The New Era for Reno-Cardiovascular Treatment in Type 2 Diabetes
Abstract
1. Introduction
2. Classical Pharmacological Reno-Cardiovascular Approaches in Diabetes
3. Reno-Cardiovascular Protection of SGLT2 Inhibition
4. Reno-Cardiovascular Protection of GLP1 Receptor Agonists
5. Combination of SGLT2 Inhibitors and GLP1 Receptor Agonists in Diabetes
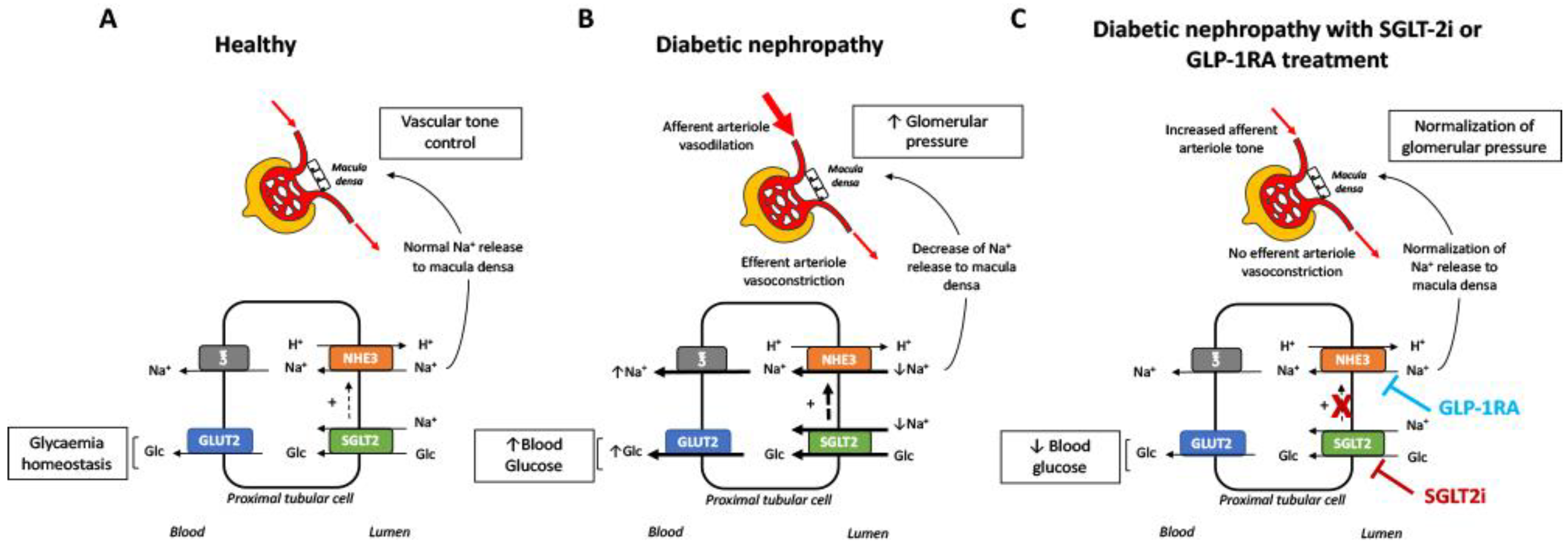
6. Potential Nephroprotective Mechanisms of SGLT2 Inhibitors and GLP1 Receptor Agonists
7. Current Role of DPP-4 Inhibitors in Patients with Type 2 Diabetes and CKD
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guideline Development Group; Bilo, H.; Coentrao, L.; Couchoud, C.; Covic, A.; De Sutter, J.; Drechsler, C.; Gnudi, L.; Goldsmith, D.; Heaf, J.; et al. Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR < 45 mL/min). Nephrol. Dial. Transplant. 2015, 30, 1–142. [Google Scholar]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Anguiano, L.; Riera, M.; Pascual, J.; Soler, M.J. Endothelin Blockade in Diabetic Kidney Disease. J. Clin. Med. 2015, 4, 1171–1192. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.E.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B.; LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Malham, S.B.; Herrick, C.J. New Pharmacologic Agents for Diabetes Treatment. Mo Med. 2016, 113, 361–366. [Google Scholar]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C.; Sowers, J.R. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Miller, J.A.; Floras, J.S.; Zinman, B.; Skorecki, K.L.; Logan, A.G. Effect of hyperglycaemia on arterial pressure, plasma renin activity and renal function in early diabetes. Clin. Sci. 1996, 90, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar]
- Matsusaka, H.; Kinugawa, S.; Ide, T.; Matsushima, S.; Shiomi, T.; Kubota, T.; Sunagawa, K.; Tsutsui, H. Angiotensin II type 1 receptor blocker attenuates exacerbated left ventricular remodeling and failure in diabetes-associated myocardial infarction. J. Cardiovasc. Pharmacol. 2006, 48, 95–102. [Google Scholar] [CrossRef]
- CONSENSUS Trial Study Group. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N. Engl. J. Med. 1987, 316, 1429–1435. [Google Scholar] [CrossRef]
- SOLVD Investigators; Yusuf, S.; Pitt, B.; Davis, C.E.; Hood, W.B.; Cohn, J.N. Effect of Enalapril on Survival in Patients with Reduced Left Ventricular Ejection Fractions and Congestive Heart Failure. N. Engl. J. Med. 1991, 325, 293–302. [Google Scholar]
- Delanaye, P.; Scheen, A.J. Preventing and treating kidney disease in patients with type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 277–294. [Google Scholar] [CrossRef]
- Zoccali, C.; Blankestijn, P.J.; Bruchfeld, A.; Capasso, G.; Fliser, D.; Fouque, D.; Goumenos, D.; Ketteler, M.; Massy, Z.; Rychlık, I.; et al. Children of a lesser god: Exclusion of chronic kidney disease patients from clinical trials. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef]
- Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar]
