Fully Automated Support System for Diagnosis of Breast Cancer in Contrast-Enhanced Spectral Mammography Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. CESM Examination
2.1.2. Inclusion and Exclusion Criteria
2.1.3. Experimental Dataset
2.2. Methods
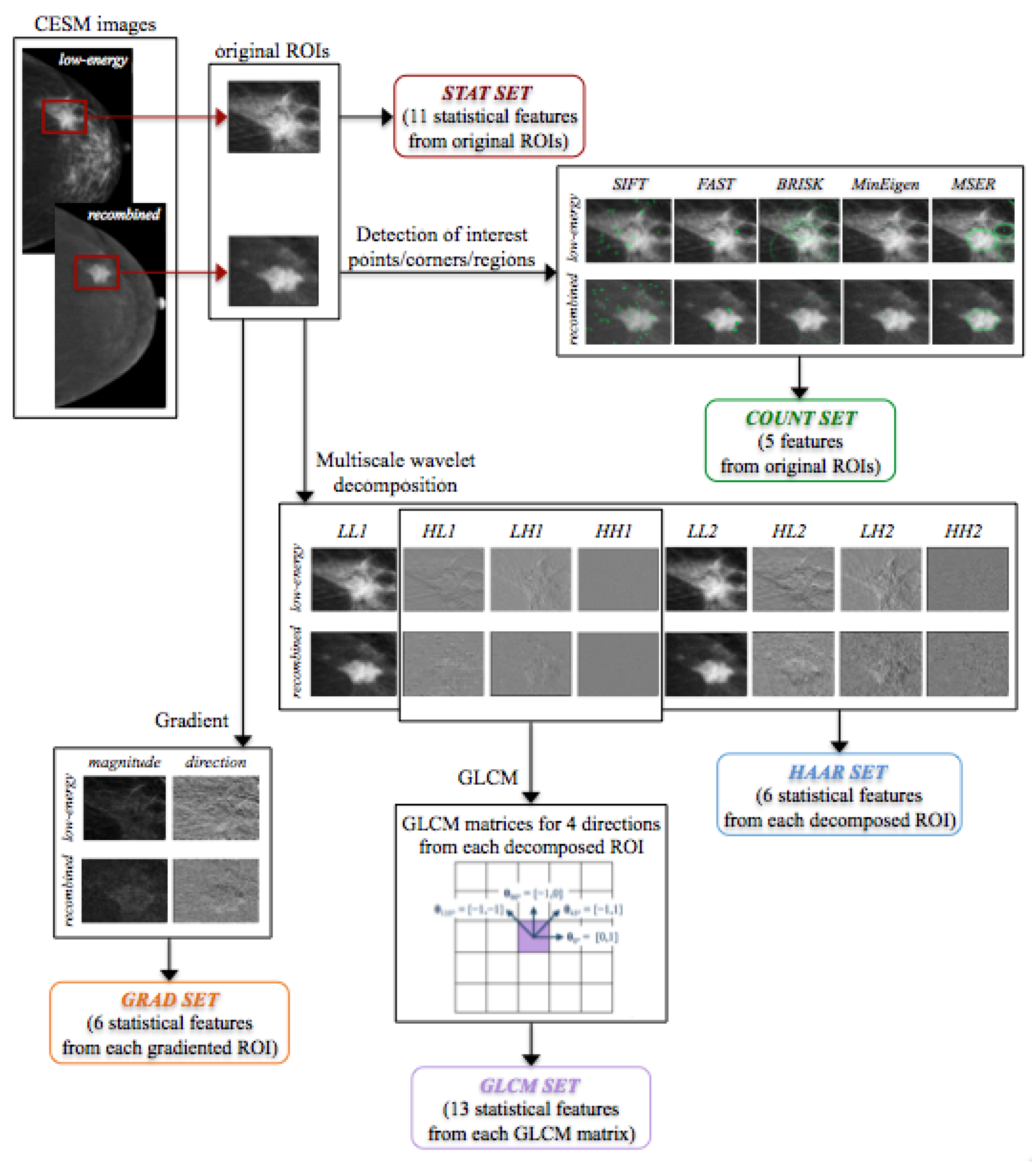
2.2.1. Feature Extraction
Statistical Features
Interest Point, Corner and Region Detection
Gradient Image
Haar Wavelet Transform
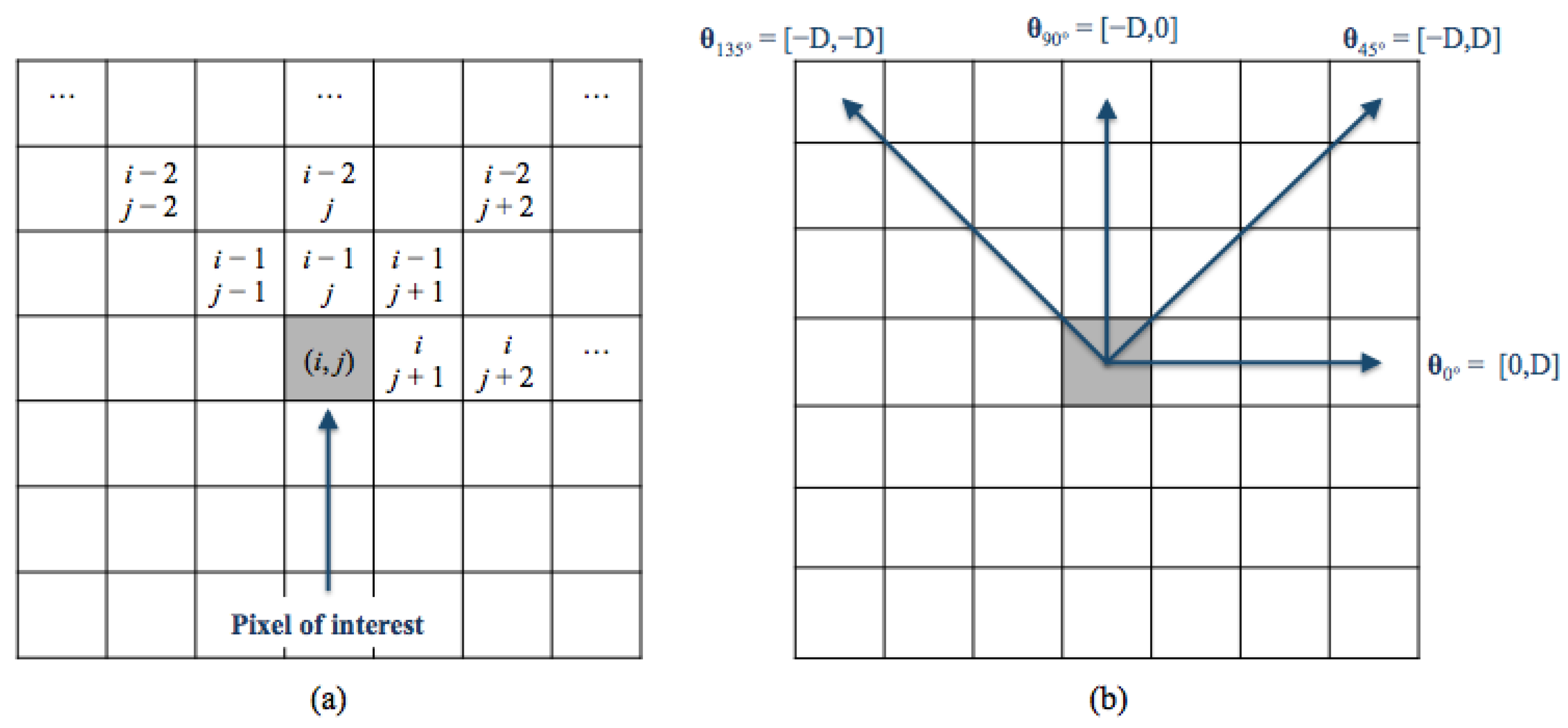
Gray-Level Co-Occurence Matrix
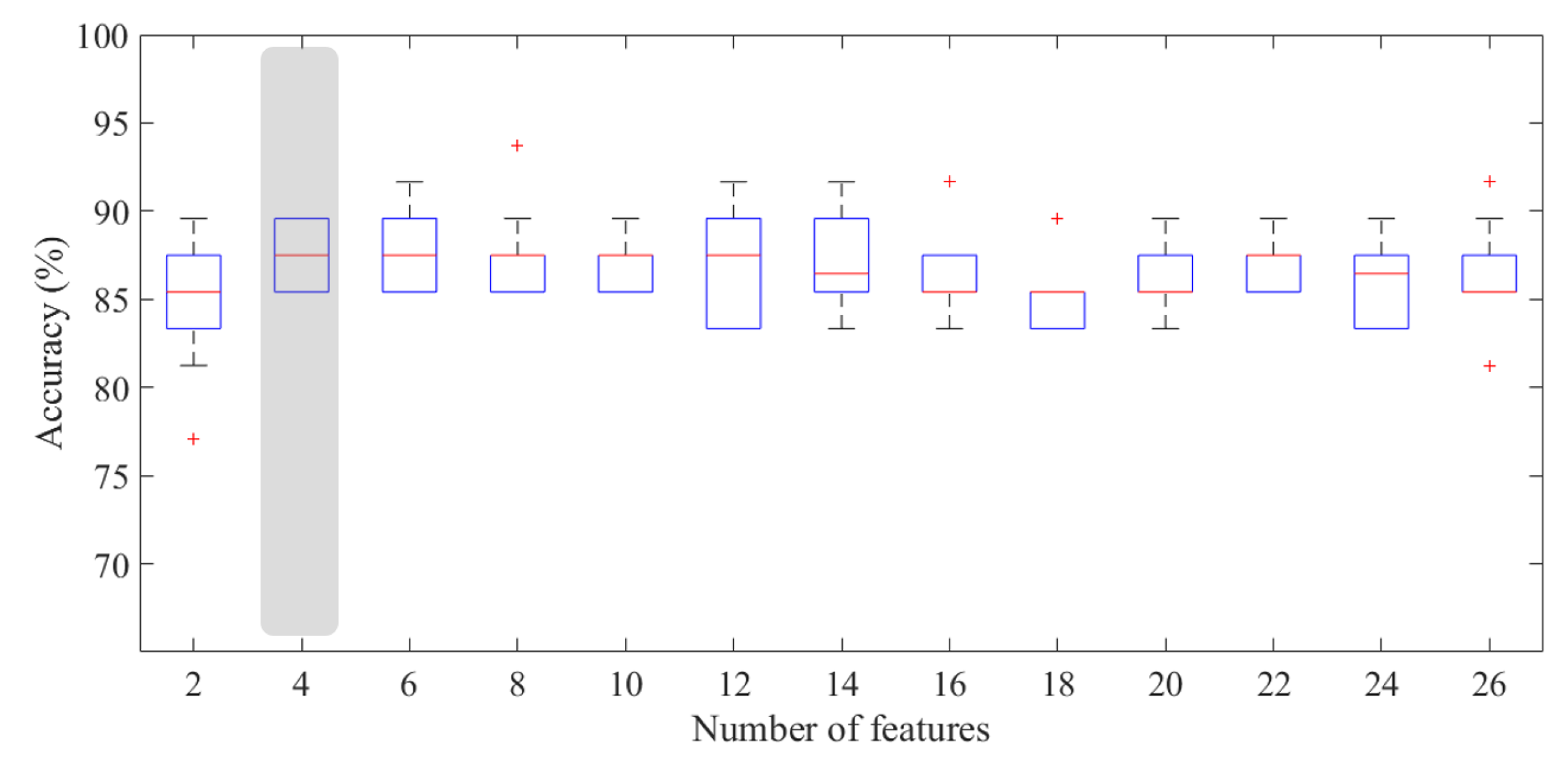
2.2.2. Classification Model
3. Results
3.1. Human-Reader Diagnostic Accuracy
3.2. Prediction Accuracy of Benign and Malignant ROIs
3.3. Prediction Accuracy of Normal and Abnormal ROIs Characterized by Mild/High BPE
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| AUC | Area Under the Curve |
| BPE | Background Parenchymal Enhancement |
| BRISK | Binary Robust Invariant Scalable Keypoints |
| CADx | Computer Automated Diagnosis |
| CC | CranioCaudal |
| CESM | Contrast-Enhanced Spectral Mammography |
| CI | Confidence Interval |
| CM | Contrast Medium |
| CT | Computerized Tomography |
| DWI | Diffusion-Weighted Imaging |
| FAST | Features from Accelerated Segment Test |
| FFDM | Full-Field Digital Mammography |
| FN | False Negative |
| FP | False Positive |
| Gdir | Gradient direction |
| Gmag | Gradient magnitude |
| GLCM | Gray-Level Co-occurence Matrix |
| HE | High-Energy |
| HH | High-High |
| HL | High-Low |
| IQR | InterQuartile Range |
| LE | Low-Energy |
| LH | Low-High |
| LL | Low-Low |
| MinEigen | Minimum Eigenvalue |
| MCC | Matthews Correlation Coefficient |
| MLO | MedioLateral Oblique |
| MRI | Magnetic Resonance Imaging |
| MSER | Maximally Stable Extremal Regions |
| RC | ReCombined |
| RF | Random Forest |
| ROC | Receiver Operating Characteristic |
| ROI | Region Of Interest |
| SD | Standard Deviation |
| SIFT | Scale Invariant Feature Transform |
| SVM | Support Vector Machine |
| TN | True Negative |
| TP | True Positive |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef]
- Cerello, P.; Bagnasco, S.; Bottigli, U.; Cheran, S.C.; Delogu, P.; Fantacci, M.E.; Fauci, F.; Forni, G.; Lauria, A.; Torres, E.L.; et al. GPCALMA: A Grid-based tool for mammographic screening. Methods Inf. Med. 2005, 44, 244–248. [Google Scholar]
