Indoleamine-2,3-Dioxygenase in Thyroid Cancer Cells Suppresses Natural Killer Cell Function by Inhibiting NKG2D and NKp46 Expression via STAT Signaling Pathways
Abstract
:1. Introduction
2. Experimental Section
2.1. Cell Lines and Culture
2.2. NK Cell Isolation and Culture
2.3. Evaluation of NK Cell Cytotoxicity
2.4. Flow Cytometry Analysis
2.5. IFN-γ and Kynurenine Measurement
2.6. Western Blot Analysis
2.7. Quantitative Real-Time PCR
2.8. Luciferase Assay
2.9. Statistical Analysis
3. Results
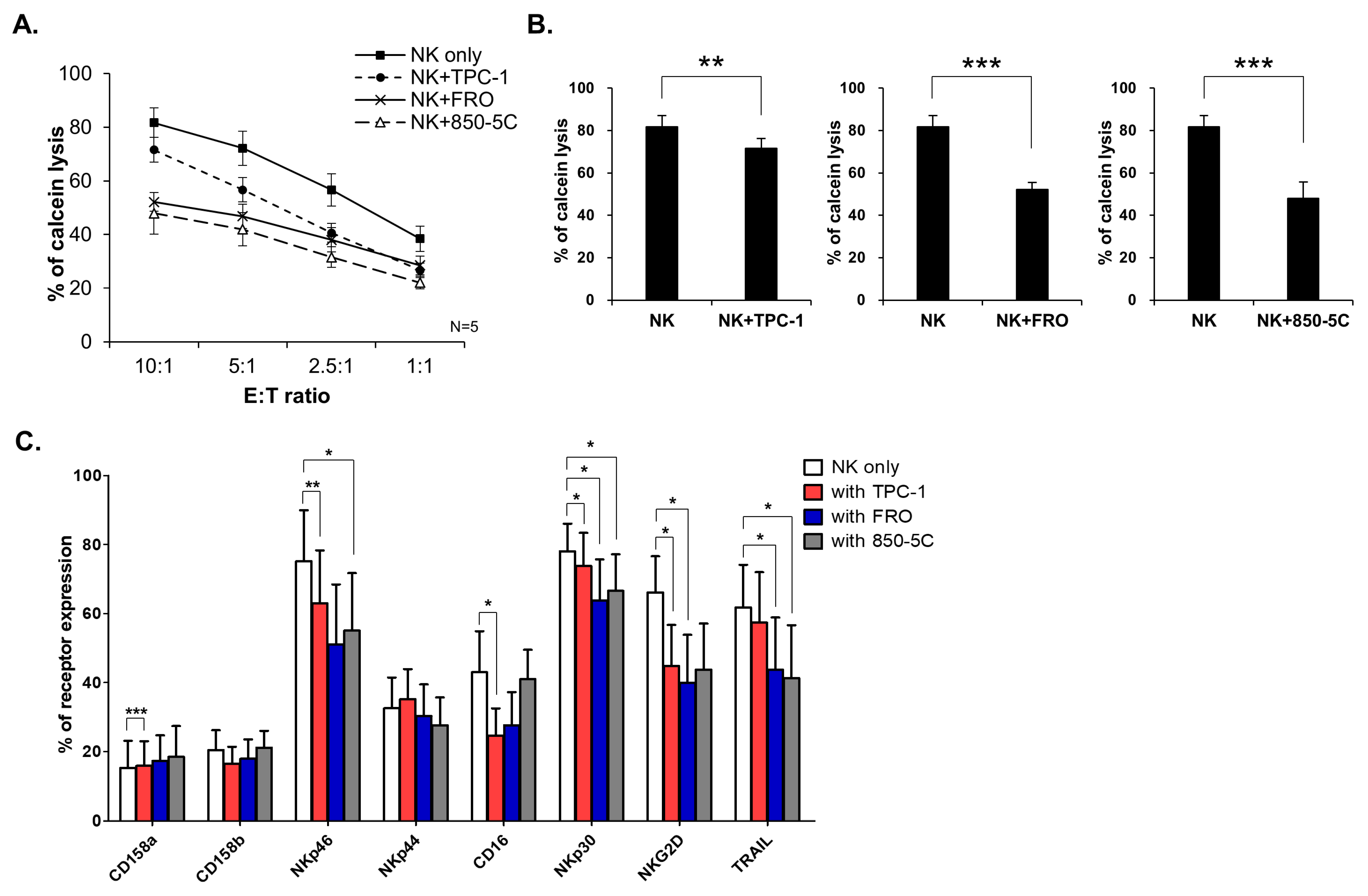
3.1. Thyroid Cancer Cells Inhibit NK Cell Cytolytic Function and NK Receptor Expression
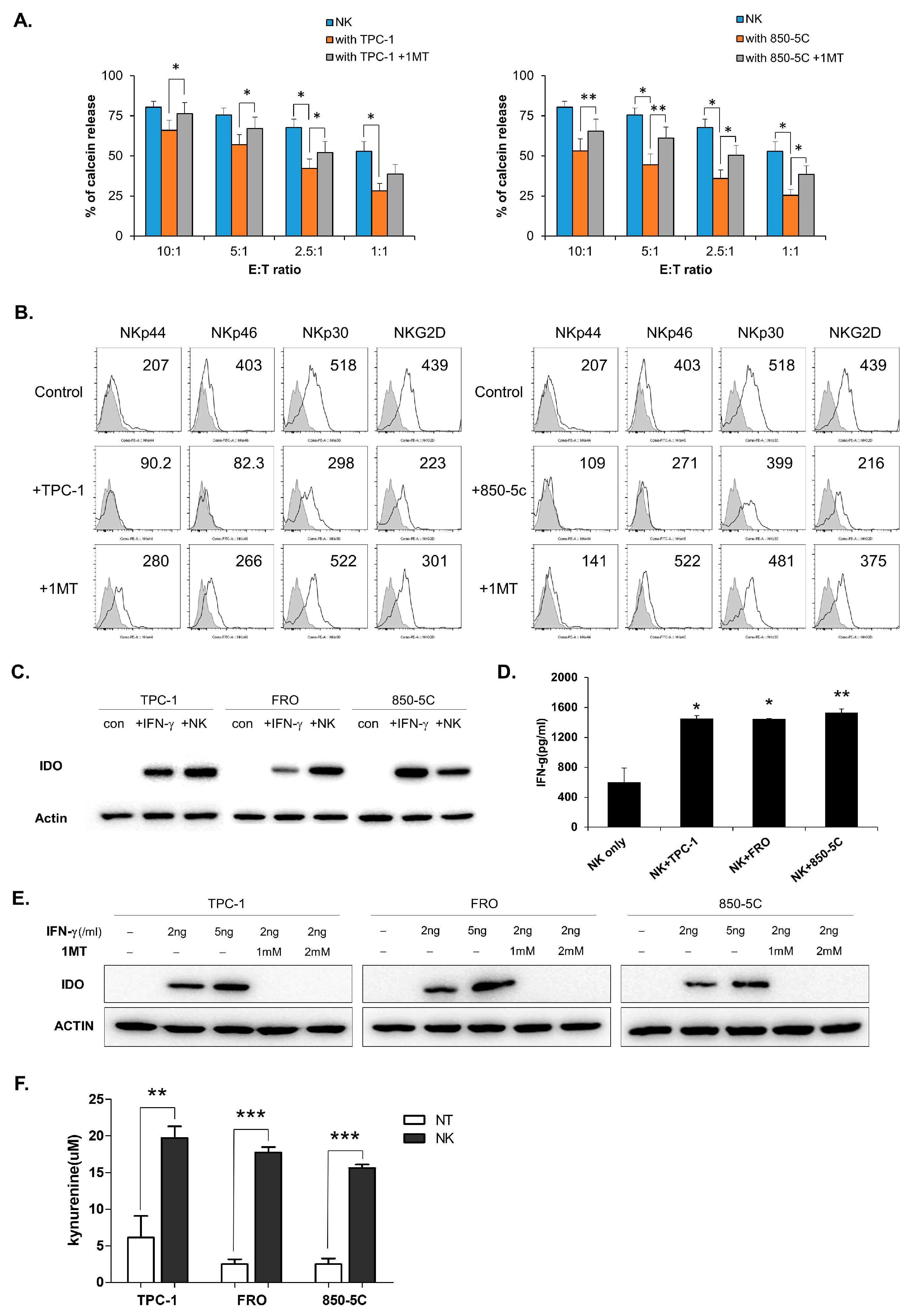
3.2. IDO Expression in Thyroid Cancer Cells is Induced by Co-Culture With NK Cells
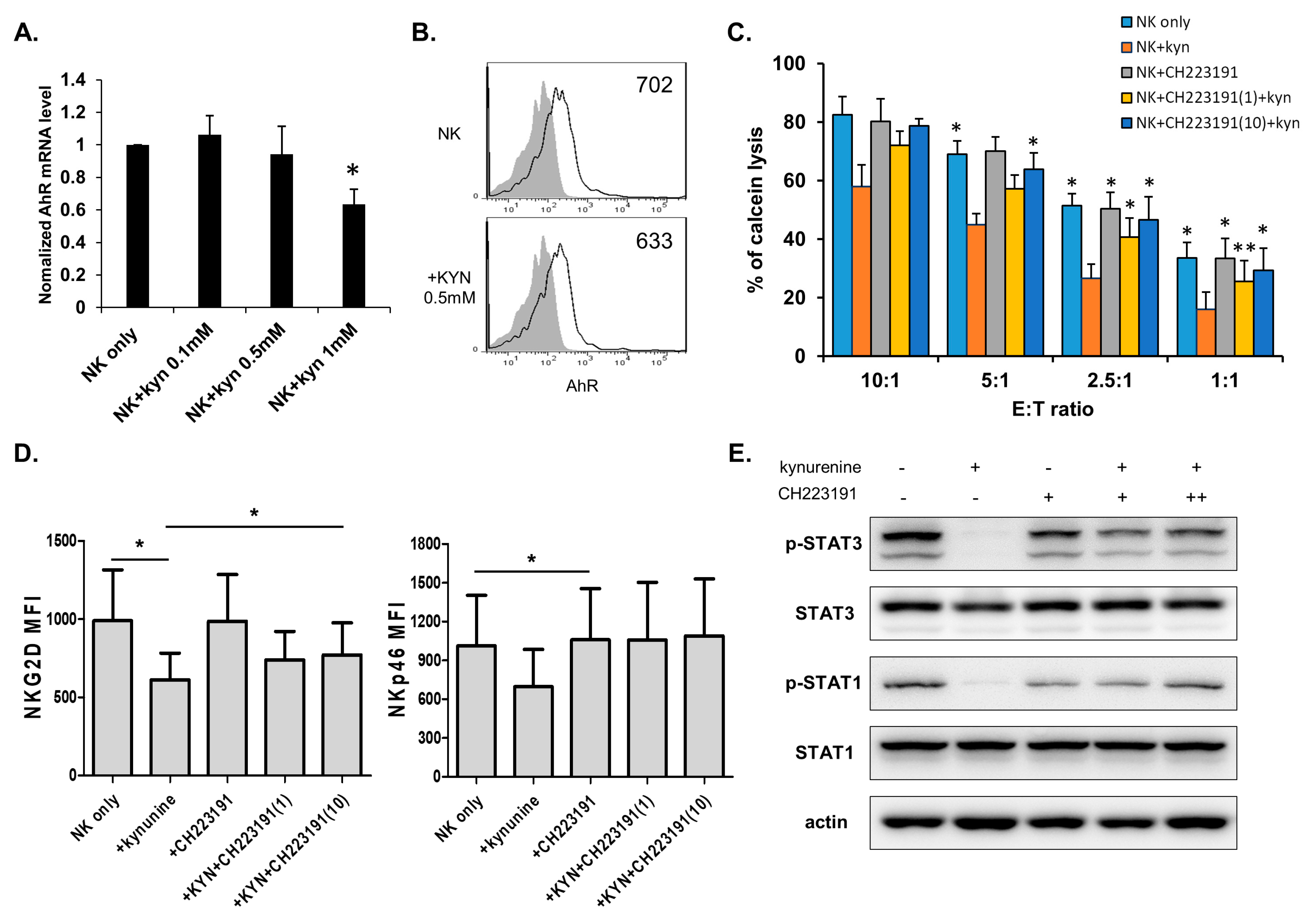
3.3. Kynurenine Inhibits NK Cell Activity via STAT Signaling Pathways.
3.4. Kynurenine Affects NK Cell Activity Through the Aryl Hydrocarbon Receptor (AhR)
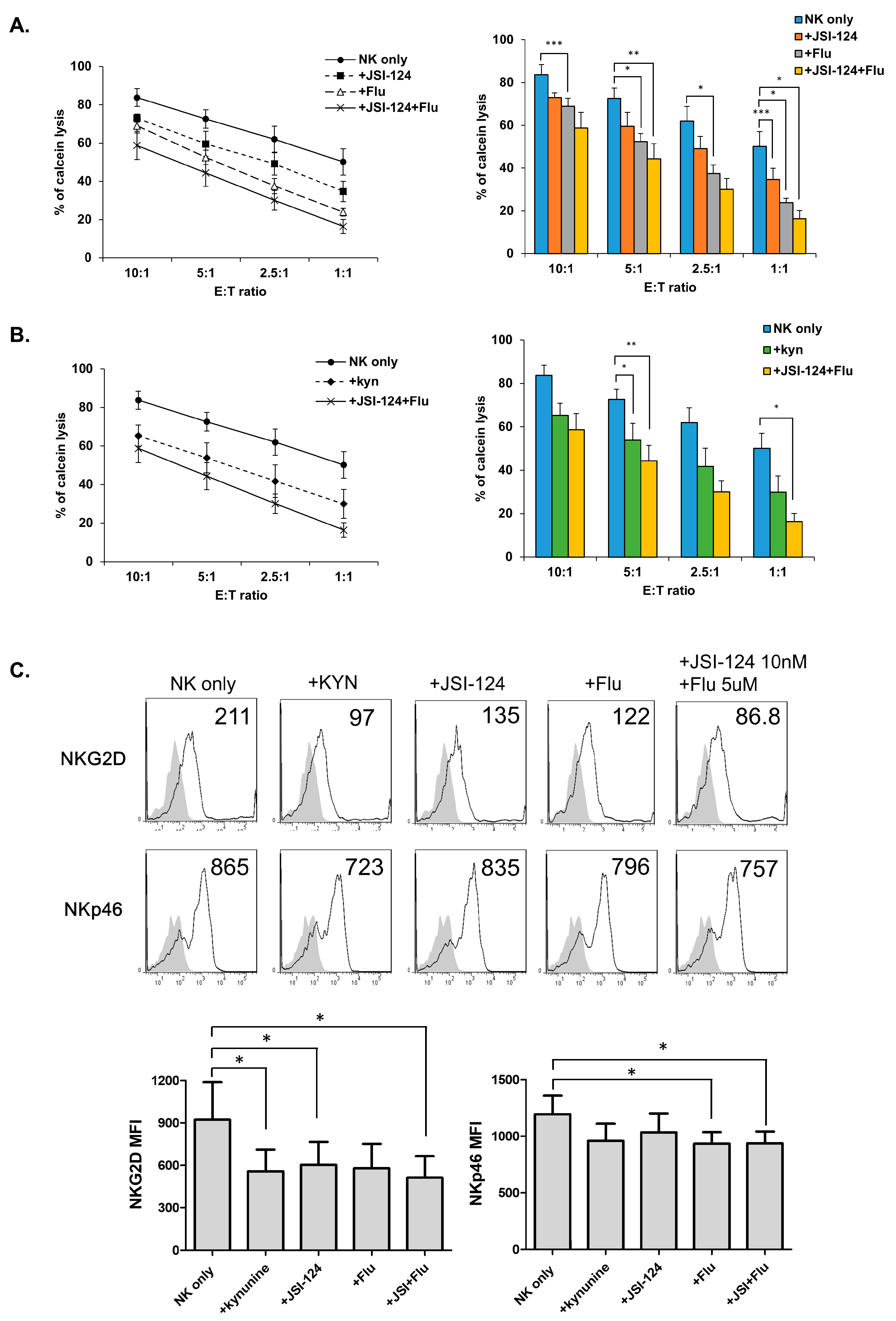
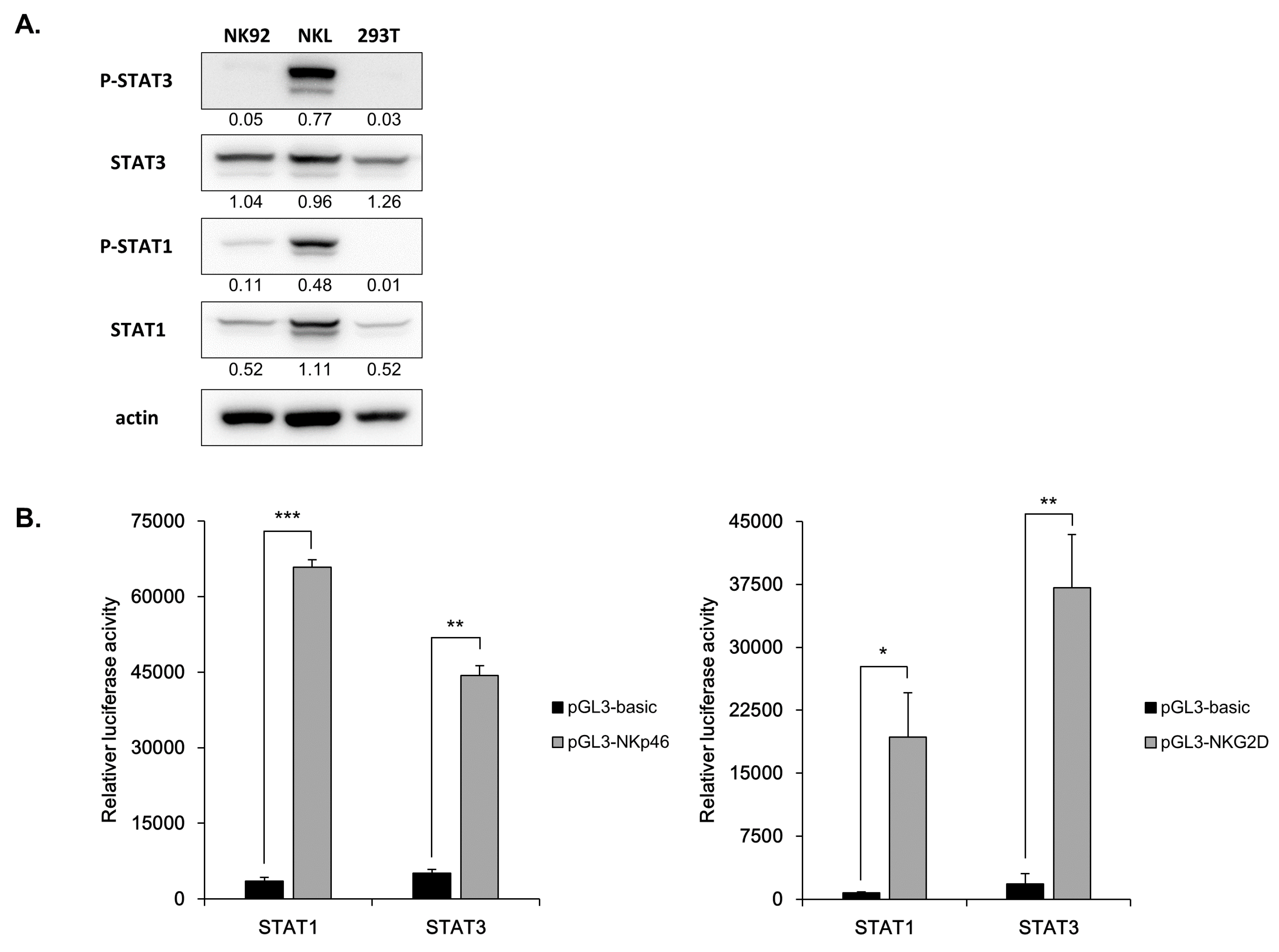
3.5. STATs Regulate NK Cytolytic Activity and Receptor Expression.
3.6. Direct Involvement of STATs in NK Receptor Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Golden-Mason, L.; Rosen, H.R. Natural killer cells: Multifaceted players with key roles in hepatitis c immunity. Immunol. Rev. 2013, 255, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.A. Molecular basis of human natural killer cell recognition of hla-e (human leucocyte antigen-e) and its relevance to clearance of pathogen-infected and tumour cells. Clin. Sci. (Lond.) 2000, 99, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Nk cell receptors. Annu. Rev. Immunol. 