- Parving, H.-H.; Brenner, B.M.; McMurray, J.J.V.; de Zeeuw, D.; Haffner, S.M.; Solomon, S.D.; Chaturvedi, N.; Persson, F.; Desai, A.S.; Nicolaides, M.; et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 2012, 367, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Isaji, M. SGLT2 inhibitors: Molecular design and potential differences in effect. Kidney Int. Suppl. 2011, 79, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Bashier, A.; Khalifa, A.A.; Rashid, F.; Abdelgadir, E.I.; Al Qaysi, A.A.; Ali, R.; Eltinay, A.; Nafach, J.; Alsayyah, F.; Alawadi, F. Efficacy and Safety of SGLT2 Inhibitors in Reducing Glycated Hemoglobin and Weight in Emirati Patients with Type 2 Diabetes. J. Clin. Med. Res. 2017, 9, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2018, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Haring, H.-U.; Merker, L.; Seewaldt-Becker, E.; Weimer, M.; Meinicke, T.; Woerle, H.J.; Broedl, U.C.; EMPA-REG METSU Trial Investigators. Empagliflozin as Add-on to Metformin Plus Sulfonylurea in Patients with Type 2 Diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013, 36, 3396–3404. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Seshiah, V.; Swallow, R.; Jones, R.; Rattunde, H.; Woerle, H.J.; Broedl, U.C.; EMPA-REG PIOTM trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: A 24-week, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2014, 16, 147–158. [Google Scholar] [CrossRef]
- Wanner, C.; Heerspink, H.J.L.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; Hantel, S.; Woerle, H.-J.; Broedl, U.C.; von Eynatten, M.; et al. Empagliflozin and Kidney Function Decline in Patients with Type 2 Diabetes: A Slope Analysis from the EMPA-REG OUTCOME Trial. J. Am. Soc. Nephrol. 2018, 29, 2755–2769. [Google Scholar] [CrossRef]
- Paternoster, S.; Falasca, M. Dissecting the Physiology and Pathophysiology of Glucagon-Like Peptide-1. Front. Endocrinol. 2018, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Guja, C.; Hardy, E.; Ahmed, A.; Dong, F.; Öhman, P.; Jabbour, S.A. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): A 28 week, multicentre, double-blind, phase 3, randomised control. Lancet. Diabetes Endocrinol. 2016, 4, 1004–1016. [Google Scholar] [CrossRef]
- Ludvik, B.; Frías, J.P.; Tinahones, F.J.; Wainstein, J.; Jiang, H.; Robertson, K.E.; García-Pérez, L.-E.; Woodward, D.B.; Milicevic, Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): A 24-week, randomised, double-blind, placebo-controlled trial. Lancet. Diabetes Endocrinol. 2018, 6, 370–381. [Google Scholar] [CrossRef]
- Philis-Tsimikas, A.; Billings, L.K.; Busch, R.; Portillo, C.M.; Sahay, R.; Halladin, N.; Eggert, S.; Begtrup, K.; Harris, S. Superior efficacy of insulin degludec/liraglutide versus insulin glargine U100 as add-on to sodium-glucose co-transporter-2 inhibitor therapy: A randomized clinical trial in people with uncontrolled type 2 diabetes. Diabetes Obes. Metab. 2019, 21, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Distribution of glucose transporters in renal diseases. J. Biomed. Sci. 2017, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Armando, I.; Upadhyay, K.; Pascua, A.; Jose, P.A. The regulation of proximal tubular salt transport in hypertension: An update. Curr. Opin. Nephrol. Hypertens. 2009, 18, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Umino, H.; Hasegawa, K.; Minakuchi, H.; Muraoka, H.; Kawaguchi, T.; Kanda, T.; Tokuyama, H.; Wakino, S.; Itoh, H. High Basolateral Glucose Increases Sodium-Glucose Cotransporter 2 and Reduces Sirtuin-1 in Renal Tubules through Glucose Transporter-2 Detection. Sci. Rep. 2018, 8, 6791. [Google Scholar] [CrossRef]