- Fauci, F.; Raso, G.; Magro, R.; Forni, G.; Lauria, A.; Bagnasco, S.; Cerello, P.; Cheran, S.C.; Torres, E.L.; Bellotti, R.; et al. A massive lesion detection algorithm in mammography. Phys. Med. 2005, 21, 23–30. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Lin, Y.C.; Wan, Y.L.; Yeow, K.M.; Huang, P.C.; Lo, Y.F.; Tsai, H.P.; Ueng, S.H.; Chang, C.J. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: Interobserver blind-reading analysis. Eur. Radiol. 2014, 24, 2394–2403. [Google Scholar] [CrossRef]
- Vestito, A.; Lorusso, V.; Faggian, A.; Gaballo, A.; Garasto, E.; Mangieri, F.F.; La Forgia, D.; Ancona, A. Contrast Enhanced Spectral Mammography: la nostra esperienza. Il Giornale Italiano di Radiol. Med. 2014, 24, 1002–1008. [Google Scholar]
- Masala, G.; Tangaro, S.; Golosio, B.; Oliva, P.; Stumbo, S.; Bellotti, R.; De Carlo, F.; Gargano, G.; Cascio, D.; Fauci, F.; et al. Comparative study of feature classification methods for mass lesion recognition in digitized mammograms. Image 2007, 7, 8. [Google Scholar]
- Tagliafico, A.S.; Mariscotti, G.; Valdora, F.; Durando, M.; Nori, J.; La Forgia, D.; Rosenberg, I.; Caumo, F.; Gandolfo, N.; Sormani, M.P.; et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2). Eur. J. Cancer 2018, 104, 39–46. [Google Scholar] [CrossRef]
- Fallenberg, E.; Dromain, C.; Diekmann, F.; Engelken, F.; Krohn, M.; Singh, J.; Ingold-Heppner, B.; Winzer, K.; Bick, U.; Renz, D.M. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur. Radiol. 2014, 24, 256–264. [Google Scholar] [CrossRef]
- Patel, B.K.; Lobbes, M.; Lewin, J. Contrast enhanced spectral mammography: A review. In Seminars in Ultrasound, CT and MRI; Elsevier: Amsterdam, The Netherlands, 2018; Volume 39, pp. 70–79. [Google Scholar]
- Lalji, U.; Jeukens, C.; Houben, I.; Nelemans, P.; van Engen, R.; van Wylick, E.; Beets-Tan, R.; Wildberger, J.; Paulis, L.; Lobbes, M. Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur. Radiol. 2015, 25, 2813–2820. [Google Scholar] [CrossRef]
- Fallenberg, E.M.; Dromain, C.; Diekmann, F.; Renz, D.M.; Amer, H.; Ingold-Heppner, B.; Neumann, A.U.; Winzer, K.J.; Bick, U.; Hamm, B.; et al. Contrast-enhanced spectral mammography: Does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res. Treat. 2014, 146, 371–381. [Google Scholar] [CrossRef]
- James, J.; Tennant, S. Contrast-enhanced spectral mammography (CESM). Clin. Radiol. 2018, 24, 256–264. [Google Scholar] [CrossRef]
