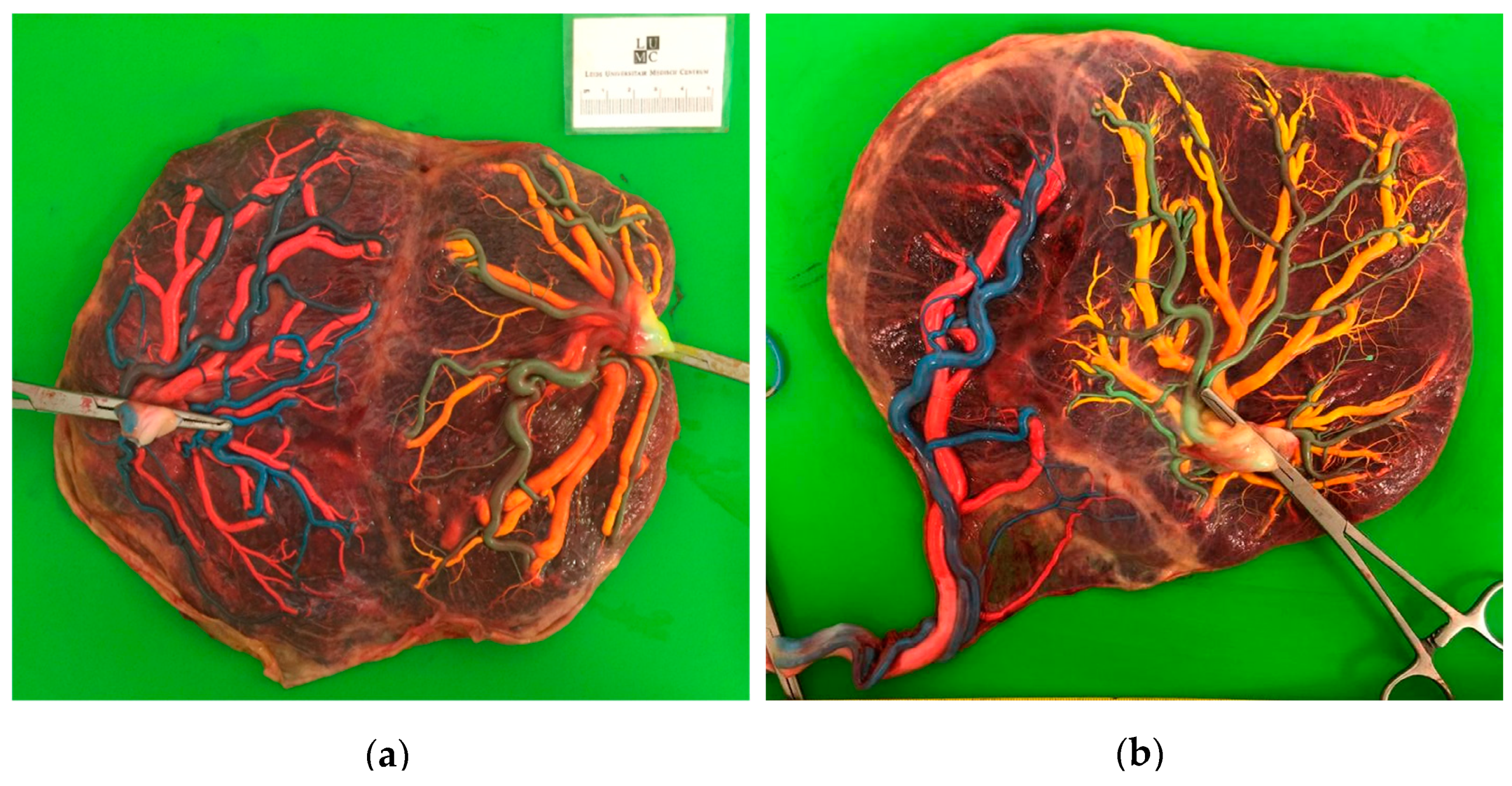
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benoit, R.M.; Baschat, A.A. Twin-to-twin transfusion syndrome: Prenatal diagnosis and treatment. Am. J. Perinatol. 2014, 31, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, C.; Gardiner, H.M.; Diehl, W.; Hecher, K. Fetal cardiovascular hemodynamics in twin-twin transfusion syndrome. Acta. Obstet. Gynecol. Scand. 2016, 95, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghella, V.; Kaufmann, M. Natural history of twin-twin transfusion syndrome. J. Reprod. Med. 2001, 46, 480–484. [Google Scholar] [PubMed]
- Van Klink, J.M.; Koopman, H.M.; Rijken, M.; Middeldorp, J.M.; Oepkes, D.; Lopriore, E. Long-Term Neurodevelopmental Outcome in Survivors of Twin-to-Twin Transfusion Syndrome. Twin Res. Hum. Genet. 2016, 19, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaghekke, F.; Lopriore, E.; Lewi, L.; Middeldorp, J.M.; van Zwet, E.W.; Weingertner, A.S.; Klumper, F.J.; DeKoninck, P.; Devlieger, R.; Kilby, M.D.; et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: An open-label randomised controlled trial. Lancet 2014, 383, 2144–2151. [Google Scholar] [CrossRef]
- Slaghekke, F.; Oepkes, D. Solomon Technique Versus Selective Coagulation for Twin-Twin Transfusion Syndrome. Twin Res. Hum. Genet. 2016, 19, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Lewi, L.; Cannie, M.; Blickstein, I.; Jani, J.; Huber, A.; Hecher, K.; Dymarkowski, S.; Gratacos, E.; Lewi, P.; Deprest, J. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. Am. J. Obstet. Gynecol. 2007, 197, 587.e1–587.e8. [Google Scholar] [CrossRef]
- Khalil, A.; Beune, I.; Hecher, K.; Wynia, K.; Ganzevoort, W.; Reed, K.; Lewi, L.; Oepkes, D.; Gratacos, E.; Thilaganathan, B.; et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: A Delphi procedure. Ultrasound Obstet. Gynecol. 2019, 53, 47–54. [Google Scholar] [CrossRef]
- Lopriore, E.; Sluimers, C.; Pasman, S.A.; Middeldorp, J.M.; Oepkes, D.; Walther, F.J. Neonatal morbidity in growth-discordant monochorionic twins: Comparison between the larger and the smaller twin. Twin Res. Hum. Genet. 2012, 15, 541–546. [Google Scholar] [CrossRef]
- Adegbite, A.L.; Castille, S.; Ward, S.; Bajoria, R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am. J. Obstet. Gynecol. 2004, 190, 156–163. [Google Scholar] [CrossRef]
- Swamy, R.S.; McConachie, H.; Ng, J.; Rankin, J.; Korada, M.; Sturgiss, S.; Embleton, N.D. Cognitive outcome in childhood of birth weight discordant monochorionic twins: The long-term effects of fetal growth restriction. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F512–F516. [Google Scholar] [CrossRef] [PubMed]
