Optical Quality Assessment in Patients with Macular Diseases Using Optical Quality Analysis System
Abstract
1. Introduction
2. Methods
Statistics
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OQAS | Optical Quality Analysis System |
| AMD | age-related macular degeneration |
| OCT | optical coherence tomography |
| OSI | objective scattering index |
| MTF | modulation transfer function |
| PVAs | predicted visual acuities |
| DR | diabetic retinopathy |
| RVO | retinal vein occlusion |
| VEGF | vascular endothelial growth factor |
| logMAR | logarithm of the minimum angle of resolution |
| ANOVA | analysis of variance |
| IS/OS | inner segment/outer segment |
References
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.R.; Omri, S.; Gelize, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2017, 63, 20–68. [Google Scholar] [CrossRef]
- De Jong, P.T. Age-related macular degeneration. New Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Kanagasingam, Y.; Bhuiyan, A.; Abramoff, M.D.; Smith, R.T.; Goldschmidt, L.; Wong, T.Y. Progress on retinal image analysis for age related macular degeneration. Prog. Retin. Eye Res. 2014, 38, 20–42. [Google Scholar] [CrossRef]
- Guell, J.L.; Pujol, J.; Arjona, M.; Diaz-Douton, F.; Artal, P. Optical Quality Analysis System; Instrument for objective clinical evaluation of ocular optical quality. J. Cataract Refract. Surg. 2004, 30, 1598–1599. [Google Scholar]
- Diaz-Douton, F.; Benito, A.; Pujol, J.; Arjona, M.; Guell, J.L.; Artal, P. Comparison of the retinal image quality with a Hartmann-Shack wavefront sensor and a double-pass instrument. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1710–1716. [Google Scholar] [CrossRef]
- Cabot, F.; Saad, A.; McAlinden, C.; Haddad, N.M.; Grise-Dulac, A.; Gatinel, D. Objective assessment of crystalline lens opacity level by measuring ocular light scattering with a double-pass system. Am. J. Ophthalmol. 2013, 155, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.W.; Hao, J.; Zhang, H.; Tian, F. Optical quality of toric intraocular lens implantation in cataract surgery. Int. J. Ophthalmol. 2015, 8, 66–71. [Google Scholar] [PubMed]
- Benito, A.; Perez, G.M.; Mirabet, S.; Vilaseca, M.; Pujol, J.; Marin, J.M.; Artal, P. Objective optical assessment of tear-film quality dynamics in normal and mildly symptomatic dry eyes. J. Cataract Refract. Surg. 2011, 37, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Goni, N.; Bidaguren, A.; Macias-Murelaga, B.; Alberdi, T.; Martinez-Soroa, I.; Mendicute, J. Objective optical quality analysis using