- Cohen, M.; Kitsberg, D.; Tsytkin, S.; Shulman, M.; Aroeti, B.; Nahmias, Y. Live imaging of GLUT2 glucose-dependent trafficking and its inhibition in polarized epithelial cysts. Open Biol. 2014, 4, 140091. [Google Scholar] [CrossRef] [PubMed]
- Hinden, L.; Udi, S.; Drori, A.; Gammal, A.; Nemirovski, A.; Hadar, R.; Baraghithy, S.; Permyakova, A.; Geron, M.; Cohen, M.; et al. Modulation of Renal GLUT2 by the Cannabinoid-1 Receptor: Implications for the Treatment of Diabetic Nephropathy. J. Am. Soc. Nephrol. JASN 2018, 29, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, T.D.; Campos, L.C.G.; Carraro-Lacroix, L.; Girardi, A.C.C.; Malnic, G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J. Am. Soc. Nephrol. JASN 2014, 25, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Kim, S.; Son, M.; Kim, M.; Koh, E.S.; Shin, S.J.; Ko, S.-H.; Kim, H.-S. Empagliflozin Contributes to Polyuria via Regulation of Sodium Transporters and Water Channels in Diabetic Rat Kidneys. Front. Physiol. 2019, 10, 271. [Google Scholar] [CrossRef]
- Carraro-Lacroix, L.R.; Malnic, G.; Girardi, A.C.C. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2009, 297, F1647–F1655. [Google Scholar] [CrossRef]
- Farah, L.X.S.; Valentini, V.; Pessoa, T.D.; Malnic, G.; McDonough, A.A.; Girardi, A.C.C. The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am. J. Physiol. Ren. Physiol. 2016, 310, F123–F127. [Google Scholar] [CrossRef]
- Crajoinas, R.O.; Oricchio, F.T.; Pessoa, T.D.; Pacheco, B.P.M.; Lessa, L.M.A.; Malnic, G.; Girardi, A.C.C. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am. J. Physiol. Ren. Physiol. 2011, 301, F355–F363. [Google Scholar] [CrossRef]
- Packer, M. Contrasting effects on the risk of macrovascular and microvascular events of antihyperglycemic drugs that enhance sodium excretion and lower blood pressure. Diabet. Med. 2018, 35, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Skov, J. Effects of GLP-1 in the Kidney. Rev. Endocr. Metab. Disord. 2014, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef] [PubMed]
- Cassis, P.; Locatelli, M.; Cerullo, D.; Corna, D.; Buelli, S.; Zanchi, C.; Villa, S.; Morigi, M.; Remuzzi, G.; Benigni, A.; et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, U.; Pegg, K.; Gross, S.; Komala, M.G.; Mudaliar, H.; Forbes, J.; Pollock, C.; Mather, A. Effects of SGLT2 Inhibition in Human Kidney Proximal Tubular Cells—Renoprotection in Diabetic Nephropathy? PLoS ONE 2013, 8, e54442. [Google Scholar] [CrossRef]
- Abbas, N.A.T.; El. Salem, A.; Awad, M.M. Empagliflozin, SGLT2inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: Potential role of klotho expression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1347–1360. [Google Scholar] [CrossRef]
- Zhang, Y.; Nakano, D.; Guan, Y.; Hitomi, H.; Uemura, A.; Masaki, T.; Kobara, H.; Sugaya, T.; Nishiyama, A. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor–dependent pathway after renal injury in mice. Kidney Int. 2018, 94, 524–535. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Liu, S.; Liao, G.; Li, L.; Chen, Y.; Cheng, J.; Lu, Y.; Liu, J. GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS ONE 2018, 13, e0193473. [Google Scholar] [CrossRef]