- Rustico, M.A.; Consonni, D.; Lanna, M.; Faiola, S.; Schena, V.; Scelsa, B.; Introvini, P.; Righini, A.; Parazzini, C.; Lista, G.; et al. Selective intrauterine growth restriction in monochorionic twins: Changing patterns in umbilical artery Doppler flow and outcomes. Ultrasound Obstet. Gynecol. 2017, 49, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Van Winden, K.R.; Quintero, R.A.; Kontopoulos, E.V.; Korst, L.M.; Llanes, A.; Chmait, R.H. Perinatal survival in cases of twin-twin transfusion syndrome complicated by selective intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2015, 28, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Senat, M.V.; Deprest, J.; Boulvain, M.; Paupe, A.; Winer, N.; Ville, Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N. Engl. J. Med. 2004, 351, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Quintero, R.A.; Morales, W.J.; Allen, M.H.; Bornick, P.W.; Johnson, P.K.; Kruger, M. Staging of twin-twin transfusion syndrome. J. Perinatol. 1999, 19, 550–555. [Google Scholar] [CrossRef]
- Tollenaar, L.S.A.; Lopriore, E.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.J.; Oepkes, D.; Slaghekke, F. Improved antenatal prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: A new antenatal classification system. Ultrasound Obstet. Gynecol. 2018, 53, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E.; Slaghekke, F.; Middeldorp, J.M.; Klumper, F.J.; van Lith, J.M.; Walther, F.J.; Oepkes, D. Accurate and simple evaluation of vascular anastomoses in monochorionic placenta using colored dye. J. Vis. Exp. 2011, 55, e3208. [Google Scholar] [CrossRef]
- Di Salvo, D.N.; Benson, C.B.; Laing, F.C.; Brown, D.L.; Frates, M.C.; Doubilet, P.M. Sonographic evaluation of the placental cord insertion site. AJR Am. J. Roentgenol. 1998, 170, 1295–1298. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- De Vries, L.S.; Eken, P.; Dubowitz, L.M. The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 1992, 49, 1–6. [Google Scholar] [CrossRef]
- Volpe, J.J. Intraventricular hemorrhage and brain injury in the premature infant. Diagnosis, prognosis, and prevention. Clin. Perinatol. 1989, 16, 387–411. [Google Scholar] [CrossRef]
- Levene, M.I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 1981, 56, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Groene, S.G.; Tollenaar, L.S.A.; Slaghekke, F.; Middeldorp, J.M.; Haak, M.; Oepkes, D.; Lopriore, E. Placental characteristics in monochorionic twins with selective intrauterine growth restriction in relation to the umbilical artery Doppler classification. Placenta 2018, 71, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chmait, R.H.; Chon, A.H.; Schrager, S.M.; Kontopoulos, E.V.; Quintero, R.A.; Vanderbilt, D.L. Donor catch-up growth after laser surgery for twin-twin transfusion syndrome. Early Hum. Dev. 2015, 91, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, S.J.; Boons, L.S.; Wolterbeek, R.; Middeldorp, J.M.; Klumper, F.J.; Lopriore, E.; Oepkes, D.; Haak, M.C. Prediction of single fetal demise after laser therapy for twin-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2016, 47, 356–362. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | TTTS-only (n = 430; 215 Pregnancies) | TTTS+sFGR (n = 624; 312 Pregnancies) | p-Value |
|---|---|---|---|
| Maternal age—years | 32.0 (29.0–35.0) | 31.0 (27.0–34.8) | 0.334 |
| Gravidity | 2 (1–3) | 2 (1–3) | 0.587 |
| Parity | 1 (0–1) | 1 (0–1) | 0.298 |
| Female | 196/424 (46) | 330/618 (53) | 0.023 |
| UA Doppler abnormalities | <0.0001 | ||
| Persistent A/REDF | 16/410 (4) | 73/592 (12) | |
| Intermittent A/REDF | 10/410 (2) | 24/592 (4) | |
| Quintero stage | <0.0001 | ||
| I | 39 (18) | 36 (12) | |
| II | 94 (44) | 87 (28) | |
| III | 77 (36) | 177 (57) | |
| IV | 5 (2) | 12 (4) | |
| Gestational age at birth—weeks | 33.1 (29.3–35.9) | 33.0 (29.7–35.7) | 0.780 |
| Caesarean | 150 (36) | 198 (33) | 0.262 |
| Birth weight—g | |||
| Donor | 1950 (1363–2415) | 1640 (1093–2125) | <0.0001 |
| Recipient | 2005 (1540–2475) | 1919 (1450–2303) | 0.136 |
| Birth weight discordance—% | 8.1 (3.3–13.7) | 15.6 (6.8–26.7) | <0.0001 |
| Birth weight discordance >25% | 10/161 (6) | 61/205 (30) | <0.0001 |
| Small for gestational age | 73/352 (21) | 231/465 (49) | <0.0001 |
| Donor is smaller twin at birth | 106/161 (66) | 172/206 (84) | <0.0001 |
| Characteristics | TTTS-only (n = 430; 215 Pregnancies) | TTTS + sFGR (n = 624; 312 Pregnancies) | p-Value |
|---|---|---|---|
| Gestational age at laser—weeks | 19.3 (17.4–22.0) | 20.0 (17.9–22.1) | 0.149 |
| Total anastomoses at fetoscopy—n | 6 (4–8) | 6 (4–7) | 0.412 |
| AV anastomoses—n | 4 (3–5) | 3 (3–4) | 0.176 |
| VA anastomoses—n | 2 (1–3) | 2 (1–3) | 0.822 |
| Presence of AA anastomoses—n/N (%) | 19/203 (9) | 51/296 (17) | 0.013 |
| Presence of VV anastomoses—n/N (%) | 19/203 (9) | 28/296 (10) | 0.970 |