double-pass technique in pterygium surgery. Cornea 2015, 34, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Sohn, J.; Choi, J.G.; Chung, S.K. Optical quality in central serous chorioretinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8598–8603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, C.W.; Kim, H.; Joo, C.K. Assessment of Optical Quality at Different Contrast Levels in Pseudophakic Eyes. J. Ophthalmol. 2016, 2016, 4247973. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Roda, J.A.; Vilaseca, M.; Ondategui, J.C.; Giner, A.; Burgos, F.J.; Cardona, G.; Pujol, J. Optical quality and intraocular scattering in a healthy young population. Clin. Exp. Optom. 2011, 94, 223–229. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ranganathan, P. Common pitfalls in statistical analysis: The use of correlation techniques. Perspect. Clin. Res. 2016, 7, 187–190. [Google Scholar]
- Yohannan, J.; Bittencourt, M.; Sepah, Y.J.; Hatef, E.; Sophie, R.; Moradi, A.; Liu, H.; Ibrahim, M.; Do, D.V.; Coulantuoni, E.; et al. Association of retinal sensitivity to integrity of photoreceptor inner/outer segment junction in patients with diabetic macular edema. Ophthalmology 2013, 120, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, A.; Alkin, Z.; Karakucuk, Y.; Karatas, G.; Fazil, K.; Gurkan