- Łuczyńska, E.; Heinze-Paluchowska, S.; Hendrick, E.; Dyczek, S.; Ryś, J.; Herman, K.; Blecharz, P.; Jakubowicz, J. Comparison between breast MRI and contrast-enhanced spectral mammography. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1358. [Google Scholar]
- Prionas, N.D.; Lindfors, K.K.; Ray, S.; Huang, S.Y.; Beckett, L.A.; Monsky, W.L.; Boone, J.M. Contrast-enhanced dedicated breast CT: Initial clinical experience. Radiology 2010, 256, 714–723. [Google Scholar] [CrossRef]
- Onega, T.; Tosteson, A.N.; Weiss, J.; Alford-Teaster, J.; Hubbard, R.A.; Henderson, L.M.; Kerlikowske, K.; Goodrich, M.E.; O’Donoghue, C.; Wernli, K.J.; et al. Costs of diagnostic and preoperative workup with and without breast MRI in older women with a breast cancer diagnosis. BMC Health Serv. Res. 2016, 16, 76. [Google Scholar] [CrossRef]
- Sankatsing, V.D.; Heijnsdijk, E.A.; van Luijt, P.A.; van Ravesteyn, N.T.; Fracheboud, J.; de Koning, H.J. Cost-effectiveness of digital mammography screening before the age of 50 in T he N etherlands. Int. J. Cancer 2015, 137, 1990–1999. [Google Scholar] [CrossRef]
- Gocgun, Y.; Banjevic, D.; Taghipour, S.; Montgomery, N.; Harvey, B.; Jardine, A.; Miller, A. Cost-effectiveness of breast cancer screening policies using simulation. Breast 2015, 24, 440–448. [Google Scholar] [CrossRef]
- Losurdo, L.; Basile, T.M.A.; Fanizzi, A.; Bellotti, R.; Bottigli, U.; Carbonara, R.; Dentamaro, R.; Diacono, D.; Didonna, V.; Lombardi, A.; et al. A Gradient-Based Approach for Breast DCE-MRI Analysis. BioMed Res. Int. 2018, 2018, 10. [Google Scholar] [CrossRef]
- Morris, E.A.; Comstock, C.E.; Lee, C.H.; Lehman, C.D.; Ikeda, D.M.; Newstead, G.M. ACR BI-RADS Magnetic Resonance Imaging. In ACR BI-RADS, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Savaridas, S.; Taylor, D.; Gunawardana, D.; Phillips, M. Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? Correlation with known radiology risk factors. Clin. Radiol. 2017, 72, 1085-e1. [Google Scholar] [CrossRef]
- King, V.; Brooks, J.D.; Bernstein, J.L.; Reiner, A.S.; Pike, M.C.; Morris, E.A. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011, 260, 50–60. [Google Scholar] [CrossRef]
- Sogani, J.; Morris, E.A.; Kaplan, J.B.; D’Alessio, D.; Goldman, D.; Moskowitz, C.S.; Jochelson, M.S. Comparison of background parenchymal enhancement at contrast-enhanced spectral mammography and breast MR imaging. Radiology 2016, 282, 63–73. [Google Scholar] [CrossRef]