| Residual anastomoses at birth—n/N (%) | 13/146 (9) | 18/209 (9) | 0.924 |
| Cord insertion—n/N (%) | |||
| Velamentous | 61/330 (19) | 116/440 (26) | 0.007 |
| Marginal | 63/330 (19) | 108/438 (25) | 0.083 |
| Placental share—% | |||
| Donor | 45.6 (41.2–50.7) | 42.6 (33.6–50.0) | 0.001 |
| Recipient | 54.4 (49.3–58.8) | 57.3 (50.0–66.4) | 0.002 |
| Placental share discordance—% | 22.1 (10.7–37.2) | 33.6 (18.1–52.0) | <0.0001 |
| TTTS-only (n = 430; 215 Pregnancies) | TTTS + sFGR (n = 624; 312 Pregnancies) | p-Value | |
|---|---|---|---|
| Donor fetal death | 36 (17) | 76 (24) | 0.036 |
| Within 24h of laser | 9 (4) | 27 (9) | 0.404 |
| Recipient fetal death | 38 (18) | 68 (22) | 0.246 |
| Within 24h of laser | 13 (6) | 31 (10) | 0.377 |
| Severe neonatal morbidity | 66/287 (23) | 86/418 (21) | 0.593 |
| Donor | 32/144 (22) | 36/204 (18) | 0.289 |
| Recipient | 34/143 (24) | 50/214 (23) | 0.928 |
| Respiratory distress syndrome | 56/297 (19) | 71/429 (17) | 0.610 |
| Donor | 29/150 (19) | 29/209 (14) | 0.166 |
| Recipient | 27/147 (18) | 42/220 (19) | 0.862 |
| Patent ductus arteriosus | 14/295 (5) | 8/429 (2) | 0.100 |
| Donor | 6/149 (4) | 2/209 (1) | 0.053 |
| Recipient | 8/146 (6) | 6/220 (3) | 0.179 |
| Necrotizing enterocolitis | 9/292 (3) | 11/429 (3) | 0.770 |
| Donor | 4/147 (3) | 4/209 (2) | 0.613 |
| Recipient | 5/145 (3) | 7/220 (3) | 0.889 |
| Severe cerebral injury | 15/296 (5) | 30/439 (7) | 0.350 |
| Donor | 5/149 (3) | 14/213 (7) | 0.177 |
| Recipient | 10/147 (7) | 16/226 (7) | 0.918 |
| Neonatal mortality | 16/355 (5) | 14/478 (3) | 0.249 |
| Donor | 6/179 (3) | 11/235 (5) | 0.500 |
| Recipient | 10/176 (6) | 3/243 (1) | 0.010 |
| Perinatal survival | 339/429 (79) | 464/622 (75) | 0.176 |
| Donor | 173/215 (81) | 224/311 (72) | 0.027 |
| Recipient | 166/214 (78) | 240/311 (77) | 0.914 |
| Severe NDI at 2-year follow-up | 13/198 (7) | 27/299 (9) | 0.385 |
| Donor | 6/101 (6) | 13/150 (9) | 0.423 |
| Recipient | 7/97 (7) | 14/149 (9) | 0.550 |
| Disease-free survival | 185/288 (64) | 269/457 (59) | 0.176 |
| Donor | 95/143 (66) | 135/237 (57) | 0.067 |
| Recipient | 90/145 (62) | 134/220 (61) | 0.824 |
| Characteristics | Perinatal Survival (N = 464) | Perinatal Death (n = 158) | Univariate Analysis OR (95% CI) | SE | p-Value | Multivariate Analysis OR (95% CI) | SE | p-Value |
|---|---|---|---|---|---|---|---|---|
| Severe sFGR (EFW <3rd centile)—n/N (%) | 104/464 (22) | 40/158 (25) | 1.3 (0.9–1.9) | 0.197 | 0.197 | |||
| EFW discordance >25%—n/N (%) | 209/464 (45) | 67/158 (42) | 0.9 (0.6–1.4) | 0.217 | 0.620 | |||