1998, 16, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Kruse, P.H.; Matta, J.; Ugolini, S.; Vivier, E. Natural cytotoxicity receptors and their ligands. Immunol. Cell Biol. 2014, 92, 221–229. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef]
- Balsamo, M.; Scordamaglia, F.; Pietra, G.; Manzini, C.; Cantoni, C.; Boitano, M.; Queirolo, P.; Vermi, W.; Facchetti, F.; Moretta, A.; et al. Melanoma-associated fibroblasts modulate nk cell phenotype and antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 20847–20852. [Google Scholar] [CrossRef]
- Ozaki, Y.; Edelstein, M.P.; Duch, D.S. Induction of indoleamine 2,3-dioxygenase: A mechanism of the antitumor activity of interferon gamma. Proc. Natl. Acad. Sci. USA 1988, 85, 1242–1246. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, Y.; Yin, Z.; Feng, S.; Sun, L.; Li, Z. Research progress of indoleamine 2,3-dioxygenase inhibitors. Future Med. Chem. 2015, 7, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.H.; Ma, Z.X.; Feltenberger, J.B.; Chen, H.; Chen, H.; Scarlett, C.; Lin, Z.; Satyshur, K.A.; Cortopassi, M.; Jefcoate, C.R.; et al. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (ahr). J. Biol. Chem. 2018, 293, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Naka, T.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor regulates stat1 activation and participates in the development of th17 cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Quintana, F.J.; Pot, C.; Joller, N.; Xiao, S.; Kumar, D.; Burns, E.J.; Sherr, D.H.; Weiner, H.L.; Kuchroo, V.K.; et al. The aryl hydrocarbon receptor interacts with c-maf to promote the differentiation of type 1 regulatory t cells induced by il-27. Nat. Immunol. 2010, 11, 854–861. [Google Scholar] [CrossRef]