- Patel, B.K.; Ranjbar, S.; Wu, T.; Pockaj, B.A.; Li, J.; Zhang, N.; Lobbes, M.; Zhang, B.; Mitchell, J.R. Computer-aided diagnosis of contrast-enhanced spectral mammography: A feasibility study. Eur. J. Radiol. 2018, 98, 207–213. [Google Scholar] [CrossRef]
- Steinwart, I.; Christmann, A. Support Vector Machines; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Perek, S.; Kiryati, N.; Zimmerman-Moreno, G.; Sklair-Levy, M.; Konen, E.; Mayer, A. Classification of contrast-enhanced spectral mammography (CESM) images. Int. J. Comput. Assist. Radiol. Surg. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Lobbes, M.B.; Lalji, U.C.; Nelemans, P.J.; Houben, I.; Smidt, M.L.; Heuts, E.; De Vries, B.; Wildberger, J.E.; Beets-Tan, R.G. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J. Cancer 2015, 6, 144. [Google Scholar] [CrossRef]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Köbrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef]
- Sardanelli, F.; Fallenberg, E.M.; Clauser, P.; Trimboli, R.M.; Camps-Herrero, J.; Helbich, T.H.; Forrai, G. Mammography: An update of the EUSOBI recommendations on information for women. Insights Imaging 2017, 8, 11–18. [Google Scholar] [CrossRef]
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. 2013 ACR BI-RADS Atlas: Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2014. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Lowe, D.G. Object recognition from local scale-invariant features. In Proceedings of the Seventh IEEE International Conference on Computer Vision, Corfu, Greece, 20–25 September 1999; IEEE Computer Society: Washington, DC, USA, 1999; Volume 2, pp. 1150–1157. [Google Scholar]
- Lindeberg, T. Scale invariant feature transform. Scholarpedia 2012, 7, 10491. [Google Scholar] [CrossRef]
- Shi, J.; Tomasi, C. Good features to track. In Proceedings of the Ninth IEEE Conference on Computer Vision and Pattern Recognition, Seattle, WA, USA, 21–23 June 1994; pp. 593–600. [Google Scholar]
- Rosten, E.; Drummond, T. Fusing points and lines for high performance tracking. In Proceedings of the Tenth IEEE International Conference on Computer Vision, Beijing, China, 17–20 October 2005; pp. 1508–1515. [Google Scholar]
- Rosten, E.; Drummond, T. Machine learning for high-speed corner detection. In Proceedings of the 9th European conference on Computer Vision, Graz, Austria, 7–13 May 2006; Springer: Berlin/Heidelberg, Germany, 2006; Volume 3951, pp. 430–443. [Google Scholar]
