Effect of Statin on Cancer Incidence: An Umbrella Systematic Review and Meta-Analysis
Abstract
:1. Introduction
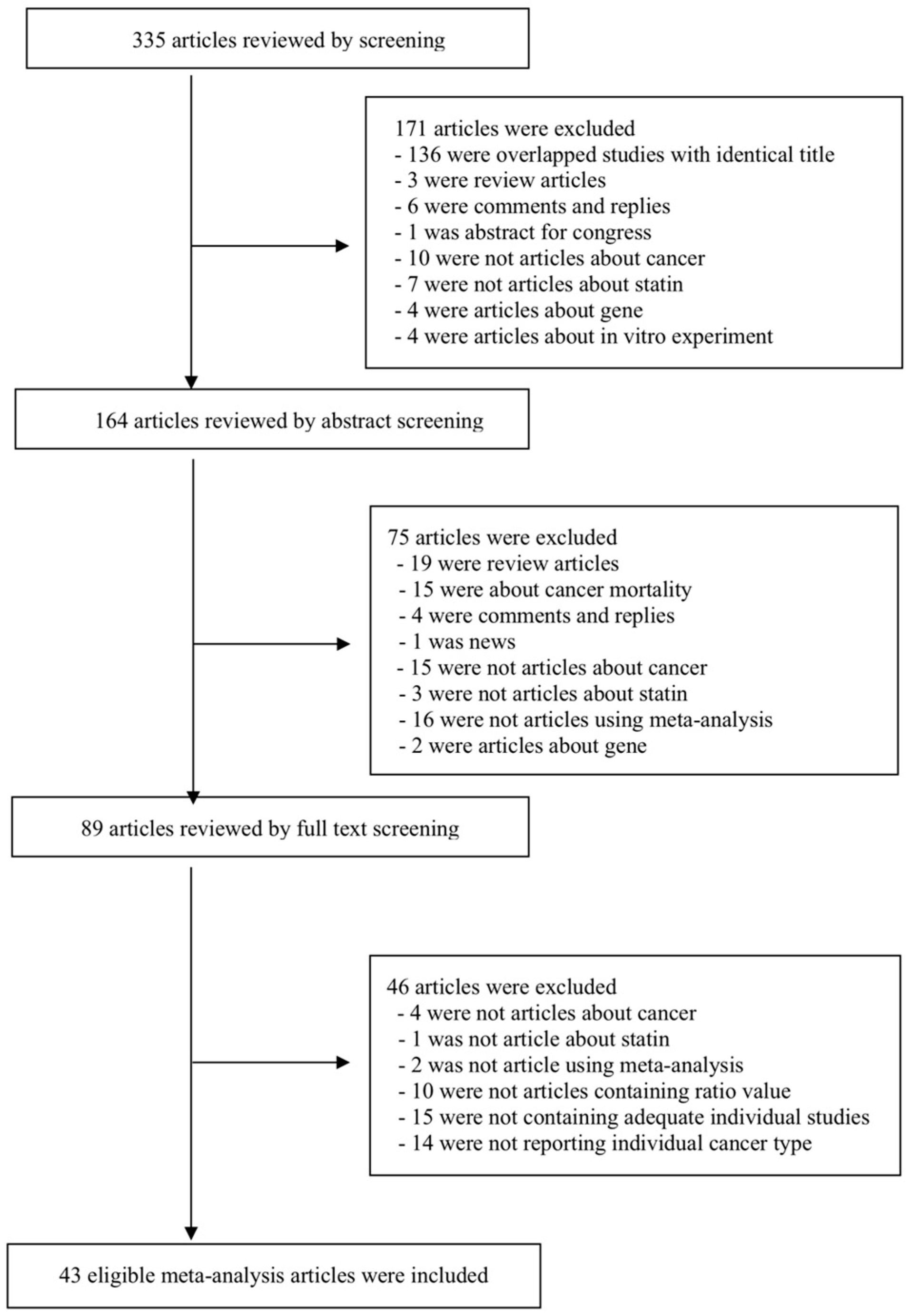
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria and