Impact of Transitory ROSC Events on Neurological Outcome in Patients with Out-of-Hospital Cardiac Arrest
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Characteristics of Study Setting and OHCA Management
2.3. CA Data Processing
- -
- -
- “sustained” (s-ROSC), if the condition persisted until arrival at the emergency department [4].
2.4. Other Collected Variables
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhan, L.; Yang, L.J.; Huang, Y.; He, Q.; Liu, G.J. Continuous chest compression versus interrupted chest compression for cardiopulmonary resuscitation of non-asphyxial out-of-hospital cardiac arrest. Cochrane Database Syst. Rev. 2017, 3, CD010134. [Google Scholar] [CrossRef]
- Sasson, C.; Rogers, M.A.; Dahl, J.; Kellermann, A.L. Predictors of survival from out-of-hospital cardiac arrest: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Stub, D.; Nehme, Z.; Bernard, S.; Lijovic, M.; Kaye, D.M.; Smith, K. Exploring which patients without return of spontaneous circulation following ventricular fibrillation out-of-hospital cardiac arrest should be transported to hospital? Resuscitation 2014, 85, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Jacobs, I.G.; Nadkarni, V.M.; Berg, R.A.; Bhanji, F.; Biarent, D.; Bossaert, L.L.; Brett, S.J.; Chamberlain, D.; de Caen, A.R.; et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update of the Utstein resuscitation registry templates for out-of-hospital cardiac arrest. Resuscitation 2015, 96, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Wik, L.; Kramer-Johansen, J.; Myklebust, H.; Sorebo, H.; Svensson, L.; Fellows, B.; Steen, P.A. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. Jama 2005, 293, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Skogvoll, E.; Eftestol, T.; Gundersen, K.; Kvaloy, J.T.; Kramer-Johansen, J.; Olasveengen, T.M.; Steen, P.A. Dynamics and state transitions during resuscitation in out-of-hospital cardiac arrest. Resuscitation 2008, 78, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, D. Predictors of survival from out-of-hospital cardiac arrest. Heart (Br. Card. Soc.) 2010, 96, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Monsieurs, K.G.; Nolan, J.P.; Bossaert, L.L.; Greif, R.; Maconochie, I.K.; Nikolaou, N.I.; Perkins, G.D.; Soar, J.; Truhlar, A.; Wyllie, J.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation 2015, 95, 1–80. [Google Scholar] [CrossRef]
- Nolan, J.P.; Soar, J.; Wenzel, V.; Paal, P. Cardiopulmonary resuscitation and management of cardiac arrest. Nat. Reviews. Cardiol. 2012, 9, 499–511. [Google Scholar] [CrossRef]