- Leutenegger, S.; Chli, M.; Siegwart, R.Y. BRISK: Binary robust invariant scalable keypoints. In Proceedings of the 2011 IEEE International Conference on Computer Vision (ICCV), Barcelona, Spain, 6–13 November 2011; pp. 2548–2555. [Google Scholar]
- Matas, J.; Chum, O.; Urban, M.; Pajdla, T. Robust wide-baseline stereo from maximally stable extremal regions. Image Vis. Comput. 2004, 22, 761–767. [Google Scholar] [CrossRef]
- Losurdo, L.; Fanizzi, A.; Basile, T.M.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Fausto, A.; Massafra, R.; Monaco, A.; et al. Combined Approach of Multiscale Texture Analysis and Interest Point/Corner Detectors for Microcalcifications Diagnosis. In International Conference on Bioinformatics and Biomedical Engineering; Springer: Cham, Switzerland, 2018; pp. 302–313. [Google Scholar]
- Tagliafico, A.S.; Valdora, F.; Mariscotti, G.; Durando, M.; Nori, J.; La Forgia, D.; Rosenberg, I.; Caumo, F.; Gandolfo, N.; Houssami, N.; et al. An exploratory radiomics analysis on digital breast tomosynthesis in women with mammographically negative dense breasts. Breast 2018, 40, 92–96. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E. Image processing. In Digital Image Processing; Gonzalez, R.C., Woods, R.E., Eds.; Prebtice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Mallat, S.G. A theory for multiresolution signal decomposition: The wavelet representation. IEEE Trans. Pattern Anal. Mach. Intell. 1989, 11, 674–693. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef]
- Pathak, B.; Barooah, D. Texture analysis based on the gray-level co-occurrence matrix considering possible orientations. Int. J. Adv. Res. Electr. Electron. Instrum. Eng. 2013, 2, 4206–4212. [Google Scholar]
- Mohanaiah, P.; Sathyanarayana, P.; GuruKumar, L. Image texture feature extraction using GLCM approach. Int. J. Sci. Res. Publ. 2013, 3, 1. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Aha, D.W.; Bankert, R.L. A comparative evaluation of sequential feature selection algorithms. In Learning from Data; Springer: Berlin, Germany, 1996; pp. 199–206. [Google Scholar]
- Youden, W. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Matthews, B.W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim. Biophys. Acta Protein Struct. 1975, 405, 442–451. [Google Scholar] [CrossRef]
- Boughorbel, S.; Jarray, F.; El-Anbari, M. Optimal classifier for imbalanced data using Matthews Correlation Coefficient metric. PLoS ONE 2017, 12, e0177678. [Google Scholar] [CrossRef]
