Platelet Function Disturbance During Veno-Venous ECMO in ARDS Patients Assessed by Multiple Electrode Aggregometry—A Prospective, Observational Cohort Study
Abstract
1. Introduction
2. Experimental Section
2.1. Trial Design and Patients
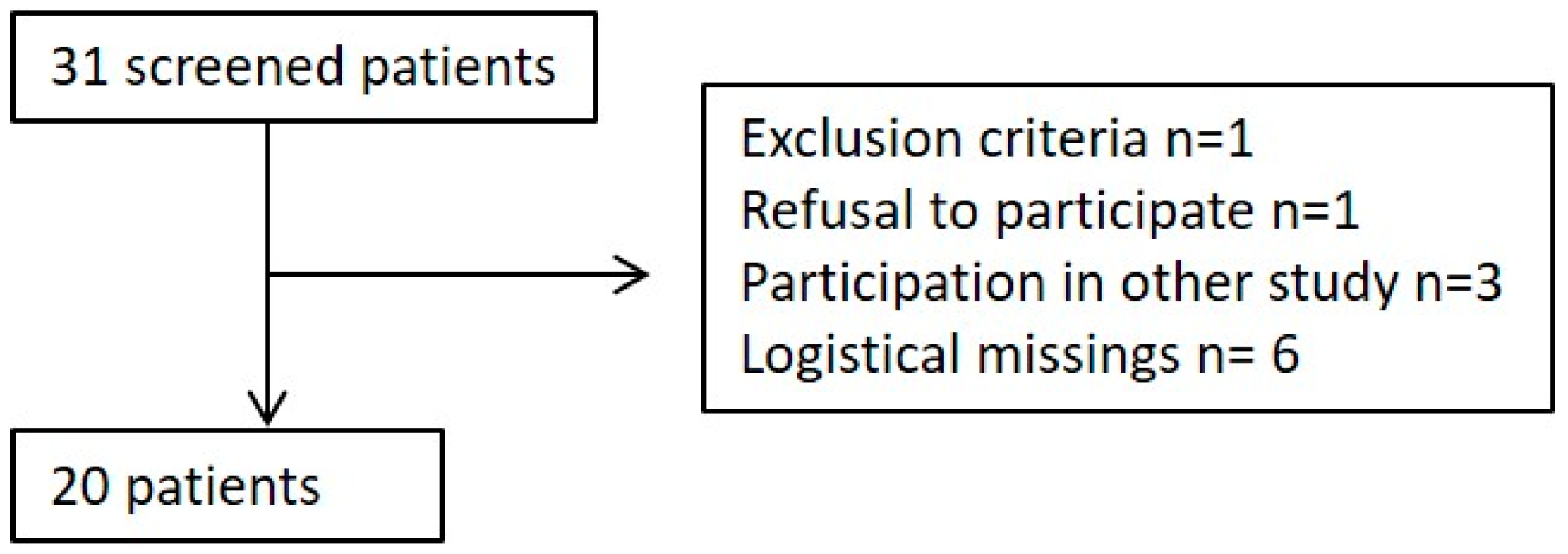
2.2. Patient Enrollment
2.3. ECMO Setup
2.4. Hematological/Hemostaseological Data Collection and Time Points
2.5. Multiple Electrode Aggregometry (MEA)
2.6. Sample Size and Statistical Analyses
3. Results
3.1. Population
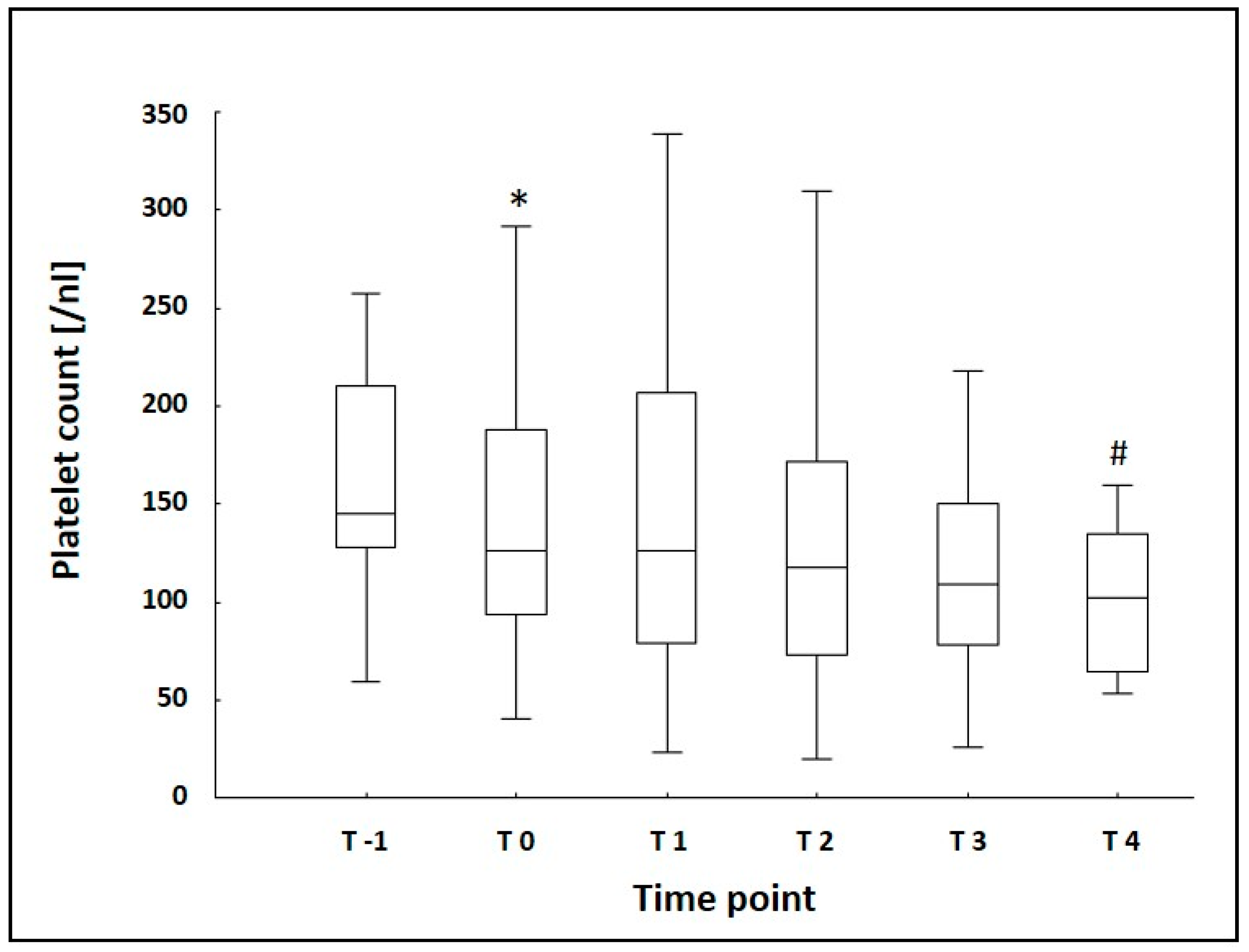
3.2. Laboratory Data
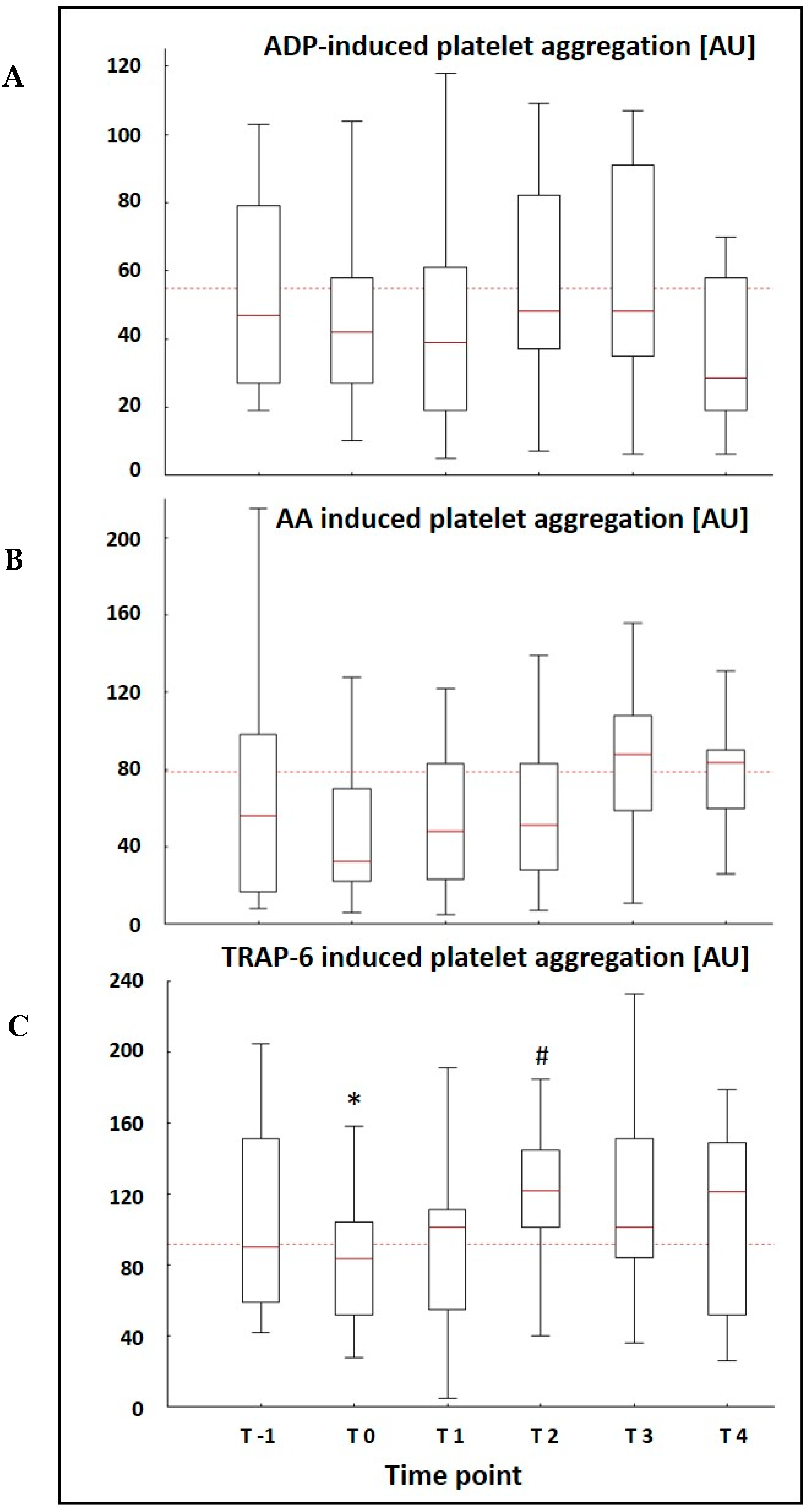
3.3. MEA Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karagiannidis, C.; Brodie, D.; Strassmann, S.; Stoelben, E.; Philipp, A.; Bein, T.; Muller, T.; Windisch, W. Extracorporeal membrane oxygenation: Evolving epidemiology and mortality. Intensive Care Med. 2016, 42, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoue, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, F.; Moerer, O.; Laudi, S.; Weber-Carstens, S.; Nothacker, M.; Kaisers, U. Clinical practice guideline: Mechanical ventilation and extracorporeal membrane oxygenation in acute respiratory insufficiency. Dtsch. Ärzteblatt Int. 2018, 115, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L.; Centers, E.M. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Landoni, G.; Biondi-Zoccai, G.; Greco, M.; Greco, T.; Frati, G.; Patroniti, N.; Antonelli, M.; Pesenti, A.; Pappalardo, F. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2013, 15, 172–178. [Google Scholar]
- Panigada, M.; Artoni, A.; Passamonti, S.M.; Maino, A.; Mietto, C.; L’Acqua, C.; Cressoni, M.; Boscolo, M.; Tripodi, A.; Bucciarelli, P.; et al. Hemostasis changes during veno-venous extracorporeal membrane oxygenation for respiratory support in adults. Minerva Anestesiol. 2016, 82, 170–179. [Google Scholar] [PubMed]