- Masuda, K.; Kimura, A.; Hanieh, H.; Nguyen, N.T.; Nakahama, T.; Chinen, I.; Otoyo, Y.; Murotani, T.; Yamatodani, A.; Kishimoto, T.; et al. Aryl hydrocarbon receptor negatively regulates lps-induced il-6 production through suppression of histamine production in macrophages. Int. Immunol. 2011, 23, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Maekawa, Y.; Kataoka, K.; Ishifune, C.; Nishida, J.; Arimochi, H.; Kitamura, A.; Yoshimoto, T.; Tomita, S.; Nagahiro, S.; et al. The arnt-stat3 axis regulates the differentiation of intestinal intraepithelial tcralphabeta(+)cd8alphaalpha(+) cells. Nat. Commun. 2013, 4, 2112. [Google Scholar] [CrossRef]
- Litzenburger, U.M.; Opitz, C.A.; Sahm, F.; Rauschenbach, K.J.; Trump, S.; Winter, M.; Ott, M.; Ochs, K.; Lutz, C.; Liu, X.; et al. Constitutive ido expression in human cancer is sustained by an autocrine signaling loop involving il-6, stat3 and the ahr. Oncotarget 2014, 5, 1038–1051. [Google Scholar] [CrossRef]
- Imada, K.; Leonard, W.J. The jak-stat pathway. Mol. Immunol. 2000, 37, 1–11. [Google Scholar] [CrossRef]
- Kallal, L.E.; Biron, C.A. Changing partners at the dance: Variations in stat concentrations for shaping cytokine function and immune responses to viral infections. JAK-STAT 2013, 2, e23504. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Schachterle, W.; Oberle, K.; Aichele, P.; Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 2007, 26, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.; Putz, E.M.; Straka, E.; Kudweis, P.; Biaggio, M.; Poli, V.; Strobl, B.; Muller, M.; Sexl, V. Loss of stat3 in murine nk cells enhances nk cell-dependent tumor surveillance. Blood 2014, 124, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.; Sexl, V. Stats in nk-cells: The good, the bad, and the ugly. Front. Immunol. 2016, 7, 694. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Frank, D.A.; Ritz, J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and stat4. Blood 2000, 95, 3183–3190. [Google Scholar] [PubMed]
- Matsui, M.; Kishida, T.; Nakano, H.; Yoshimoto, K.; Shin-Ya, M.; Shimada, T.; Nakai, S.; Imanishi, J.; Yoshimoto, T.; Hisa, Y.; et al. Interleukin-27 activates natural killer cells and suppresses nk-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009, 69, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Vosshenrich, C.A.; Ranson, T.; Samson, S.I.; Corcuff, E.; Colucci, F.; Rosmaraki, E.E.; Di Santo, J.P. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of nk cell precursors and peripheral nk cells in vivo. J. Immunol. 2005, 174, 1213–1221. [Google Scholar] [CrossRef]
- Park, A.; Lee, Y.; Kim, M.S.; Kang, Y.J.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R.; et al. Prostaglandin e2 secreted by thyroid cancer cells contributes to immune escape through the suppression of natural killer (nk) cell cytotoxicity and nk cell differentiation. Front. Immunol. 2018, 9, 1859. [Google Scholar] [CrossRef] [PubMed]