- Lobbes, M.B.; Lalji, U.; Houwers, J.; Nijssen, E.C.; Nelemans, P.J.; van Roozendaal, L.; Smidt, M.L.; Heuts, E.; Wildberger, J.E. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur. Radiol. 2014, 24, 1668–1676. [Google Scholar] [CrossRef]
- Lalji, U.; Houben, I.; Prevos, R.; Gommers, S.; van Goethem, M.; Vanwetswinkel, S.; Pijnappel, R.; Steeman, R.; Frotscher, C.; Mok, W.; et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: Validation of results in a large multireader, multicase study. Eur. Radiol. 2016, 26, 4371–4379. [Google Scholar] [CrossRef]
- Jochelson, M.; Lobbes, M.B.; Bernard-Davila, B. Reply to Tagliafico AS, Bignotti B, Rossi F, et al. Breast 2017, 32, 267. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Bignotti, B.; Rossi, F.; Signori, A.; Sormani, M.P.; Valdora, F.; Calabrese, M.; Houssami, N. Diagnostic performance of contrast-enhanced spectral mammography: Systematic review and meta-analysis. Breast 2016, 28, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, J.M.; Zhang, K.; Xia, L.J.; Feng, L.; Yang, P.; Zhang, M.Y.; Xiao, W.; Lin, H.X.; Yu, Y.H. Diagnostic value of Contrast-enhanced Spectral Mammography for screening Breast Cancer: A Systematic Review and Meta analysis. Clin. Breast Cancer 2018, 18, e985–e995. [Google Scholar] [CrossRef] [PubMed]
- Luczyńska, E.; Heinze-Paluchowska, S.; Dyczek, S.; Blecharz, P.; Rys, J.; Reinfuss, M. Contrast-enhanced spectral mammography: Comparison with conventional mammography and histopathology in 152 women. Korean J. Radiol. 2014, 15, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Roth, R.; Germaine, P.; Ren, S.; Lee, M.; Hunter, K.; Tinney, E.; Liao, L. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn. Interv. Imaging 2017, 98, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.M.; Taylor, D.B.; Buzynski, S.; Peake, R.E. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance. J. Med. Imaging Radiat. Oncol. 2015, 59, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Miller, M.M.; Mehta, T.S.; Fein-Zachary, V.; Nathanson, A.; Hori, W.; Monahan-Earley, R.; Slanetz, P.J. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: Patient preferences and attitudes. Clin. Imaging 2017, 42, 193–197. [Google Scholar] [CrossRef]
- Houben, I.; Van de Voorde, P.; Jeukens, C.; Wildberger, J.; Kooreman, L.; Smidt, M.; Lobbes, M. Contrast-enhanced spectral mammography as work-up tool in patients recalled from breast cancer screening has low risks and might hold clinical benefits. Eur. J. Radiol. 2017, 94, 31–37. [Google Scholar] [CrossRef]