- Malfertheiner, M.V.; Philipp, A.; Lubnow, M.; Zeman, F.; Enger, T.B.; Bein, T.; Lunz, D.; Schmid, C.; Muller, T.; Lehle, K. Hemostatic Changes During Extracorporeal Membrane Oxygenation: A Prospective Randomized Clinical Trial Comparing Three Different Extracorporeal Membrane Oxygenation Systems. Crit. Care Med. 2016, 44, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.; Burns, J.; Retter, A.; Salt, G.; Camporota, L.; Meadows, C.I.; Langrish, C.C.; Wyncoll, D.; Glover, G.; Ioannou, N.; et al. Prevalence of Venous Thrombosis Following Venovenous Extracorporeal Membrane Oxygenation in Patients With Severe Respiratory Failure. Crit. Care Med. 2015, 43, e581–e584. [Google Scholar] [CrossRef]
- Velik-Salchner, C.; Maier, S.; Innerhofer, P.; Kolbitsch, C.; Streif, W.; Mittermayr, M.; Praxmarer, M.; Fries, D. An assessment of cardiopulmonary bypass-induced changes in platelet function using whole blood and classical light transmission aggregometry: The results of a pilot study. Anesth. Analg. 2009, 108, 1747–1754. [Google Scholar] [CrossRef]
- Weber, C.F.; Zacharowski, K.; Brun, K.; Volk, T.; Martin, E.O.; Hofer, S.; Kreuer, S. Basic algorithm for Point-of-Care based hemotherapy: Perioperative treatment of coagulopathic patients. Der Anaesthesist 2013, 62, 464–472. [Google Scholar] [CrossRef]
- Weber, C.F.; Gorlinger, K.; Meininger, D.; Herrmann, E.; Bingold, T.; Moritz, A.; Cohn, L.H.; Zacharowski, K. Point-of-care testing: A prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012, 117, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Johnson, R.I.; Kirmani, B.H. Pre- and Post-Bypass Platelet Function Testing With Multiple Electrode Aggregometry and TEG Platelet Mapping in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Passmore, M.R.; Fung, Y.L.; Simonova, G.; Foley, S.R.; Diab, S.D.; Dunster, K.R.; Spanevello, M.M.; McDonald, C.I.; Tung, J.P.; Pecheniuk, N.M.; et al. Evidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation support. Crit. Care (Lond. Engl.) 2017, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Wand, S.; Huber-Petersen, F.; Schaeper, J.; Binder, C.; Moerer, O. 971: Extracorporeal membrane oxygenation does not lead to a longer-term impairment of platelet function. Crit. Care Med. 2016, 44, 319. [Google Scholar] [CrossRef]
- Cardinal, D.C.; Flower, R.J. The electronic aggregometer: A novel device for assessing platelet behavior in blood. J. Pharmacol. Methods 1980, 3, 135–158. [Google Scholar] [CrossRef]
- Toth, O.; Calatzis, A.; Penz, S.; Losonczy, H.; Siess, W. Multiple electrode aggregometry: A new device to measure platelet aggregation in whole blood. Thromb. Haemost. 2006, 96, 781–788. [Google Scholar]
- Kalbhenn, J.; Wittau, N.; Schmutz, A.; Zieger, B.; Schmidt, R. Identification of acquired coagulation disorders and effects of target-controlled coagulation factor substitution on the incidence and severity of spontaneous intracranial bleeding during veno-venous ECMO therapy. Perfusion 2015, 30, 675–682. [Google Scholar] [CrossRef]
- Peek, G.J.; Firmin, R.K. The inflammatory and coagulative response to prolonged extracorporeal membrane oxygenation. ASAIO J. 1999, 45, 250–263. [Google Scholar] [CrossRef]
- Nair, P.; Hoechter, D.J.; Buscher, H.; Venkatesh, K.; Whittam, S.; Joseph, J.; Jansz, P. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J. Cardiothorac. Vasc. Anesth. 2015, 29, 288–296. [Google Scholar] [CrossRef]
- Mutlak, H.; Reyher, C.; Meybohm, P.; Papadopoulos, N.; Hanke, A.A.; Zacharowski, K.; Weber, C.F. Multiple electrode aggregometry for the assessment of acquired platelet dysfunctions during extracorporeal circulation. Thorac. Cardiovasc. Surg. 2015, 63, 21–27. [Google Scholar] [CrossRef]
- Kalbhenn, J.; Schlagenhauf, A.; Rosenfelder, S.; Schmutz, A.; Zieger, B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J. Heart Lung Transpl. 2018, 37, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Bochsen, L.; Johansson, P.I.; Kristensen, A.T.; Daugaard, G.; Ostrowski, S.R. The influence of platelets, plasma and red blood cells on functional haemostatic assays. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2011, 22, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Timsit, J.F.; Vesin, A.; Garrouste-Orgeas, M.; de Lassence, A.; Zahar, J.R.; Adrie, C.; Vincent, F.; Cohen, Y.; Schlemmer, B.; et al. Platelet count decline: An early prognostic marker in critically ill patients with prolonged ICU stays. Chest 2007, 131, 1735–1741. [Google Scholar] [CrossRef]
- Levi, M.; Schultz, M. Coagulopathy and platelet disorders in critically ill patients. Minerva Anestesiol. 2010, 76, 851–859. [Google Scholar] [PubMed]
- Adamzik, M.; Gorlinger, K.; Peters, J.; Hartmann, M. Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis. Crit. Care (Lond. Engl.) 2012, 16, R204. [Google Scholar] [CrossRef]
- Lundahl, T.H.; Petersson, J.; Fagerberg, I.H.; Berg, S.; Lindahl, T.L. Impaired platelet function correlates with multi-organ dysfunction. A study of patients with sepsis. Platelets 1998, 9, 223–225. [Google Scholar] [CrossRef]
- Davies, G.R.; Mills, G.M.; Lawrence, M.; Battle, C.; Morris, K.; Hawkins, K.; Williams, P.R.; Davidson, S.; Thomas, D.; Evans, P.A. The role of whole blood impedance aggregometry and its utilisation in the diagnosis and prognosis of patients with systemic inflammatory response syndrome and sepsis in acute critical illness. PLoS ONE 2014, 9, e108589. [Google Scholar] [CrossRef][Green Version]
- Hayes, R.A.; Foley, S.; Shekar, K.; Diab, S.; Dunster, K.R.; McDonald, C.; Fraser, J.F. Ovine platelet function is unaffected by extracorporeal membrane oxygenation within the first 24 h. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2015, 26, 816–822. [Google Scholar] [CrossRef]