- Ristagno, G.; Semeraro, F.; Radeschi, G.; Pellis, T.; Gordini, G.; Ferro, S.; Cerchiari, E. The “Italian Registry of Cardiac Arrest—RIAC”, a National achievement to portrait the Italian reality and to contribute to the wider European vision by “EuReCa”. Resuscitation 2014, 85, e193–e194. [Google Scholar] [CrossRef]
- Arrich, J.; Holzer, M.; Havel, C.; Mullner, M.; Herkner, H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 2016, 2, Cd004128. [Google Scholar] [CrossRef] [PubMed]
- Stecher, F.S.; Olsen, J.A.; Stickney, R.E.; Wik, L. Transthoracic impedance used to evaluate performance of cardiopulmonary resuscitation during out of hospital cardiac arrest. Resuscitation 2008, 79, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Losert, H.; Risdal, M.; Sterz, F.; Nysaether, J.; Kohler, K.; Eftestol, T.; Wandaller, C.; Myklebust, H.; Uray, T.; Aase, S.O.; et al. Thoracic-impedance changes measured via defibrillator pads can monitor signs of circulation. Resuscitation 2007, 73, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Losert, H.; Risdal, M.; Sterz, F.; Nysaether, J.; Kohler, K.; Eftestol, T.; Wandaller, C.; Myklebust, H.; Uray, T.; Sodeck, G.; et al. Thoracic impedance changes measured via defibrillator pads can monitor ventilation in critically ill patients and during cardiopulmonary resuscitation. Crit. Care Med. 2006, 34, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Stickney, R.; Marx, R.; Olsufka, M.; Doherty, A.; McMahon, M.; Walker, C.; O’Grady, S. Accurate measurement of chest compressions from thoracic impedance signals. Circulation 2005, 112, II-326. [Google Scholar]
- Kramer-Johansen, J.; Edelson, D.P.; Losert, H.; Kohler, K.; Abella, B.S. Uniform reporting of measured quality of cardiopulmonary resuscitation (CPR). Resuscitation 2007, 74, 406–417. [Google Scholar] [CrossRef]
- Wik, L.; Olsen, J.-A.; Persse, D.; Sterz, F.; Lozano, M., Jr.; Brouwer, M.A.; Westfall, M.; Souders, C.M.; Travis, D.T.; Herken, U.R.; et al. Why do some studies find that CPR fraction is not a predictor of survival? Resuscitation 2016, 104, 59–62. [Google Scholar] [CrossRef]
- Cromie, N.A.; Allen, J.D.; Turner, C.; Anderson, J.M.; Adgey, A.A. The impedance cardiogram recorded through two electrocardiogram/defibrillator pads as a determinant of cardiac arrest during experimental studies. Crit. Care Med. 2008, 36, 1578–1584. [Google Scholar] [CrossRef]
- Pellis, T.; Bisera, J.; Tang, W.; Weil, M.H. Expanding automatic external defibrillators to include automated detection of cardiac, respiratory, and cardiorespiratory arrest. Crit. Care Med. 2002, 30, S176–S178. [Google Scholar] [CrossRef]
