Incidence and Cost of Acute Kidney Injury in Hospitalized Patients with Infective Endocarditis
Abstract
1. Introduction
2. Materials and Methods
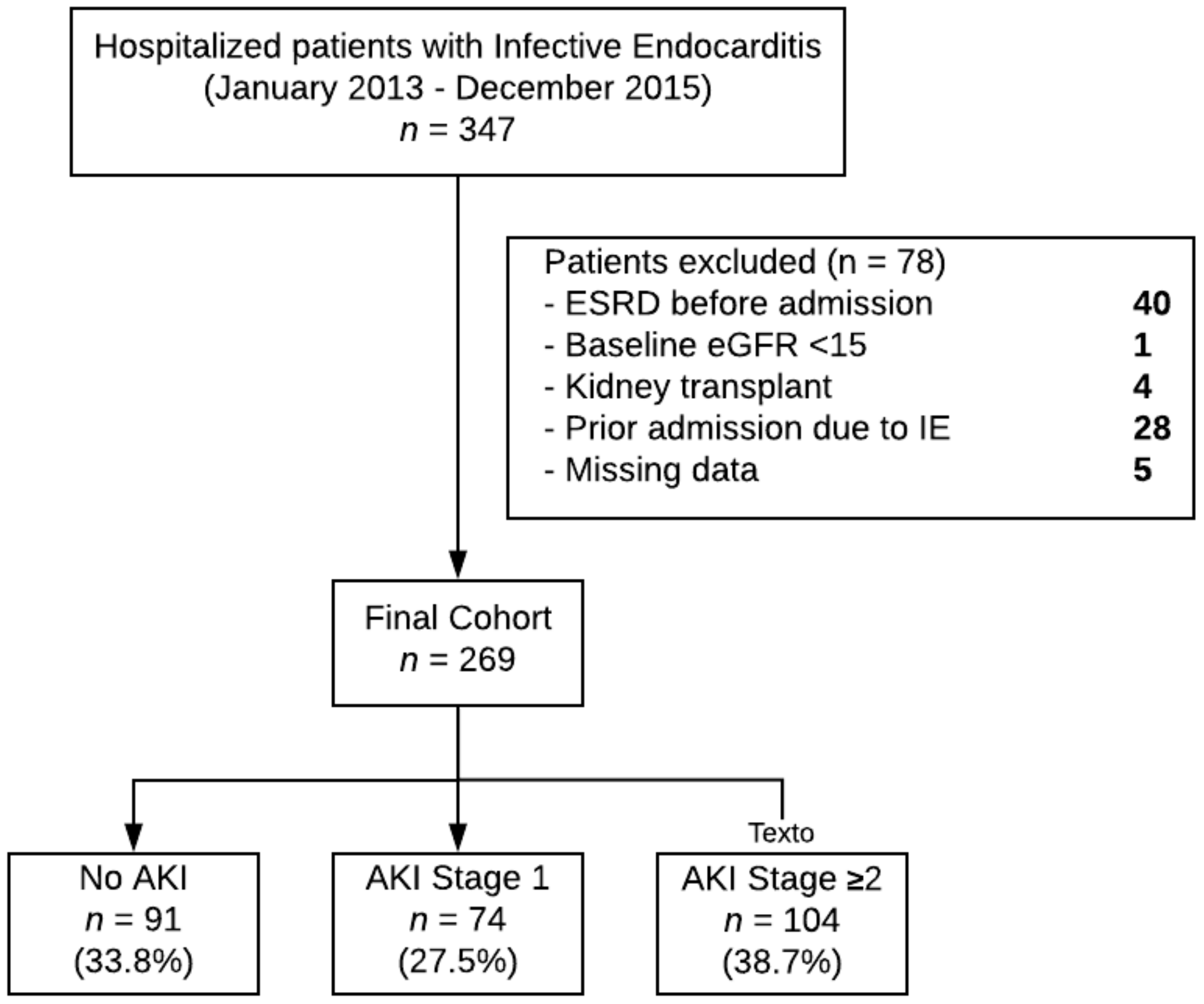
2.1. Study Design and Participants
2.2. Study Variables and Definitions
2.3. Study Outcomes
2.3.1. Clinical Outcomes
2.3.2. Healthcare Cost Outcomes
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Clinical Parameters Associated with AKI
3.3. Clinical Outcomes Associated with AKI
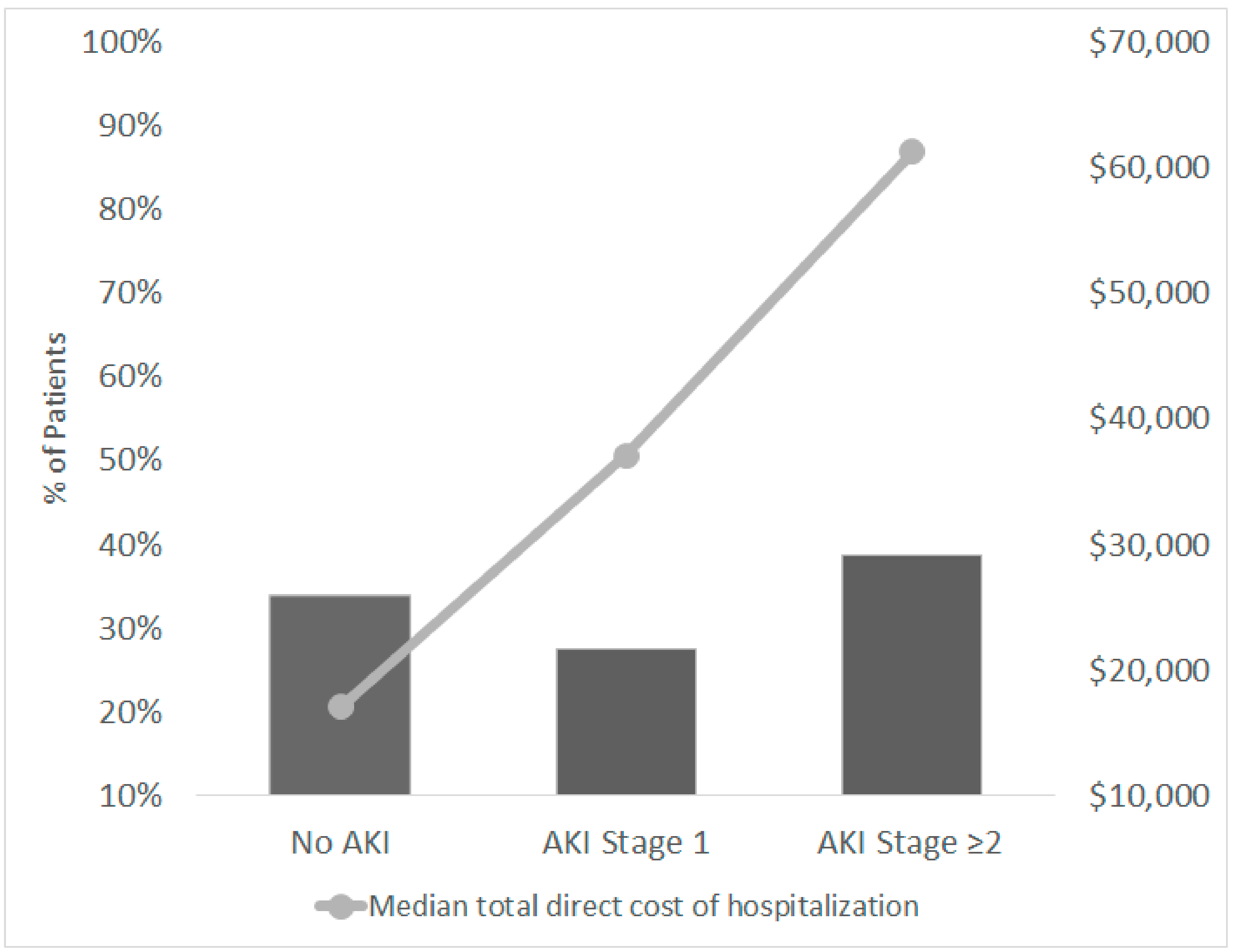
3.4. Healthcare Costs Outcomes
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Grisoli, D.; Cautela, J.; Riberi, A.; Raoult, D.; Habib, G. Infective endocarditis: Prevention, diagnosis, and management. Can. J. Cardiol. 2014, 30, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Bolger, A.F.; Levison, M.E.; Ferrieri, P.; Gerber, M.A.; Tani, L.Y.; Gewitz, M.H.; et al. Infective endocarditis: Diagnosis, antimicrobial therapy, and management of complications: A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation 2005, 111, e394–e434. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Patel, N.J.; Deshmukh, A.; Golwala, H.; Patel, N.; Badheka, A.; Hirsch, G.A.; Mehta, J.L. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J. Am. Coll. Cardiol. 2015, 65, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, N.; Chikwe, J.; Itagaki, S.; Gelijns, A.C.; Adams, D.H.; Egorova, N.N. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA 2017, 317, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Fleischauer, A.T.; Ruhl, L.; Rhea, S.; Barnes, E. Hospitalizations for Endocarditis and Associated Health Care Costs Among Persons with Diagnosed Drug Dependence—North Carolina, 2010–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 569–573. [Google Scholar] [CrossRef]
- Vivolo-Kantor, A.M.; Seth, P.; Gladden, R.M.; Mattson, C.L.; Baldwin, G.T.; Kite-Powell, A.; Coletta, M.A. Vital Signs: Trends in Emergency Department Visits for Suspected Opioid Overdoses—United States, July 2016–September 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 279–285. [Google Scholar] [CrossRef]