- Baumgarten, A.; Wilhelmi, M.; Kalbantner, K.; Ganter, M.; Mischke, R. Measurement of platelet aggregation in ovine blood using a new impedance aggregometer. Vet. Clin. Pathol. 2010, 39, 149–156. [Google Scholar] [CrossRef]
- Foley, S.R.; Solano, C.; Simonova, G.; Spanevello, M.M.; Bird, R.J.; Semple, J.W.; Jackson, D.E.; Schibler, A.; Fraser, J.F.; Fung, Y.L. A comprehensive study of ovine haemostasis to assess suitability to model human coagulation. Thromb. Res. 2014, 134, 468–473. [Google Scholar] [CrossRef]
- Cornet, A.D.; Kooter, A.J.; Peters, M.J.; Smulders, Y.M. The potential harm of oxygen therapy in medical emergencies. Crit. Care (Lond. Engl.) 2013, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; Grocott, M.P. Oxygen therapy in critical illness: Precise control of arterial oxygenation and permissive hypoxemia. Crit. Care Med. 2013, 41, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Shekar, K.; Fraser, J.F. Is hyperoxaemia helping or hurting patients during extracorporeal membrane oxygenation? Review of a complex problem. Perfusion 2013, 28, 184–193. [Google Scholar] [CrossRef] [PubMed]
| Age [years, median (IQR)] | 57 (53/64) |
| Gender [Male, n (%)] | 13 (65%) |
| BMI [kg/m2, median (IQR)] | 30.7 (26.2/36.9) |
| SAPS II [median (IQR)] | 40 (29/55) |
| Reason for ARDS [n] | 1. Pneumonia n = 8 2. Aspiration n = 2 3. Postoperative ARDS n = 3 4. Smoke inhalation n = 1 5. Thorax trauma n = 1 6. Internal disease n = 5 |
| Duration of ECMO-Therapy [days, median (IQR)] | 9.5 (7/13.5) |
| Duration of ICU-Therapy at the ARDS Center [days, median (IQR)] | 14.5 (11.2/35.6) |
| Total Duration of ICU-Therapy [days, median (IQR)] | 19 (13.5/51) |
| 28-day Mortality [n (%)] | 9 (45%) |
| ICU Mortality [n (%)] | 11 (55%) |
| Hospital Mortality [n (%)] | 12 (60%) |
| Parameter | T-1 (n = 13) | T0 (n = 20) | T1 (n = 19) | T2 (n = 18) | T3 (n = 18) | T4 (n = 16) |
|---|---|---|---|---|---|---|
| Hemoglobin [g/dL] | 10.1 (8.6/13) | 8.9 * (8.3/10.1) | 9.2 (8.4/10.1) | 8.7 (8.3/9.5) | 8.6 (8.3/9.1) | 8.7 (8.2/9.7) |