- Ruiz, J.; Alonso, E.; Aramendi, E.; Kramer-Johansen, J.; Eftestol, T.; Ayala, U.; Gonzalez-Otero, D. Reliable extraction of the circulation component in the thoracic impedance measured by defibrillation pads. Resuscitation 2013, 84, 1345–1352. [Google Scholar] [CrossRef]
- Nordseth, T.; Bergum, D.; Edelson, D.P.; Olasveengen, T.M.; Eftestol, T.; Wiseth, R.; Abella, B.S.; Skogvoll, E. Clinical state transitions during advanced life support (ALS) in in-hospital cardiac arrest. Resuscitation 2013, 84, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Safar, P. Resuscitation after brain ischemia. In Brain Failure and Resuscitation; Grenvik, A., Safar, P., Eds.; Churchill Livingstone: New York, NY, USA, 1981; pp. 155–184. [Google Scholar]
- Arntz, H.R.; Wenzel, V.; Dissmann, R.; Marschalk, A.; Breckwoldt, J.; Muller, D. Out-of-hospital thrombolysis during cardiopulmonary resuscitation in patients with high likelihood of ST-elevation myocardial infarction. Resuscitation 2008, 76, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Doan, J.; Blackwood, J.; Coult, J.; Kudenchuk, P.; Sherman, L.; Rea, T.; Kwok, H. Rhythm profiles and survival after out-of-hospital ventricular fibrillation cardiac arrest. Resuscitation 2018, 125, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Nordseth, T.; Olasveengen, T.M.; Kvaloy, J.T.; Wik, L.; Steen, P.A.; Skogvoll, E. Dynamic effects of adrenaline (epinephrine) in out-of-hospital cardiac arrest with initial pulseless electrical activity (PEA). Resuscitation 2012, 83, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, T.P. The problem with and benefit of ventilations: Should our approach be the same in cardiac and respiratory arrest? Curr. Opin. Crit. Care 2006, 12, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pellis, T.; Sanfilippo, F.; Ristagno, G. The optimal hemodynamics management of post-cardiac arrest shock. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Stub, D.; Bernard, S.; Pellegrino, V.; Smith, K.; Walker, T.; Sheldrake, J.; Hockings, L.; Shaw, J.; Duffy, S.J.; Burrell, A.; et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 2015, 86, 88–94. [Google Scholar] [CrossRef]
- Tsou, P.Y.; Kurbedin, J.; Chen, Y.S.; Chou, E.H.; Lee, M.G.; Lee, M.C.; Ma, M.H.; Chen, S.C.; Lee, C.C. Accuracy of point-of-care focused echocardiography in predicting outcome of resuscitation in cardiac arrest patients: A systematic review and meta-analysis. Resuscitation 2017, 114, 92–99. [Google Scholar] [CrossRef]
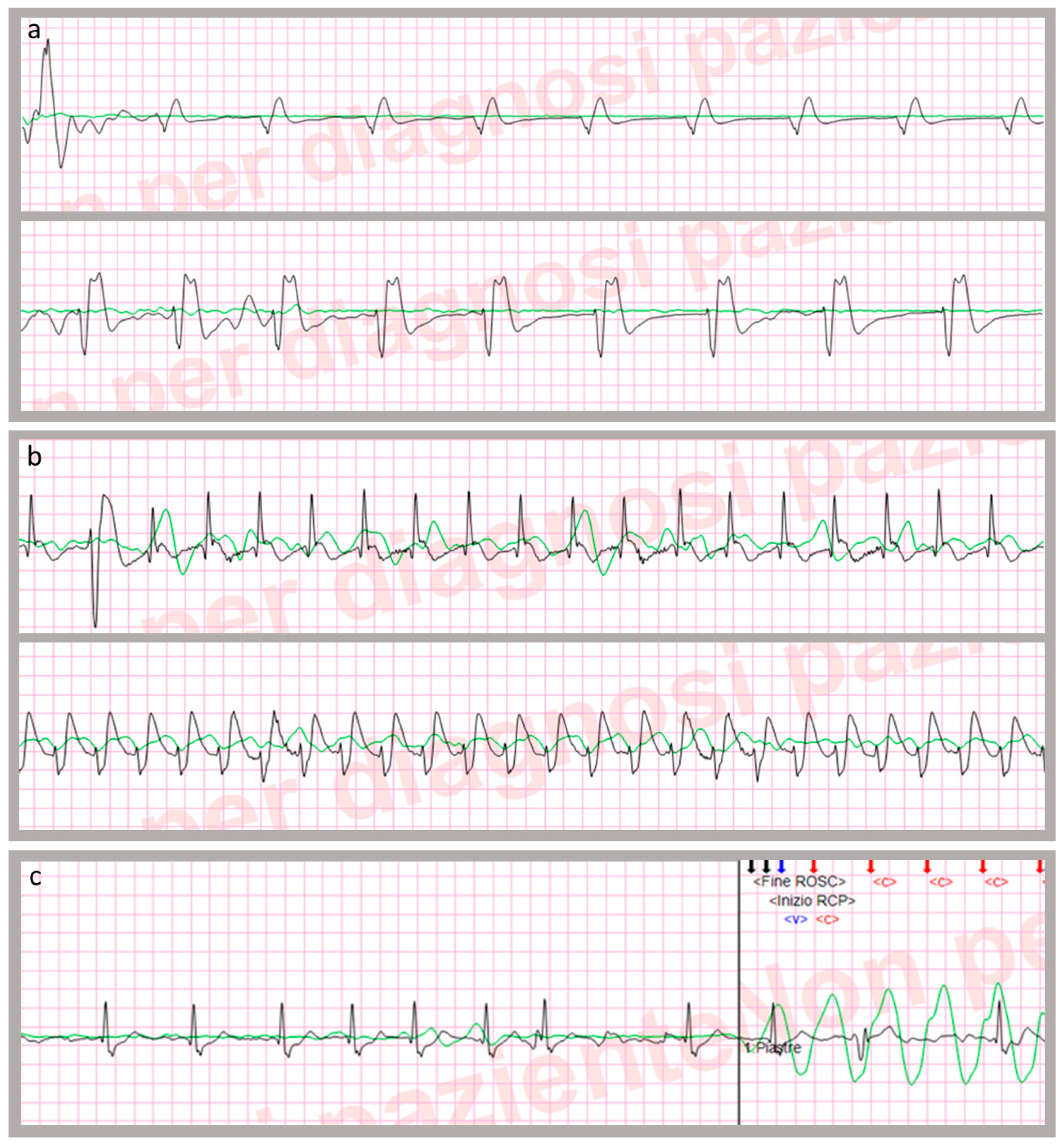
| Start of cardiac arrest episode | The first therapeutic event after EMS arrival and corresponding to the beginning of CPR manoeuvres (e.g., first recorded chest compression or first rhythm analysis) |