- Jones, C.M. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers—United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013, 132, 95–100. [Google Scholar] [CrossRef]
- Scheidegger, C.; Zimmerli, W. Incidence and spectrum of severe medical complications among hospitalized HIV-seronegative and HIV-seropositive narcotic drug users. AIDS 1996, 10, 1407–1414. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef]
- Szczech, L.A.; Harmon, W.; Hostetter, T.H.; Klotman, P.E.; Powe, N.R.; Sedor, J.R.; Smedberg, P.; Himmelfarb, J. World Kidney Day 2009: Problems and challenges in the emerging epidemic of kidney disease. J. Am. Soc. Nephrol. 2009, 20, 453–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silver, S.A.; Long, J.; Zheng, Y.; Chertow, G.M. Cost of Acute Kidney Injury in Hospitalized Patients. J. Hosp. Med. 2017, 12, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rewa, O.; Bagshaw, S.M. Acute kidney injury—Epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Gallo, G.R.; Baldwin, D.S. Glomerulonephritis in bacterial endocarditis. Am. J. Kidney. Dis. 1984, 3, 371–379. [Google Scholar] [CrossRef]
- Majumdar, A.; Chowdhary, S.; Ferreira, M.A.; Hammond, L.A.; Howie, A.J.; Lipkin, G.W.; Littler, W.A. Renal pathological findings in infective endocarditis. Nephrol. Dial. Transplant. 2000, 15, 1782–1787. [Google Scholar] [CrossRef]
- Boils, C.L.; Nasr, S.H.; Walker, P.D.; Couser, W.G.; Larsen, C.P. Update on endocarditis-associated glomerulonephritis. Kidney Int. 2015, 87, 1241–1249. [Google Scholar] [CrossRef]
- Conlon, P.J.; Jefferies, F.; Krigman, H.R.; Corey, G.R.; Sexton, D.J.; Abramson, M.A. Predictors of prognosis and risk of acute renal failure in bacterial endocarditis. Clin. Nephrol. 1998, 49, 96–101. [Google Scholar]
- Ritchie, B.M.; Hirning, B.A.; Stevens, C.A.; Cohen, S.A.; DeGrado, J.R. Risk factors for acute kidney injury associated with the treatment of bacterial endocarditis at a tertiary academic medical center. J. Chemother. 2017, 29, 292–298. [Google Scholar] [CrossRef]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef]