- Ito, M.; Abe, M.; Okada, K.; Sasaki, H.; Maruyama, N.; Tsuchida, M.; Higuchi, T.; Kikuchi, F.; Soma, M. The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Endocr. J. 2011, 58, 979–987. [Google Scholar] [CrossRef]
- Ito, H.; Mifune, M.; Matsuyama, E.; Furusho, M.; Omoto, T.; Shinozaki, M.; Nishio, S.; Antoku, S.; Abe, M.; Togane, M.; et al. Vildagliptin is Effective for Glycemic Control in Diabetic Patients Undergoing either Hemodialysis or Peritoneal Dialysis. Diabetes Ther. 2013, 4, 321–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujii, Y.; Abe, M.; Higuchi, T.; Mizuno, M.; Suzuki, H.; Matsumoto, S.; Ito, M.; Maruyama, N.; Okada, K.; Soma, M. The dipeptidyl peptidase-4 inhibitor alogliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Expert Opin. Pharmacother. 2013, 14, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Arjona Ferreira, J.C.; Corry, D.; Mogensen, C.E.; Sloan, L.; Xu, L.; Golm, G.T.; Gonzalez, E.J.; Davies, M.J.; Kaufman, K.D.; Goldstein, B.J. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: A 54-week randomized trial. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2013, 61, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Rosenstock, J.; Groop, P.-H.; Barnett, A.H.; Gallwitz, B.; Hehnke, U.; Tamminen, I.; Patel, S.; von Eynatten, M.; Woerle, H.-J. Treatment with the Dipeptidyl Peptidase-4 Inhibitor Linagliptin or Placebo Followed by Glimepiride in Patients with Type 2 Diabetes With Moderate to Severe Renal Impairment: A 52-Week, Randomized, Double-Blind Clinical Trial: Figure 1. Diabetes Care 2015, 38, e15–e17. [Google Scholar] [CrossRef] [PubMed]
- Udell, J.A.; Bhatt, D.L.; Braunwald, E.; Cavender, M.A.; Mosenzon, O.; Steg, P.G.; Davidson, J.A.; Nicolau, J.C.; Corbalan, R.; Hirshberg, B.; et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus and Moderate or Severe Renal Impairment: Observations From the SAVOR-TIMI 53 Trial. Diabetes Care 2014, 38, dc141850. [Google Scholar] [CrossRef] [PubMed]
- Groop, P.-H.; Cooper, M.E.; Perkovic, V.; Hocher, B.; Kanasaki, K.; Haneda, M.; Schernthaner, G.; Sharma, K.; Stanton, R.C.; Toto, R.; et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: The randomized MARLINA-T2D trial. Diabetes Obes. Metab. 2017, 19, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Groop, P.-H.; Cooper, M.E.; Perkovic, V.; Emser, A.; Woerle, H.-J.; von Eynatten, M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 2013, 36, 3460–3468. [Google Scholar] [CrossRef]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39, S165–S171. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z.I. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.; Ferro, C.J.; Morales, E.; Ortiz, A.; Malyszko, J.; Hojs, R.; Khazim, K.; Ekart, R.; Valdivielso, J.; Fouque, D.; et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial. Transplant. 2019, 34, 208–230. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Vaduganathan, M. Practical Guide to Prescribing Sodium-Glucose Cotransporter 2 Inhibitors for Cardiologists. JACC Heart Fail. 2019, 7, 169–172. [Google Scholar] [CrossRef] [PubMed]
| Pharmacological Class | SGLT2 Inhibitors | GLP-1 Receptor Agonists | ||||
|---|---|---|---|---|---|---|
| Trials | EMPA-REG OUTCOME | CANVAS Program | DECLARE-TIMI 58 | CREDENCE | LEADER | SUSTAIN-6 |
| Antidiabetic agent | Empagliflozin | Canagliflozin | Dapagliflozin | Canagliflozin | Liraglutide | Semaglutide |