| End of cardiac arrest episode | The last registered chest compression, coinciding with having obtained a sustained ROSC or patient declared dead. |
| CPR time | Length of low-flow time (interval between “start” and “end” of CPR episode, after subtracting cumulative t-ROSCs duration) |
| Uninterrupted chest compression rate | The mean chest compression frequency per minute provided during uninterrupted chest compressions cycles, considering consecutive compressions which follow one another with pauses of less than 1.5 s |
| Chest compression fraction | The ratio between time spent delivering uninterrupted chest compressions and CPR time |
| Ventilation rate | The mean ventilation frequency per minute calculated by dividing the total number of ventilations by CPR time |
| Variable | Data |
|---|---|
| Age (years) § | 74.1 ± 14.7 (76.0) |
| Sex (male) ¥** | 178 (64.5%) |
| OHCA–CPR interval (min:sec) § | 9:12 ± 6:00 (8:00) |
| CPR started before the EMS arrival ¥* | 44 (15.5%) |
| CPR time (min:sec) § | 23:40 ± 14:00 (21:50) |
| First documented ECG rhythm ¥ | |
| VF/VT | 81 (28.4%) |
| asystole | 111 (38.9%) |
| PEA | 93 (32.6%) |
| Chest compression fraction (%) § | 70.0 ± 12.4 (72.4) |
| Uninterrupted CC rate (CCs min−1) § | 115.4 ± 18.4 (110.1) |
| Ventilation rate (ventilations min−1) § | 7.7 ± 3.9 (7.1) |
| t-ROSC: number of events ¥ | |
| 0 | 240 (84.2%) |
| 1 | 30 (10.5%) |
| 2 | 7 (2.5%) |
| 3–5 | 8 (2.8%) |
| t-ROSC: cumulative length of events §*** | 10:35 ± 9:14 (7:32) |
| Sustained ROSC ¥ | 81 (28.2%) |
| Cumulative outcome at hospital discharge ¥**** | |
| CPC 1 | 15 (5.3%) |
| CPC 2 | 1 (0.4%) |
| CPC 4 | 3 (1.1%) |
| CPC 5 or dead | 267 (94.3%) |
| Variable | No t-ROSC Event (n = 240) | One or More t-ROSC Events (n = 45) | p-Value |
|---|---|---|---|
| Age (years) §* | 74.4 ± 15.0 (76.5) | 72.9 ± 12.7 (72.0) | 0.547 |
| Sex (male) ¥* | 150 (64.9%) | 28 (62.2%) | 0.728 |
| OHCA–CPR interval (min) §** | 9.2 ± 5.7 (9.0) | 9.0 ± 7.7 (7.0) | 0.858 |
| CPR started before EMS ¥** | 36 (15.1%) | 8 (17.8%) | 0.644 |
| CPR time (min:sec) § | 23:24 ± 14:06 (21:30) | 24:48 ± 13:30 (23:00) | 0.549 |
| VF/VT as first documented ECG rhythm ¥ | 61 (25.4%) | 20 (44.4%) | 0.009 |
| CC fraction (%) § | 70.4 ± 11.9 (72.4) | 67.6 ± 15.0 (73.4) | 0.250 |
| Uninterrupted CC rate (CC/min−1) § | 116.4 ± 18.5 (111.3) | 110.1 ± 17.2 (106.5) | 0.035 |
| Ventilation rate (breaths/min−1) § | 7.1 ± 3.5 (6.7) | 10.4 ± 4.7 (9.7) | <0.001 |
| Variable | Dead or CPC 3–5 (n = 267) | CPC 1–2 (n = 16) | p-Value |
|---|---|---|---|