- Durack, D.T.; Lukes, A.S.; Bright, D.K. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Duke Endocarditis Service. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Keslin, M.H.; Messner, R.P.; Williams, R.C., Jr. Glomerulonephritis with subacute bacterial endocarditis. Immunofluorescent studies. Arch. Intern. Med. 1973, 132, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Zarbock, A.; Nadim, M.K. What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med. 2017, 43, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Jacob, K.A.; Srivastava, A.; Chen, M.E.; Christov, M.; Juppner, H.; Sabbisetti, V.S.; Martin, A.; Wolf, M.; Waikar, S.S. Fibroblast Growth Factor 23 Levels Associate with AKI and Death in Critical Illness. J. Am. Soc. Nephrol. 2017, 28, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, G.D.; Angus, D.C.; Pinsky, M.R.; Curtis, J.R.; Connors, A.F., Jr.; Bernard, G.R. Outcomes research in critical care: Results of the American Thoracic Society Critical Care Assembly Workshop on Outcomes Research. The Members of the Outcomes Research Workshop. Am. J. Resp. Crit. Care. 1999, 160, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Levey, A.S.; Tonelli, M.; Tan, Z.; Barry, R.; Pannu, N.; Ravani, P.; Klarenbach, S.W.; Manns, B.J.; Hemmelgarn, B.R. Incidence and Prognosis of Acute Kidney Diseases and Disorders Using an Integrated Approach to Laboratory Measurements in a Universal Health Care. JAMA Netw. Open 2019, 2, e191795. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Kellum, J.A.; Ronco, C. Subclinical AKI—An emerging syndrome with important consequences. Nat. Rev. Nephrol. 2012, 8, 735–739. [Google Scholar] [CrossRef]
- Ronco, C.; Kellum, J.A.; Haase, M. Subclinical AKI is still AKI. Crit. Care 2012, 16, 313. [Google Scholar] [CrossRef]
- Cresti, A.; Chiavarelli, M.; Scalese, M.; Nencioni, C.; Valentini, S.; Guerrini, F.; D’Aiello, I.; Picchi, A.; De Sensi, F.; Habib, G. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc. Diagn. Ther. 2017, 7, 27–35. [Google Scholar] [CrossRef]