| Median follow-up (years) | 3.1 | 2.4 | 4.2 | 2.6 | 3.84 | 2.1 |
| Number of patients (n) (active vs. placebo) | 4687 vs. 2333 | 5795 vs. 4347 | 8582 vs. 8578 | 2202 vs. 2199 | 4668 vs. 4672 | 1648 vs. 1649 |
| % Patients with moderate-to-severe renal disease a | 25.9% | NR | 7% | 59.8% | 23.1% | 28.5% |
| Hazard Ratio (95% CI) | ||||||
| Composite renal outcome | 0.61 (0.53–0.70) b | 0.60 (0.47–0.77) c | 0.76 (0.67–0.87) d | 0.70 (0.59–0.82) e | 0.78 (0.67–0.92) b | 0.64 (0.46–0.88) b |
| New onset of persistent macroalbuminuria | 0.62 (0.54–0.72) | NR | NR | NR | 0.62 (0.54–0.72) | NR |
| Persistent doubling of serum creatinine | 0.56 (0.39–0.79) f | NR | NR | 0.60 (0.48–0.76) g | 0.89 (0.67–1.19) f | NR |
| Initiation of renal-replacement therapy | 0.45 (0.21–0.97) | NR | NR | 0.74 (0.55–1.00) | 0.87 (0.61–1.24) | NR |
| Death due to renal disease | NA | NR | NR | NA | 1.59 (0.52–4.87) | NR |
| Chronic Kidney Diseases Stages (mL/min/1.73 m2) | ||||||
|---|---|---|---|---|---|---|
| 1 (≥90) | 2 (60–89) | 3a (45–59) | 3b (30–44) | 4 (15–29) | 5 (<15) | |
| DPP4 inhibitors (oral) | ||||||
| Sitagliptin | 25–100 mg/day | No dose adjustment | 50 mg/day | 25 mg/day | ||
| Vildagliptin | 50–100 mg/day | No dose adjustment | 50 mg/day | |||
| Saxagliptin | 2.5–5 mg/day | No dose adjustment | 2.5 mg/day | Avoid in Dialysis | ||
| Linagliptin | 5 mg/day | No dose adjustment | ||||
| Alogliptin | 6.5–25 mg/day | No dose adjustment | 12.5 mg/day | 6.5 mg/day | ||
| GLP-1 receptor agonists (subcutaneously) | ||||||
| Exetanide | 10μg/12 hours | No dose adjustment | Avoid | |||
| Exetanide LP | 2 mg/week | No dose adjustment | Avoid | |||
| Lixisetanide | 20μg/day | No dose adjustment | Avoid | |||
| Albiglutide | 30–50 mg/week | No dose adjustment | Avoid | |||
| Dulaglutide | 0.75–1.5 mg/week | No dose adjustment | Avoid | |||
| Liraglutide | 1.2–1.8 mg/day | No dose adjustment | Avoid | |||
| Semaglutide | 0.25–1 mg/week | No dose adjustment | Avoid | |||
| SGLT2 inhibitors (oral) | ||||||
| Empagliflozin | 10–25 mg/day | No dose adjustment | Avoid | |||
| Canagliflozin | 100–300 mg/day | No dose adjustment | Avoid | |||
| Dapagliflozin | 5–10 mg/day | No dose adjustment | Avoid | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Carro, C.; Vergara, A.; Agraz, I.; Jacobs-Cachá, C.; Espinel, E.; Seron, D.; Soler, M.J. The New Era for Reno-Cardiovascular Treatment in Type 2 Diabetes. J. Clin. Med. 2019, 8, 864. https://doi.org/10.3390/jcm8060864
García-Carro C, Vergara A, Agraz I, Jacobs-Cachá C, Espinel E, Seron D, Soler MJ. The New Era for Reno-Cardiovascular Treatment in Type 2 Diabetes. Journal of Clinical Medicine. 2019; 8(6):864. https://doi.org/10.3390/jcm8060864
Chicago/Turabian StyleGarcía-Carro, Clara, Ander Vergara, Irene Agraz, Conxita Jacobs-Cachá, Eugenia Espinel, Daniel Seron, and María José Soler. 2019. "The New Era for Reno-Cardiovascular Treatment in Type 2 Diabetes" Journal of Clinical Medicine 8, no. 6: 864. https://doi.org/10.3390/jcm8060864
APA StyleGarcía-Carro, C., Vergara, A., Agraz, I., Jacobs-Cachá, C., Espinel, E., Seron, D., & Soler, M. J. (2019). The New Era for Reno-Cardiovascular Treatment in Type 2 Diabetes. Journal of Clinical Medicine, 8(6), 864. https://doi.org/10.3390/jcm8060864