| Age (years) §* | 74.7 ± 14.3 (76.0) | 64.4 ± 18.6 (64.5) | 0.006 |
| Sex (male) ¥* | 168 (65.1%) | 10 (62.5%) | 0.831 |
| OHCA–CPR interval (min) §** | 9.3 ± 6.1 (9.0) | 6.0 ± 4.0 (5.0) | 0.032 |
| CPR started before EMS ¥** | 35 (13.2%) | 8 (50%) | <0.001 |
| CPR time (min:sec) § | 18:09 ± 11:47 (16:23) | 11:13 ± 9:47 (8:26) | 0.022 |
| VF/VT as first documented ECG rhythm ¥ | 69 (25.8%) | 12 (75.0%) | <0.001 |
| CC fraction (%) § | 70.1 ± 12.3 (72.5) | 69.3 ± 13.7 (73.4) | 0.811 |
| Uninterrupted CC rate (CC/min−1) § | 115.0 ± 18.2 (110.0) | 121.2 ± 22.0 (114.7) | 0.189 |
| Ventilation rate (breaths/min−1) § | 7.2 ± 3.4 (6.9) | 11.6 ± 5.7 (11.5) | 0.008 |
| t-ROSC periods (min:sec) § | 1:30 ± 5:12 (0:00) | 4:43 ± 7:13 (2:22) | 0.020 |
| t-ROSC occurrence (yes) ¥ | 35 (13.1%) | 9 (56.3%) | <0.001 |
| Predictor | B (SE) | Wald Test | OR (95% CI) | p-Value |
|---|---|---|---|---|
| OHCA–CPR interval (min) | −0.908 (0.325) | 7.808 | 0.403 (0.213 to 0.763) | 0.005 |
| CPR started before EMS (yes) | 2.814 (0.812) | 12.008 | 16.675 (3.395 to 91.902) | 0.001 |
| Shockable rhythm (yes) | 2.397 (0.799) | 9.004 | 10.987 (2.296 to 52.568) | 0.003 |
| CPR time (min) | −0.736 (0.245) | 8.987 | 0.479 (0.296 to 0.775) | 0.003 |
| Ventilation rate (breaths/min−1) | 1.204 (0.533) | 5.097 | 3.334 (1.172 to 9.485) | 0.024 |
| t-ROSC length (min) | 0.463 (0.228) | 4.132 | 1.588 (1.017 to 2.481) | 0.042 |
| Intercept | −3.668 (2.039) | 3.235 | 0.026 (/) | 0.072 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonaglia, V.; Pegani, C.; Caggegi, G.D.; Patsoura, A.; Xu, V.; Zambon, M.; Sanson, G. Impact of Transitory ROSC Events on Neurological Outcome in Patients with Out-of-Hospital Cardiac Arrest. J. Clin. Med. 2019, 8, 926. https://doi.org/10.3390/jcm8070926
Antonaglia V, Pegani C, Caggegi GD, Patsoura A, Xu V, Zambon M, Sanson G. Impact of Transitory ROSC Events on Neurological Outcome in Patients with Out-of-Hospital Cardiac Arrest. Journal of Clinical Medicine. 2019; 8(7):926. https://doi.org/10.3390/jcm8070926
Chicago/Turabian StyleAntonaglia, Vittorio, Carlo Pegani, Giuseppe Davide Caggegi, Athina Patsoura, Veronica Xu, Marco Zambon, and Gianfranco Sanson. 2019. "Impact of Transitory ROSC Events on Neurological Outcome in Patients with Out-of-Hospital Cardiac Arrest" Journal of Clinical Medicine 8, no. 7: 926. https://doi.org/10.3390/jcm8070926
APA StyleAntonaglia, V., Pegani, C., Caggegi, G. D., Patsoura, A., Xu, V., Zambon, M., & Sanson, G. (2019). Impact of Transitory ROSC Events on Neurological Outcome in Patients with Out-of-Hospital Cardiac Arrest. Journal of Clinical Medicine, 8(7), 926. https://doi.org/10.3390/jcm8070926