- Alkhawam, H.; Sogomonian, R.; Zaiem, F.; Vyas, N.; El-Hunjul, M.; Jolly, J.; Al-Khazraji, A.; Ashraf, A. Morbidity and mortality of infective endocarditis in a hospital system in New York City serving a diverse urban population. J. Invest. Med. 2016, 64, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.M.; Walton, B.I.; Kharbanda, R.K.; Hardy, R.; Wilson, A.P.; Swanton, R.H. Mortality from infective endocarditis: Clinical predictors of outcome. Heart 2002, 88, 53–60. [Google Scholar] [CrossRef]
- Koyner, J.L.; Davison, D.L.; Brasha-Mitchell, E.; Chalikonda, D.M.; Arthur, J.M.; Shaw, A.D.; Tumlin, J.A.; Trevino, S.A.; Bennett, M.R.; Kimmel, P.L.; et al. Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J. Am. Soc. Nephrol. 2015, 26, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.T.; Witten, J.; Shrestha, N.K.; Blackstone, E.H.; Pettersson, G.B. Tricuspid valve endocarditis. Ann. Cardiothorac. Surg. 2017, 6, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, A.; Dombrovskiy, V.; Saadat, S.; Batsides, G.; Ghaly, A.; Spotnitz, A.; Lee, L.Y. Patients with Infectious Endocarditis and Drug Dependence Have Worse Clinical Outcomes after Valvular Surgery. Surg. Infect. 2017, 18, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Collister, D.; Pannu, N.; Ye, F.; James, M.; Hemmelgarn, B.; Chui, B.; Manns, B.; Klarenbach, S.; Alberta Kidney Disease Network. Health Care Costs Associated with AKI. Clin. J. Am. Soc. Nephrol. 2017, 12, 1733–1743. [Google Scholar] [CrossRef]
| No AKI | AKI Stage 1 | AKI Stage ≥2 | p | |
|---|---|---|---|---|
| n = 91 | n = 74 | n = 104 | ||
| Demographics | ||||
| Age, years, mean ± SD | 44.6 ± 15.6 | 45.5 ± 16.5 | 45.8 ± 16.8 | 0.860 |
| Gender, male, n (%) | 62 (68.1) | 34 (46.0) | 64 (61.5) | 0.013 |
| Ethnic group, white, n (%) | 86 (94.5) | 71 (96.0) | 99 (95.2) | 0.939 |
| BMI, kg/m2, mean ± SD | 27.9 ± 10.9 | 29.8 ± 11.0 | 26.9 ± 7.1 | 0.140 |
| Comorbidity | ||||
| Elixhauser Score, median (IQ1-IQ3) | 4.0 (3.0–6.0) | 6.0 (4.0–7.0) | 7.0 (5.0–8.0) | <0.001 |