- Somanchi, S.S.; Senyukov, V.V.; Denman, C.J.; Lee, D.A. Expansion, purification, and functional assessment of human peripheral blood nk cells. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Pietra, G.; Manzini, C.; Rivara, S.; Vitale, M.; Cantoni, C.; Petretto, A.; Balsamo, M.; Conte, R.; Benelli, R.; Minghelli, S.; et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012, 72, 1407–1415. [Google Scholar] [CrossRef]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A.; et al. Transforming growth factor beta 1 inhibits expression of nkp30 and nkg2d receptors: Consequences for the nk-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef]
- Krockenberger, M.; Dombrowski, Y.; Weidler, C.; Ossadnik, M.; Honig, A.; Hausler, S.; Voigt, H.; Becker, J.C.; Leng, L.; Steinle, A.; et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating nkg2d. J. Immunol. 2008, 180, 7338–7348. [Google Scholar] [CrossRef]
- Drake, C.G.; Jaffee, E.; Pardoll, D.M. Mechanisms of immune evasion by tumors. Adv. Immunol. 2006, 90, 51–81. [Google Scholar] [PubMed]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, C.R.; Hadding, U.; Daubener, W. Interferon-gamma-induced activation of indoleamine 2,3-dioxygenase in cord blood monocyte-derived macrophages inhibits the growth of group b streptococci. J. Infect. Dis. 1998, 178, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Goto, S.; Tahara, K.; Sasaki, A.; Kawano, K.; Kitano, S. Inhibition of indoleamine 2,3-dioxygenase suppresses nk cell activity and accelerates tumor growth. J. Exp. Ther. Oncol. 2003, 3, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of t and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Terness, P.; Bauer, T.M.; Rose, L.; Dufter, C.; Watzlik, A.; Simon, H.; Opelz, G. Inhibition of allogeneic t cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: Mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002, 196, 447–457. [Google Scholar] [CrossRef]