| Platelet Count [/nL] | 145 (128/210) | 126 * (94/188) | 126 (79/207) | 118 (73/172) | 109 (78/150) | 102 # (65/135) |
| Fibrinogen [mg/dL] | 465 (366/584) | 433 (307/632) | 426 (275/567) | 434 (257/539) | 406 (233/602) | 329 (223/681) |
| PT [%] | 82 (72/88) | 74 (63/87) | 78 (61/90) | 79 (65/93) | 78 (71/95) | 77 (64/90) |
| aPTT [s] | 32 (29/35) | 32 (29/41) | 36 (30/42) | 35 (30/42) | 34 (30/40) | 37 (36/41) |
| Leukocyte Count [/nL] | 16.7 (8.7/21.3) | 12.2 * (6.7/16.2) | 10.4 (8.2/15.9) | 11.1 (7.5/14.5) | 10.5 (8.1/14.7) | 13.5 (8.4/16.8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wand, S.; Huber-Petersen, J.F.; Schaeper, J.; Binder, C.; Moerer, O. Platelet Function Disturbance During Veno-Venous ECMO in ARDS Patients Assessed by Multiple Electrode Aggregometry—A Prospective, Observational Cohort Study. J. Clin. Med. 2019, 8, 1056. https://doi.org/10.3390/jcm8071056
Wand S, Huber-Petersen JF, Schaeper J, Binder C, Moerer O. Platelet Function Disturbance During Veno-Venous ECMO in ARDS Patients Assessed by Multiple Electrode Aggregometry—A Prospective, Observational Cohort Study. Journal of Clinical Medicine. 2019; 8(7):1056. https://doi.org/10.3390/jcm8071056
Chicago/Turabian StyleWand, Saskia, Jan Felix Huber-Petersen, Joern Schaeper, Claudia Binder, and Onnen Moerer. 2019. "Platelet Function Disturbance During Veno-Venous ECMO in ARDS Patients Assessed by Multiple Electrode Aggregometry—A Prospective, Observational Cohort Study" Journal of Clinical Medicine 8, no. 7: 1056. https://doi.org/10.3390/jcm8071056
APA StyleWand, S., Huber-Petersen, J. F., Schaeper, J., Binder, C., & Moerer, O. (2019). Platelet Function Disturbance During Veno-Venous ECMO in ARDS Patients Assessed by Multiple Electrode Aggregometry—A Prospective, Observational Cohort Study. Journal of Clinical Medicine, 8(7), 1056. https://doi.org/10.3390/jcm8071056