| Diabetes, n (%) | 23 (25.3) | 14 (18.9) | 18 (17.3) | 0.361 |
| Hypertension, n (%) | 55 (60.4) | 41 (55.4) | 69 (66.4) | 0.328 |
| Hepatitis B, n (%) | 1 (1.1) | 9 (12.2) | 7 (6.7) | 0.011 |
| Hepatitis C, n (%) | 27 (29.7) | 38 (51.4) | 51 (49.0) | 0.006 |
| Congenital heart defect, n (%) | 6 (6.6) | 8 (10.8) | 9 (8.7) | 0.628 |
| Current substance use disorder, n (%) | 46 (50.5) | 42 (56.8) | 55 (52.9) | 0.672 |
| Baseline eGFR, mL/min/1.73 m2, median (IQ1-IQ3) | 105.3 (69.1–127.6) | 96.8 (45.5–123.9) | 103.2 (71.4–122.9) | 0.220 |
| Medications During Hospitalization | ||||
| Opiods, n (%) | 3 (3.3) | 6 (8.1) | 9 (8.7) | 0.279 |
| ACEI or ARB, n (%) | 36 (39.6) | 12 (16.2) | 21 (20.2) | <0.001 |
| Aminoglycosides, n (%) | 36 (39.6) | 31 (41.9) | 52 (50.0) | 0.305 |
| Diuretic, n (%) | 38 (41.8) | 51 (68.9) | 83 (79.8) | <0.001 |
| NSAIDs, n (%) | 54 (59.3) | 51 (68.9) | 77 (74.0) | 0.088 |
| Pressor or inotrope, n (%) | 0 (0.0) | 5 (6.8) | 19 (18.3) | <0.001 |
| Vancomycin, n (%) | 76 (83.5) | 69 (93.2) | 89 (85.6) | 0.142 |
| Piperacillin/tazobactam, n (%) | 30 (33.0) | 23 (31.1) | 38 (36.5) | 0.733 |
| Cefepime, n (%) | 25 (27.5) | 30 (40.5) | 39 (37.5) | 0.169 |
| Vancomycin + Pip/Tazo, n (%) | 30 (33.0) | 24 (32.4) | 36 (34.6) | 0.948 |
| Vancomycin + Cefepime, n (%) | 26 (28.6) | 32 (43.2) | 37 (35.6) | 0.146 |
| Infective endocarditis characteristics | ||||
| ICU care, n (%) | 38 (41.8) | 39 (52.7) | 46 (44.2) | 0.346 |
| Sepsis, n (%) | 32 (35.2) | 36 (48.7) | 66 (63.5) | <0.001 |
| Osteomyelitis, n (%) | 14 (15.4) | 7 (9.5) | 18 (17.3) | 0.327 |
| Number of affected cardiac valves, | 0.006 | |||
| 0, n (%) | 45 (49.5) | 26 (35.1) | 29 (27.9) | |
| 1, n (%) | 40 (44.0) | 42 (56.8) | 56 (53.8) | |
| ≥2, n (%) | 6 (6.5) | 6 (8.1) | 19 (18.3) | |
| Affected valve, | ||||
| Mitral, n (%) | 31 (34.1) | 27 (37.0) | 34 (32.7) | 0.837 |
| Aortic, n (%) | 38 (41.8) | 24 (32.9) | 35 (33.7) | 0.395 |
| Tricuspid, n (%) | 20 (22.0) | 18 (24.7) | 47 (45.2) | <0.001 |
| Pulmonic, n (%) | 1 (1.1) | 2 (2.7) | 2 (1.9) | 0.856 |
| Type of valve, prosthetic, n (%) | 23 (25.3) | 20 (27.0) | 33 (31.7) | 0.585 |