Erdogan, M.; Perente, I.; Taskapili, M. Thickness of the retinal photoreceptor outer segment layer in healthy volunteers and in patients with diabetes mellitus without retinopathy, diabetic retinopathy, or diabetic macular edema. Saudi J. Ophthalmol. 2017, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Altunel, O.; Duru, N.; Goktas, A.; Ozkose, A.; Goktas, E.; Atas, M. Evaluation of foveal photoreceptor layer in eyes with macular edema associated with branch retinal vein occlusion after ozurdex treatment. Int. Ophthalmol. 2017, 37, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Medeiros, N.E.; Millican, C.L. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Tomkins-Netzer, O.; Ismetova, F.; Bar, A.; Seguin-Greenstein, S.; Kramer, M.; Lightman, S. Functional outcome of macular edema in different retinal disorders. Prog. Retin. Eye Res. 2015, 48, 119–136. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Control | Macular Disease | p-Value |
|---|---|---|---|
| Eyes (n) | 43 | 88 | |
| Age (years) | 54.70 ± 15.03 | 65.24 ± 12.96 | <0.001 * |
| Male:female | 11:32 | 51:37 | |
| Visual acuity (logMAR) | 0.06 ± 0.19 | 0.44 ± 0.33 | <0.001 * |
| No. of pseudophakic eyes (n, %) | 4 (9.30%) | 36 (40.91%) | |
| Retinal thickness | |||