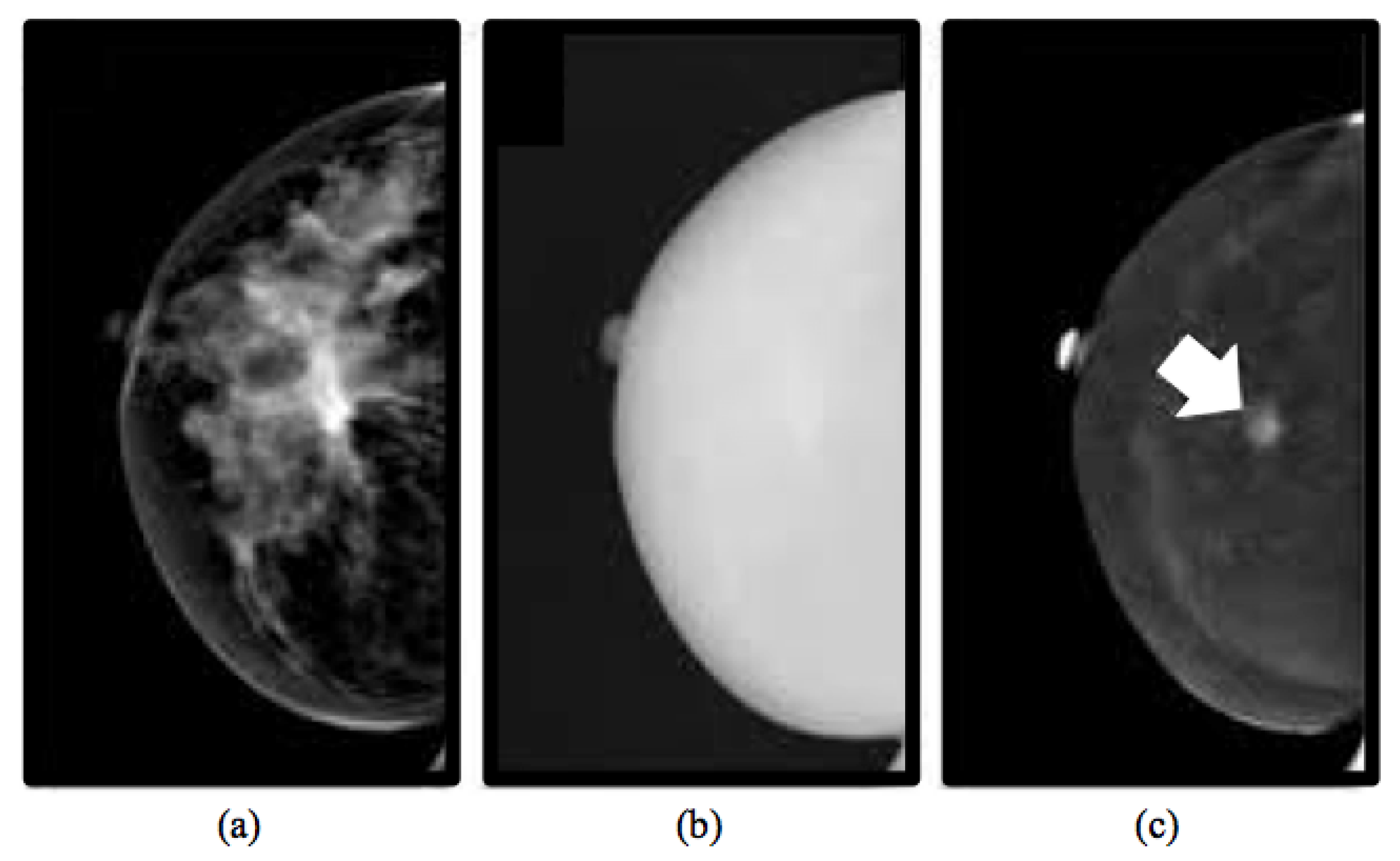
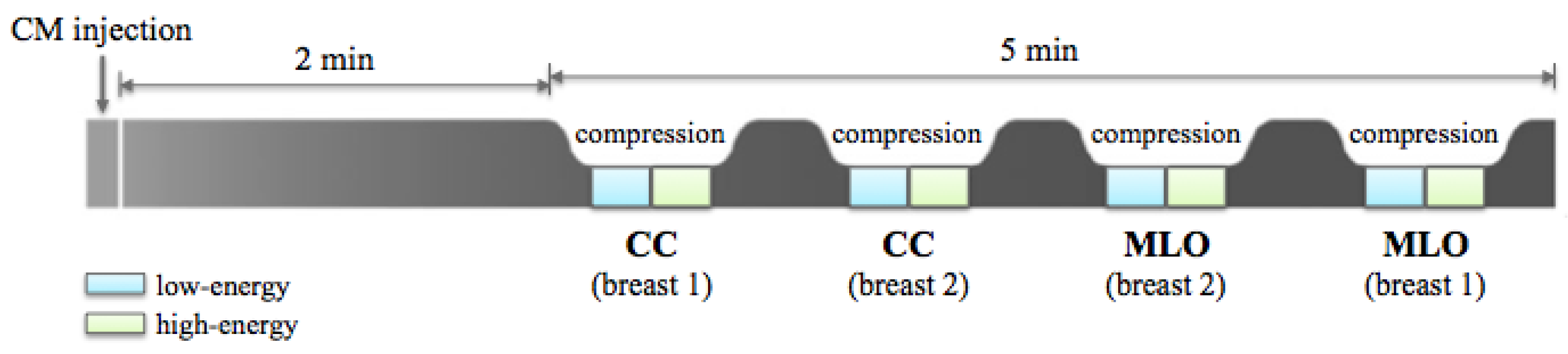

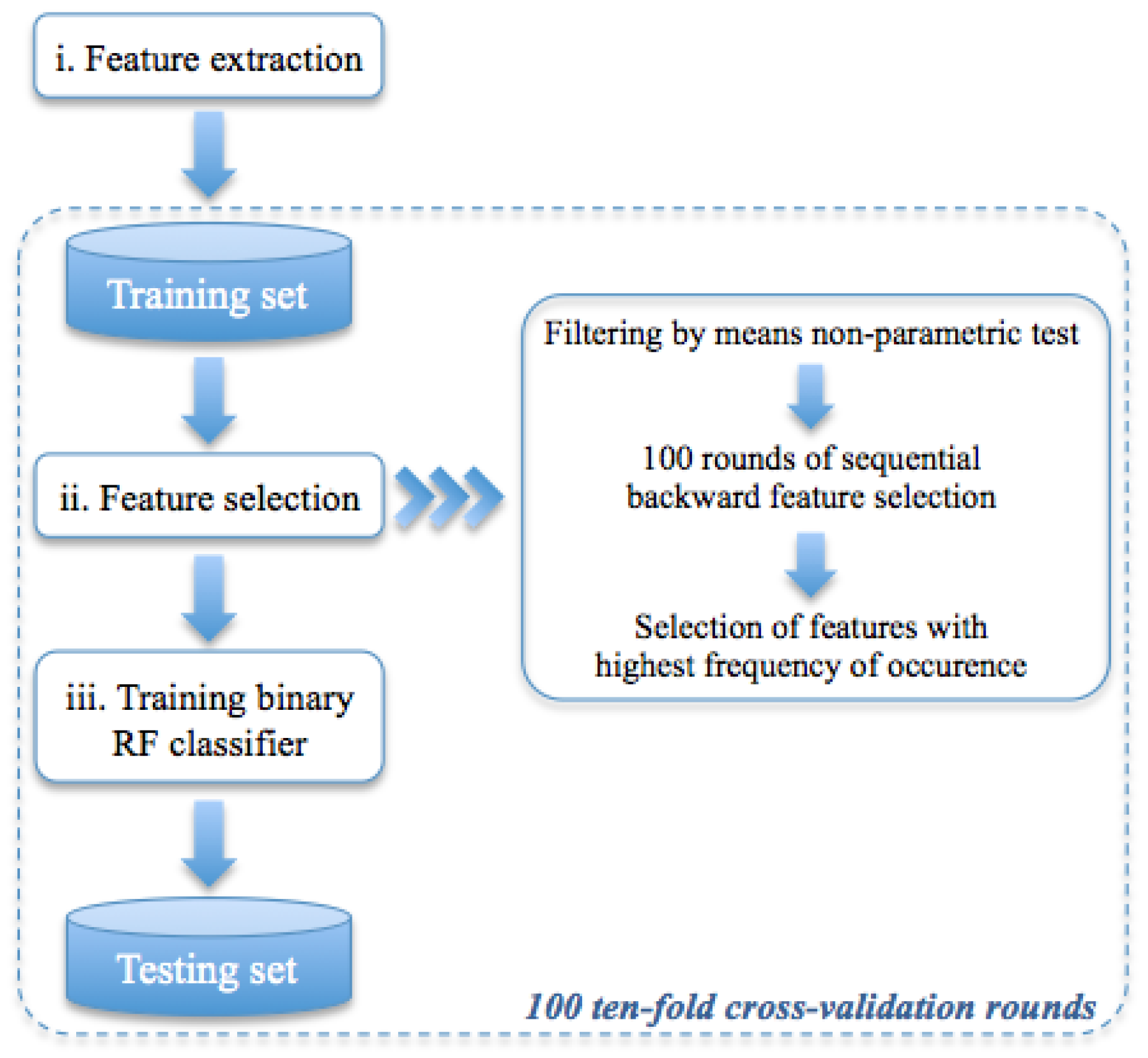
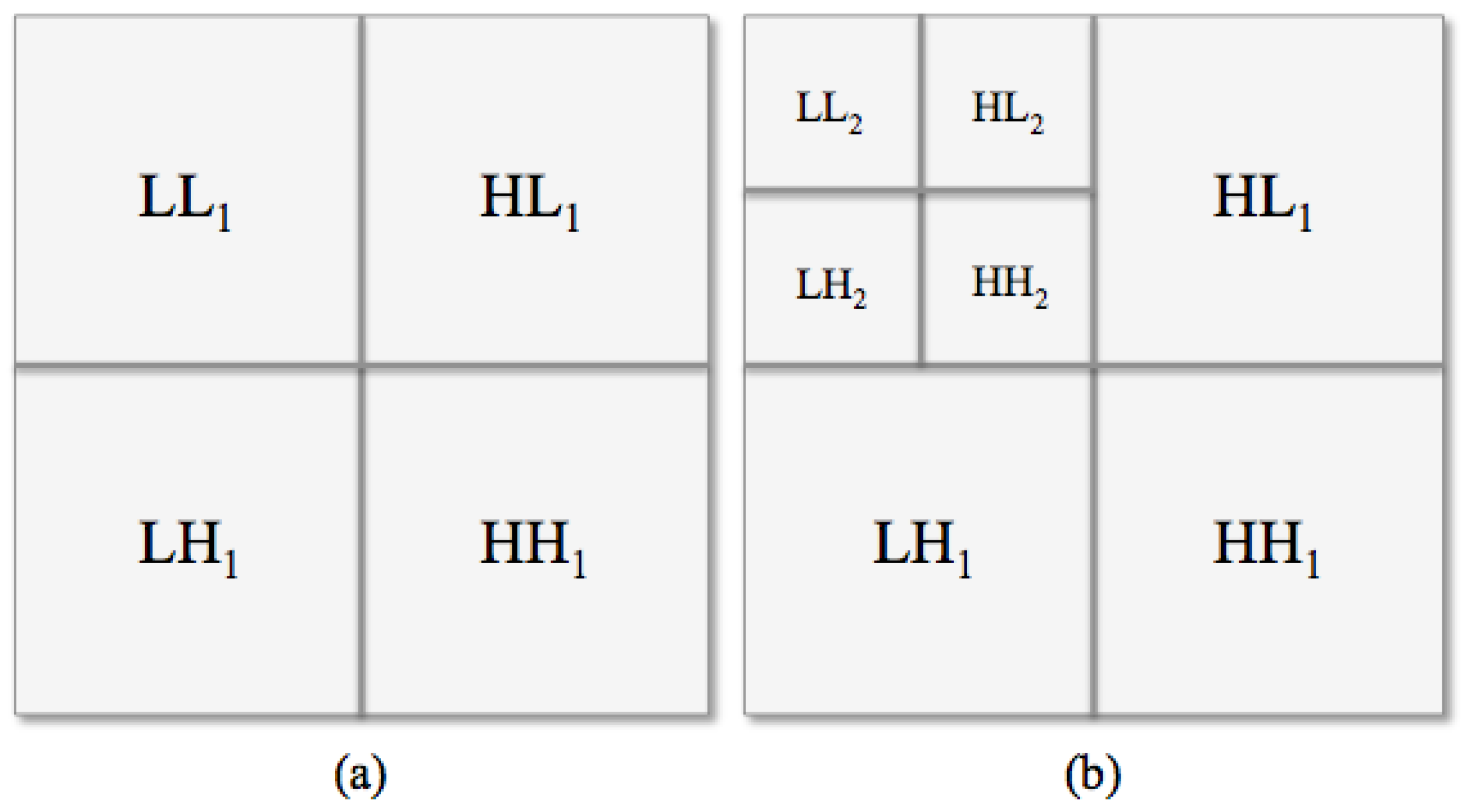

| Diagnostic Test Parameter | Only LE Images | CESM Images | Micro-Histological Results (Only LE/CESM) |
|---|---|---|---|
| No. of selected patients | 53 | 53 | 53/53 |
| No. of selected breasts | 47 | 48 | 47/48 |
| No. of selected lesions | 57 | 58 | 57/58 |
| No. of malignant lesions (TP) | 38 (34) | 38 (34) | 34/34 |
| No. of benign lesions (TN) | 16 (16) | 20 (20) | 23/24 |
| Sensitivity [CI ] | 91.2% (88.8–93.5%) | 100% (95.0–100%) | |
| Specificity [CI ] | 69.6% (67.9–71.2%) | 83.3% (81.2–85.4%) | |
| Accuracy [CI ] | 82.5% (80.4–84.6%) | 93.1% (90.7–95.5%) | |
| MCC | |||
| Size of lesions: mean ± SD (mm) | |||
| Size of the smallest lesion detected (mm) | 6 | 6 |
| BPE I (15B/10M) | BPE II (4B/7M) | BPE III-IV (5B/7M) | Overall Dataset (24B/24M) | |
|---|---|---|---|---|
| Accuracy (%) | 86.0 (84.0–88.0) | 95.5 (90.9–100) | 83.3 (81.3–84.3) | 87.5 (85.4–89.6) |
| Sensitivity (%) | 75.0 (70.0–80.0) | 100 (100–100) | 92.9 (85.7–100) | 87.5 (83.3–91.7) |
| Specificity (%) | 96.7 (86.7–100) | 100 (75.0–100) | 80.0 (60.0–80.0) | 91.7 (87.5–91.7) |
| MCC | 0.84 (0.72–0.91) | 0.91 (0.81–1) | 0.68 (0.66–0.71) | 0.76 (0.74–0.79) |
| Human Reader (24B/34M) | Proposed Model (24B/24M) | |
|---|---|---|
| Accuracy (%) | ||
| Sensitivity (%) | 100 | |
| Specificity (%) | ||
| MCC |
| BPE II (15 Normal/20 Abnormal) | BPE III–IV (10 Normal/12 Abnormal) | Overall Dataset | |
|---|---|---|---|
| Accuracy (%) | 82.9 (80.0–88.6) | 77.3 (77.3–81.8) | 82.5 (79.0–82.5) |
| Sensitivity (%) | 70.0 (65.0–80.0) | 75.0 (66.7–91.7) | 70.3 (68.8–84.4) |
| Specificity (%) | 100 (100–100) | 85.0 (85.0–90.0) | 94.0 (88.0–96.0) |
| MCC | 0.71 (0.67–0.79) | 0.57 (0.55–0.65) | 0.65 (0.63–0.69) |
| Feature Set | Feature | ROI Type | Frequency (%) |
|---|---|---|---|
| COUNT | SIFT | LE | |
| HAAR | Variance_LL2 | RC | |
| STAT | RelativeSmoothness | RC | |
| GRAD | RelativeSmoothness_Gmag | LE | |
| HAAR | Variance_LL1 | RC | |
| STAT | Variance | RC | |
| GRAD | Variance_Gmag | LE | |
| GLCM | ClusterProminence_HL1 () | RC | |
| GLCM | Correlation_LH1 () | RC | |
| HAAR | Variance_LH1 | LE | |
| HAAR | RelativeSmoothness_HL2 | RC | |
| HAAR | Variance_LH2 | LE | |