| Procedures, | ||||
| Valve replacement, n (%) | 16 (17.6) | 14 (18.9) | 33 (31.7) | 0.037 |
| Valve repair, n (%) | 11 (12.1) | 12 (16.2) | 16 (15.4) | 0.716 |
| Other, n (%) | 8 (8.8) | 3 (4.1) | 13 (12.5) | 0.165 |
| Microbiologic data | ||||
| MRSA, n (%) | 23 (25.3) | 20 (27.0) | 37 (35.6) | 0.286 |
| MSSA, n (%) | 16 (17.6) | 12 (16.2) | 27 (26.0) | 0.227 |
| MSSE, n (%) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0.458 |
| Other Staphylococcus, n (%) | 11 (12.1) | 12 (16.2) | 8 (7.7) | 0.197 |
| Streptococcus, n (%) | 24 (26.4) | 13 (17.6) | 17 (16.3) | 0.155 |
| Enterococcus, n (%) | 12 (13.2) | 15 (20.3) | 16 (15.4) | 0.460 |
| Gram-negative rods, n (%) | 10 (11.0) | 13 (17.6) | 15 (14.4) | 0.492 |
| Rothia, n (%) | 2 (2.2) | 2 (2.7) | 1 (1.0) | 0.659 |
| Candida sp., n (%) | 1 (1.1) | 5 (6.8) | 9 (8.7) | 0.068 |
| Negative culture, n (%) | 4 (4.4) | 4 (5.4) | 2 (1.9) | 0.428 |
| AKI Stage ≥1 vs. No AKI | AKI Stage ≥2 vs. AKI Stage 1 or No AKI | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| ACEI or ARB, Yes vs. No | 0.28 ** | 0.13–0.57 | 0.66 | 0.33–1.32 |
| Tricuspid valve affected, Yes vs. No | 1.71 | 0.84–3.46 | 2.97 ** | 1.59–5.51 |
| Use of diuretic, Yes Vs. No | 3.18 ** | 1.63–6.23 | 2.04 * | 1.04–4.01 |
| Hepatitis B coinfection, Yes vs. No | 6.57 | 0.78–55.16 | 0.61 | 0.19–1.97 |
| Use of NSAID, Yes vs. No | 1.95 * | 1.01–3.76 | 1.72 | 0.90–3.25 |
| Pressor or Inotrope need, Yes vs. No | - | - | 4.94 * | 1.52–16.01 |
| Diabetes, Yes vs. No | 0.47 | 0.21–1.06 | 0.55 | 0.26–1.19 |
| Elixhauser score, per 1-unit score | 1.35 ** | 1.16–1.56 | 1.35 ** | 1.17–1.55 |
| No AKI n = 91 | AKI Stage 1 n = 74 | AKI Stage ≥2 n = 104 | p Value | |
|---|---|---|---|---|
| Clinical outcomes | ||||
| Hospital mortality, n (%) | 14 (15.4) | 8 (10.8) | 20 (19.2) | 0.312 |
| Readmission due to IE, (%) | 4 (4.4) | 4 (5.4) | 3 (2.9) | 0.687 |
| MAKE50 *, n (%) | 35 (38.5) | 40 (54.1) | 62 (59.6) | 0.011 |
| MAKE25 #, n (%) | 55 (60.4) | 51 (68.9) | 77 (74.0) | 0.125 |
| Healthcare cost outcomes | ||||