| UA Dopplers prior to laser—n/N (%) | ||||||||
| Persistent A/REDF | 46/439 (11) | 27/151 (18) | 1.9 (1.1–3.1) | 0.263 | 0.018 | 1.7 (1.0–2.9) | 0.268 | 0.049 |
| Intermittent A/REDF | 18/439 (4) | 6/151 (4) | 1.0 (0.4–2.5) | 0.481 | 0.946 | |||
| Female—n/N (%) | 237/462 (51) | 93/154 (60) | 1.4 (0.9–2.2) | 0.218 | 0.090 | |||
| Quintero stage—n (%) | 0.847 | |||||||
| I | 50 (11) | 22 (14) | 0.8 (0.2–2.8) | 0.665 | ||||
| II | 132 (28) | 42 (27) | 1.0 (0.3–3.6) | 0.623 | ||||
| III | 264 (57) | 88 (56) | 1.0 (0.3–3.2) | 0.605 | ||||
| IV | 18 (4) | 6 (4) | - | - | ||||
| Gestational age at laser—weeks | 20.0 (18.0–22.4) | 19.8 (17.0–21.6) | 1.1 (1.0–1.2) | 0.033 | 0.008 | 1.1 (1.0–1.1) | 0.032 | 0.023 |
| Presence of AA anastomoses—n/N (%) | 36/222 (16) | 15/73 (21) | 1.3 (0.7–2.6) | 0.342 | 0.397 | |||
| Velamentous cord insertion—n/N (%) | 95/368 (26) | 19/70 (27) | 1.3 (0.8–2.0) | 0.227 | 0.264 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groene, S.G.; Tollenaar, L.S.A.; van Klink, J.M.M.; Haak, M.C.; Klumper, F.J.C.M.; Middeldorp, J.M.; Oepkes, D.; Slaghekke, F.; Lopriore, E. Twin-Twin Transfusion Syndrome with and without Selective Fetal Growth Restriction Prior to Fetoscopic Laser Surgery: Short and Long-Term Outcome. J. Clin. Med. 2019, 8, 969. https://doi.org/10.3390/jcm8070969
Groene SG, Tollenaar LSA, van Klink JMM, Haak MC, Klumper FJCM, Middeldorp JM, Oepkes D, Slaghekke F, Lopriore E. Twin-Twin Transfusion Syndrome with and without Selective Fetal Growth Restriction Prior to Fetoscopic Laser Surgery: Short and Long-Term Outcome. Journal of Clinical Medicine. 2019; 8(7):969. https://doi.org/10.3390/jcm8070969
Chicago/Turabian StyleGroene, Sophie G., Lisanne S. A. Tollenaar, Jeanine M. M. van Klink, Monique C. Haak, Frans J. C. M. Klumper, Johanna M. Middeldorp, Dick Oepkes, Femke Slaghekke, and Enrico Lopriore. 2019. "Twin-Twin Transfusion Syndrome with and without Selective Fetal Growth Restriction Prior to Fetoscopic Laser Surgery: Short and Long-Term Outcome" Journal of Clinical Medicine 8, no. 7: 969. https://doi.org/10.3390/jcm8070969
APA StyleGroene, S. G., Tollenaar, L. S. A., van Klink, J. M. M., Haak, M. C., Klumper, F. J. C. M., Middeldorp, J. M., Oepkes, D., Slaghekke, F., & Lopriore, E. (2019). Twin-Twin Transfusion Syndrome with and without Selective Fetal Growth Restriction Prior to Fetoscopic Laser Surgery: Short and Long-Term Outcome. Journal of Clinical Medicine, 8(7), 969. https://doi.org/10.3390/jcm8070969