| GLCM | Homogeneity_LH1 () | RC | |
| STAT | Standard Deviation | RC | |
| HAAR | Variance_HL2 | RC | |
| STAT | Maximum − Minimum | RC | |
| COUNT | MinEigen | RC |
| Feature Set | Feature | ROI Type | Frequency (%) |
|---|---|---|---|
| HAAR | Mean_LL2 | RC | |
| GLCM | SumEntropy_LH1 () | RC | |
| GLCM | Entropy_LH1 () | RC | |
| HAAR | Mean_LL2 | LE | |
| HAAR | Variance_LH1 | RC | |
| GLCM | Entropy_LH1 () | RC | |
| HAAR | Entropy_LH1 | RC | |
| GLCM | SumEntropy_LH1 () | RC | |
| HAAR | RelativeSmoothness_LH1 | RC | |
| HAAR | RelativeSmoothness_LH2 | RC | |
| HAAR | Variance_LH2 | RC | |
| GLCM | Energy_LH1 () | RC | |
| HAAR | Mean_LL1 | RC | |
| HAAR | Variance_LL2 | RC | |
| HAAR | Mean_LL1 | LE | |
| GLCM | Entropy_LH1 () | RC | |
| STAT | Minimum | RC | |
| GLCM | ClusterProminence_HH1 () | RC | |
| COUNT | MSER | RC | |
| HAAR | Variance_LL1 | RC | |
| HAAR | RelativeSmoothness_LL1 | RC | |
| GLCM | Entropy_LH1 () | RC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanizzi, A.; Losurdo, L.; Basile, T.M.A.; Bellotti, R.; Bottigli, U.; Delogu, P.; Diacono, D.; Didonna, V.; Fausto, A.; Lombardi, A.; et al. Fully Automated Support System for Diagnosis of Breast Cancer in Contrast-Enhanced Spectral Mammography Images. J. Clin. Med. 2019, 8, 891. https://doi.org/10.3390/jcm8060891
Fanizzi A, Losurdo L, Basile TMA, Bellotti R, Bottigli U, Delogu P, Diacono D, Didonna V, Fausto A, Lombardi A, et al. Fully Automated Support System for Diagnosis of Breast Cancer in Contrast-Enhanced Spectral Mammography Images. Journal of Clinical Medicine. 2019; 8(6):891. https://doi.org/10.3390/jcm8060891
Chicago/Turabian StyleFanizzi, Annarita, Liliana Losurdo, Teresa Maria A. Basile, Roberto Bellotti, Ubaldo Bottigli, Pasquale Delogu, Domenico Diacono, Vittorio Didonna, Alfonso Fausto, Angela Lombardi, and et al. 2019. "Fully Automated Support System for Diagnosis of Breast Cancer in Contrast-Enhanced Spectral Mammography Images" Journal of Clinical Medicine 8, no. 6: 891. https://doi.org/10.3390/jcm8060891
APA StyleFanizzi, A., Losurdo, L., Basile, T. M. A., Bellotti, R., Bottigli, U., Delogu, P., Diacono, D., Didonna, V., Fausto, A., Lombardi, A., Lorusso, V., Massafra, R., Tangaro, S., & La Forgia, D. (2019). Fully Automated Support System for Diagnosis of Breast Cancer in Contrast-Enhanced Spectral Mammography Images. Journal of Clinical Medicine, 8(6), 891. https://doi.org/10.3390/jcm8060891