| Total direct hospitalization cost, dollars, median (IQ1-IQ3) | 17,069 (6722–31,910) | 37,111 (20,100–58,258) | 61,357 (34,164–88,495) | <0.001 |
| Hospital length of stay, days, median (IQ1-IQ3) | 9.0 (5.0–17.5) | 23.0 (12.0–43.8) | 34.5 (16.8–48.0) | <0.001 |
| Telemetry bed days, median (IQ1-IQ3) | 3.0 (0.0–10.0) | 8.0 (2.0–17.0) | 9.5 (1.0–20.0) | <0.001 |
| ICU length of stay, median (IQ1-IQ3) | 0.0 (0.0–2.0) | 1.5 (0.0–5.0) | 6.0 (1.5–14.5) | <0.001 |
| MAKE50 | MAKE25 | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Sepsis, Yes vs. No | 2.03 ** | 1.23–3.36 | 1.56 | 0.91–2.66 |
| AKI Severity | ||||
| Stage ≥2 vs. No AKI | 1.97 | 1.09–3.58 | 1.66 | 0.89–3.10 |
| Stage 1 vs. No AKI | 1.74 | 0.92–3.28 | 1.37 | 0.71–2.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Soriano, V.; Donaldson, K.; Du, G.; Li, Y.; Lambert, J.; Rudy, M.; Cleland, D.; Thornton, A.; Fanucchi, L.C.; Huaman, M.A.; et al. Incidence and Cost of Acute Kidney Injury in Hospitalized Patients with Infective Endocarditis. J. Clin. Med. 2019, 8, 927. https://doi.org/10.3390/jcm8070927
Ortiz-Soriano V, Donaldson K, Du G, Li Y, Lambert J, Rudy M, Cleland D, Thornton A, Fanucchi LC, Huaman MA, et al. Incidence and Cost of Acute Kidney Injury in Hospitalized Patients with Infective Endocarditis. Journal of Clinical Medicine. 2019; 8(7):927. https://doi.org/10.3390/jcm8070927
Chicago/Turabian StyleOrtiz-Soriano, Victor, Katherine Donaldson, Gaixin Du, Ye Li, Joshua Lambert, Mark Rudy, Dan Cleland, Alice Thornton, Laura C. Fanucchi, Moises A. Huaman, and et al. 2019. "Incidence and Cost of Acute Kidney Injury in Hospitalized Patients with Infective Endocarditis" Journal of Clinical Medicine 8, no. 7: 927. https://doi.org/10.3390/jcm8070927
APA StyleOrtiz-Soriano, V., Donaldson, K., Du, G., Li, Y., Lambert, J., Rudy, M., Cleland, D., Thornton, A., Fanucchi, L. C., Huaman, M. A., & Neyra, J. A. (2019). Incidence and Cost of Acute Kidney Injury in Hospitalized Patients with Infective Endocarditis. Journal of Clinical Medicine, 8(7), 927. https://doi.org/10.3390/jcm8070927