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The kynurenine pathway is a double-edged sword in immune-privileged sites and in cancer: Implications for immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef]
- Platten, M.; von Knebel Doeberitz, N.; Oezen, I.; Wick, W.; Ochs, K. Cancer immunotherapy by targeting ido1/tdo and their downstream effectors. Front. Immunol. 2014, 5, 673. [Google Scholar] [CrossRef]
- Dagenais-Lussier, X.; Aounallah, M.; Mehraj, V.; El-Far, M.; Tremblay, C.; Sekaly, R.P.; Routy, J.P.; van Grevenynghe, J. Kynurenine reduces memory cd4 t-cell survival by interfering with interleukin-2 signaling early during hiv-1 infection. J. Virol. 2016, 90, 7967–7979. [Google Scholar] [CrossRef]
- Takaki, R.; Hayakawa, Y.; Nelson, A.; Sivakumar, P.V.; Hughes, S.; Smyth, M.J.; Lanier, L.L. Il-21 enhances tumor rejection through a nkg2d-dependent mechanism. J. Immunol. 2005, 175, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, Y.L.; Hoey, T. Cooperative DNA binding and sequence-selective recognition conferred by the stat amino-terminal domain. Science 1996, 273, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Stange, J.; Veldhoen, M. The aryl hydrocarbon receptor in innate t cell immunity. Semin. Immunopathol. 2013, 35, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Fu, J.; Zhou, Y. The aryl hydrocarbon receptor and tumor immunity. Front. Immunol. 