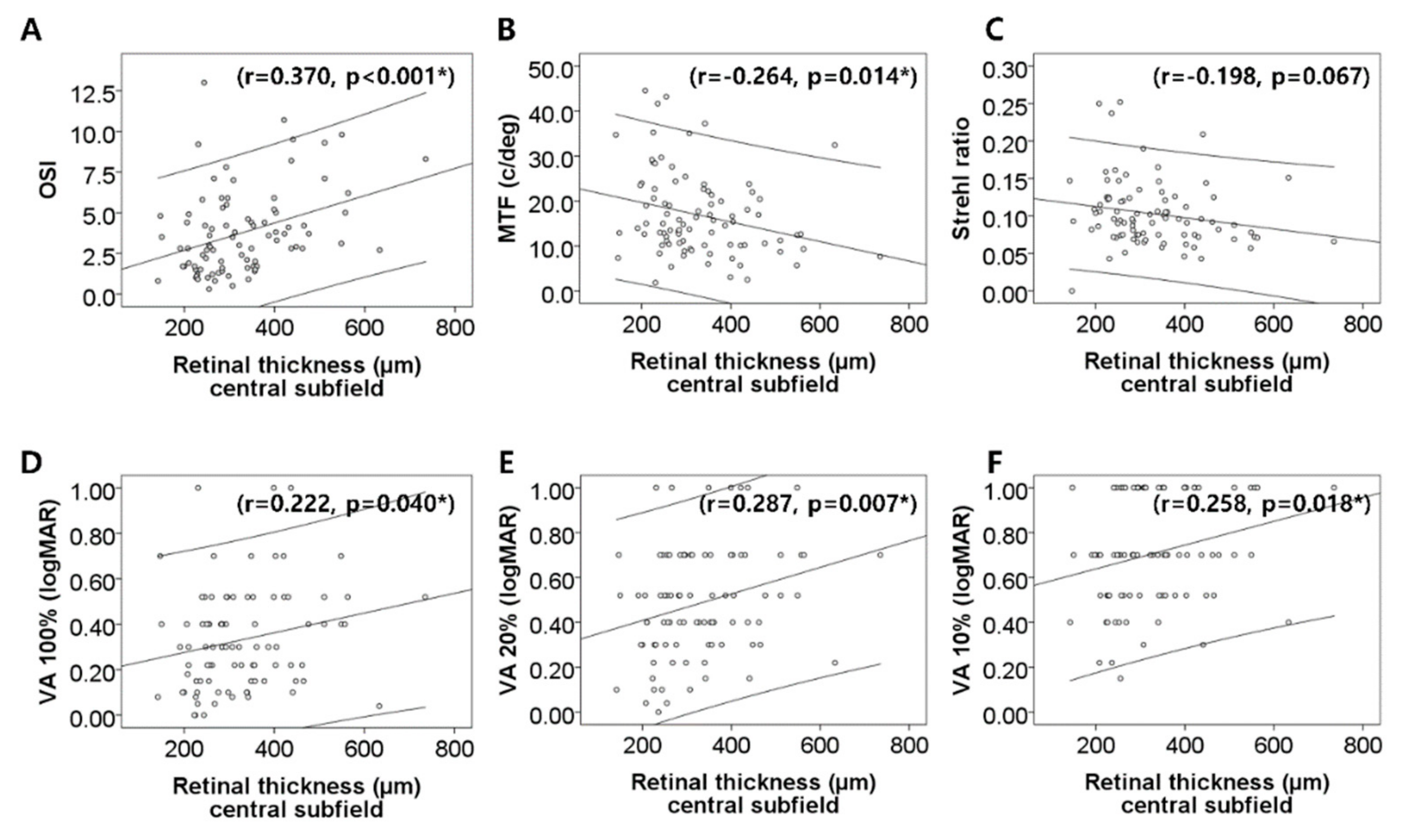
| Central subfield (µm) | 329.4 ± 113.3 | ||
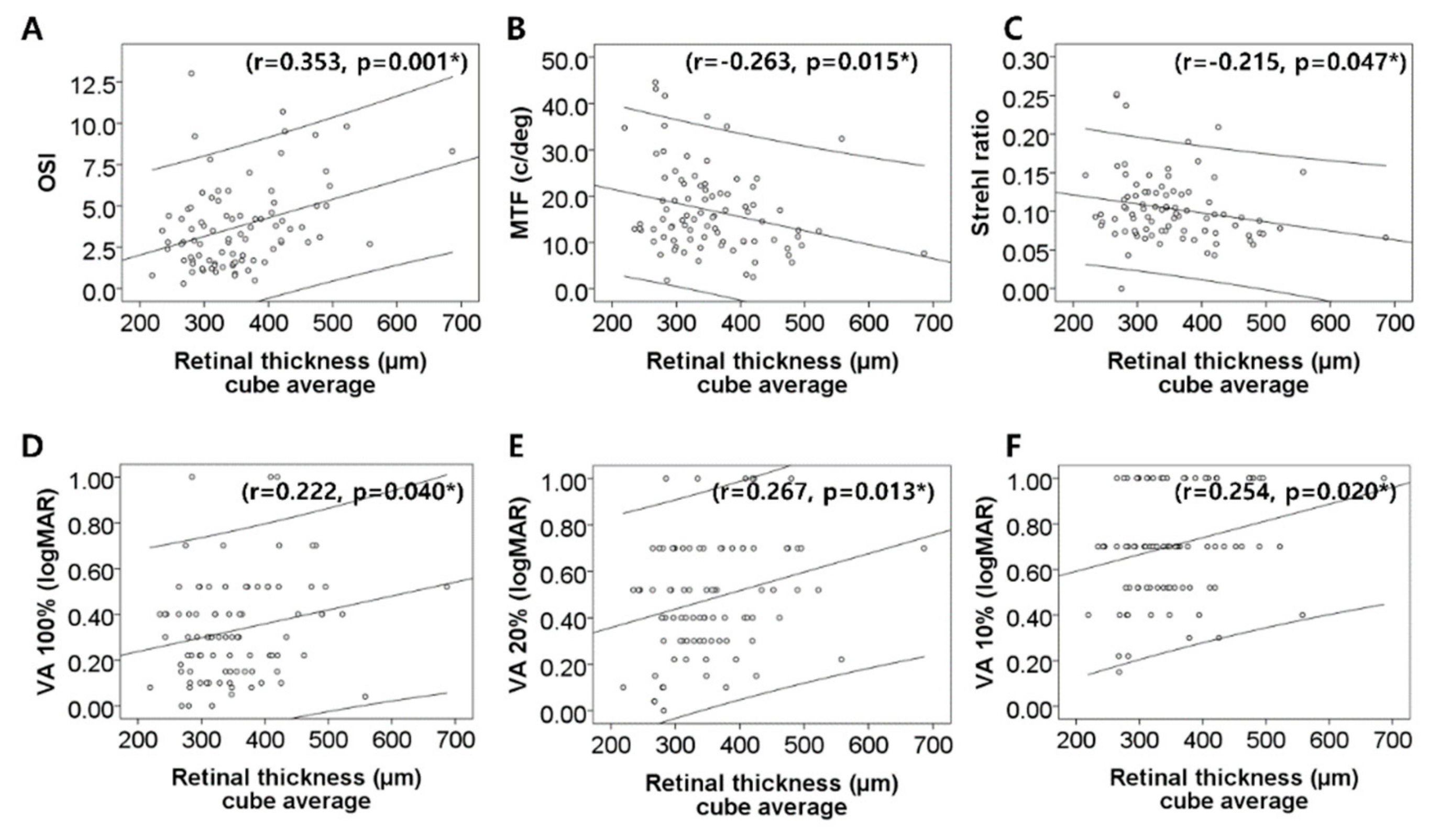
| Cube average (µm) | 353.4 ± 81.1 | ||
| Optical quality | |||
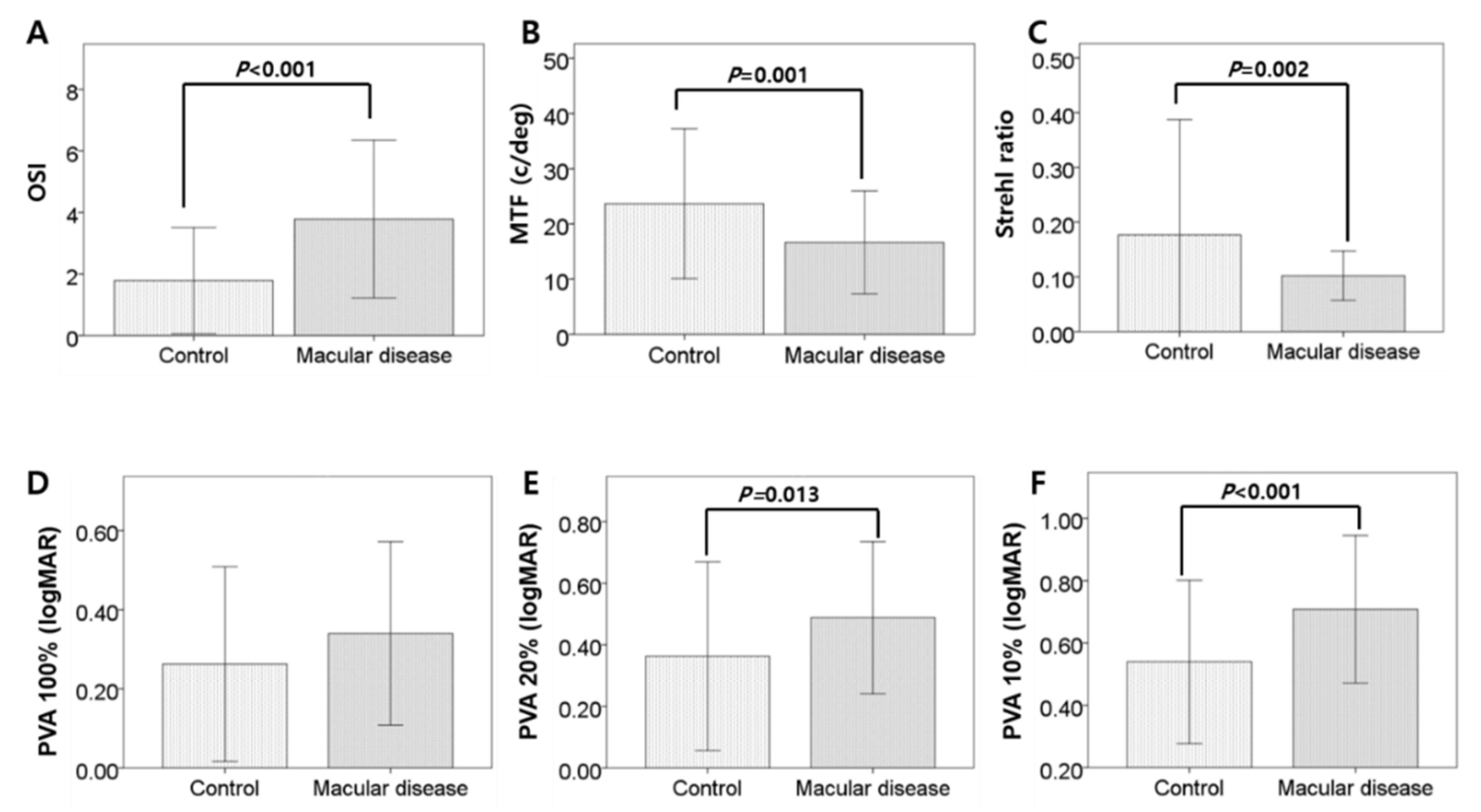
| OSI (OSI value) | 1.78 ± 1.73 | 3.78 ± 2.56 | <0.001 * |
| MTF cut-off (c/deg) | 23.64 ± 13.56 | 16.66 ± 9.32 | 0.001 * |
| Strehl ratio | 0.18 ± 0.02 | 0.10 ± 0.04 | 0.002 * |
| PVA at 100% (logMAR) | 0.26 ± 0.24 | 0.34 ± 0.23 | 0.081 |
| PVA at 20% (logMAR) | 0.36 ± 0.31 | 0.49 ± 0.25 | 0.013 * |
| PVA at 10% (logMAR) | 0.54 ± 0.26 | 0.71 ± 0.24 | <0.001 * |
| Characteristics | Subgroup of Macular Disease | p-Value | ||
|---|---|---|---|---|
| AMD | DR | RVO | ||
| Eyes (n) | 62 | 19 | 7 | |
| Age (years) | 68.35 ± 11.28 | 56.42 ± 14.01 | 61.57 ± 13.76 | 0.001 * |
| Male:female | 40:22 | 9:10 | 2:5 | |
| Visual acuity (logMAR) | 0.44 ± 0.36 | 0.49 ± 0.23 | 0.31 ± 0.22 | 0.453 |
| Retina thickness | ||||
| Subfovea (µm) | 284.1 ± 81.3 | 421.3 ± 91.2 | 488.0 ± 128.2 | <0.001 * |
| Cube average (µm) | 318.9 ± 53.8 | 427.0 ± 60.7 | 464.1 ± 105.7 | <0.001 * |
| Optical quality | ||||
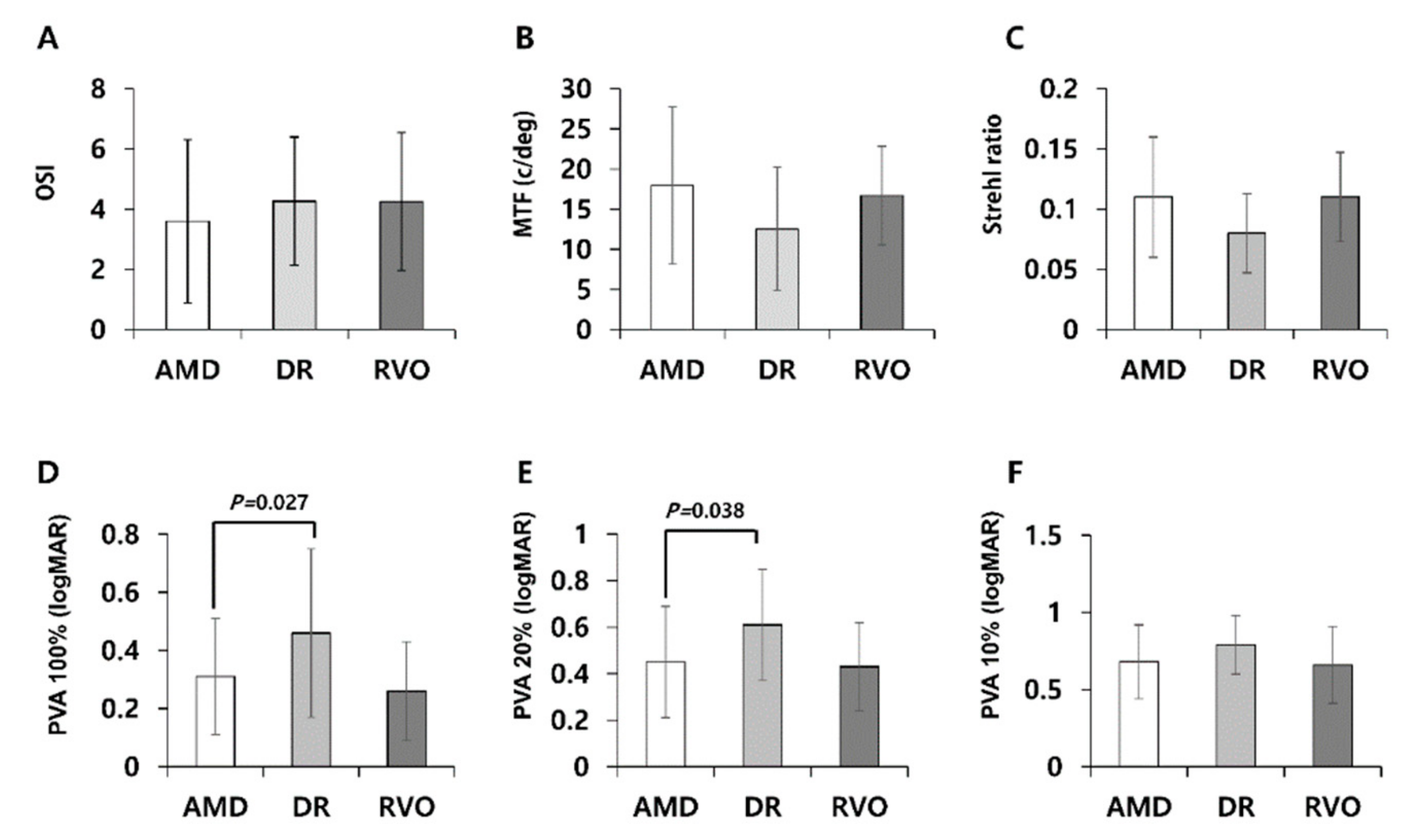
| OSI | 3.59 ± 2.71 | 4.26 ± 2.13 | 4.25 ± 2.29 | 0.534 |
| MTF cut-off (c/deg) | 17.93 ± 9.78 | 12.52 ± 7.69 | 16.68 ± 6.16 | 0.085 |