2018, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A.; Stockinger, B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009, 30, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Henry, E.C.; Kim, D.K.; Kim, Y.H.; Shin, K.J.; Han, M.S.; Lee, T.G.; Kang, J.K.; Gasiewicz, T.A.; Ryu, S.H.; et al. Novel compound 2-methyl-2h-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (ch-223191) prevents 2,3,7,8-tcdd-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol. Pharmacol. 2006, 69, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hirota, K.; Christensen, J.; O’Garra, A.; Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of th17 t cells. J. Exp. Med. 2009, 206, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bedel, R.; Thiery-Vuillemin, A.; Grandclement, C.; Balland, J.; Remy-Martin, J.P.; Kantelip, B.; Pallandre, J.R.; Pivot, X.; Ferrand, C.; Tiberghien, P.; et al. Novel role for stat3 in transcriptional regulation of nk immune cell targeting receptor mica on cancer cells. Cancer Res. 2011, 71, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Cacalano, N.A. Regulation of natural killer cell function by stat3. Front. Immunol. 2016, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr. Stats and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef]
- Schindler, C.; Darnell, J.E., Jr. Transcriptional responses to polypeptide ligands: The jak-stat pathway. Annu. Rev. Biochem. 1995, 64, 621–651. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G.B.; Reichenbach, P.; Schindler, U.; Horvath, C.M.; Fritz, S.; Nabholz, M.; Bucher, P. DNA binding specificity of different stat proteins. Comparison of in vitro specificity with natural target sites. J. Biol. Chem. 2001, 276, 6675–6688. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, A.; Yang, Y.; Lee, Y.; Kim, M.S.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. Indoleamine-2,3-Dioxygenase in Thyroid Cancer Cells Suppresses Natural Killer Cell Function by Inhibiting NKG2D and NKp46 Expression via STAT Signaling Pathways. J. Clin. Med. 2019, 8, 842. https://doi.org/10.3390/jcm8060842
Park A, Yang Y, Lee Y, Kim MS, Park Y-J, Jung H, Kim T-D, Lee HG, Choi I, Yoon SR. Indoleamine-2,3-Dioxygenase in Thyroid Cancer Cells Suppresses Natural Killer Cell Function by Inhibiting NKG2D and NKp46 Expression via STAT Signaling Pathways. Journal of Clinical Medicine. 2019; 8(6):842. https://doi.org/10.3390/jcm8060842
Chicago/Turabian StylePark, Arum, Yunjeong Yang, Yunhee Lee, Mi Sun Kim, Young-Jun Park, Haiyoung Jung, Tae-Don Kim, Hee Gu Lee, Inpyo Choi, and Suk Ran Yoon. 2019. "Indoleamine-2,3-Dioxygenase in Thyroid Cancer Cells Suppresses Natural Killer Cell Function by Inhibiting NKG2D and NKp46 Expression via STAT Signaling Pathways" Journal of Clinical Medicine 8, no. 6: 842. https://doi.org/10.3390/jcm8060842
APA StylePark, A., Yang, Y., Lee, Y., Kim, M. S., Park, Y.-J., Jung, H., Kim, T.-D., Lee, H. G., Choi, I., & Yoon, S. R. (2019). Indoleamine-2,3-Dioxygenase in Thyroid Cancer Cells Suppresses Natural Killer Cell Function by Inhibiting NKG2D and NKp46 Expression via STAT Signaling Pathways. Journal of Clinical Medicine, 8(6), 842. https://doi.org/10.3390/jcm8060842