| Strehl ratio | 0.11 ± 0.05 | 0.08 ± 0.03 | 0.11 ± 0.04 | 0.050 |
| PVA at 100% (logMAR) | 0.31 ± 0.20 | 0.46 ± 0.29 | 0.26 ± 0.17 | 0.023 * |
| PVA at 20% (logMAR) | 0.45 ± 0.24 | 0.61 ± 0.24 | 0.43 ± 0.19 | 0.036 * |
| PVA at 10% (logMAR) | 0.68 ± 0.24 | 0.79 ± 0.19 | 0.66 ± 0.25 | 0.232 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.H.; Bae, S.H.; Kim, H.K.; Shin, Y.J. Optical Quality Assessment in Patients with Macular Diseases Using Optical Quality Analysis System. J. Clin. Med. 2019, 8, 892. https://doi.org/10.3390/jcm8060892
Cho JH, Bae SH, Kim HK, Shin YJ. Optical Quality Assessment in Patients with Macular Diseases Using Optical Quality Analysis System. Journal of Clinical Medicine. 2019; 8(6):892. https://doi.org/10.3390/jcm8060892
Chicago/Turabian StyleCho, Joon Hee, So Hyun Bae, Ha Kyoung Kim, and Young Joo Shin. 2019. "Optical Quality Assessment in Patients with Macular Diseases Using Optical Quality Analysis System" Journal of Clinical Medicine 8, no. 6: 892. https://doi.org/10.3390/jcm8060892
APA StyleCho, J. H., Bae, S. H., Kim, H. K., & Shin, Y. J. (2019). Optical Quality Assessment in Patients with Macular Diseases Using Optical Quality Analysis System. Journal of Clinical Medicine, 8(6), 892. https://doi.org/10.3390/jcm8060892




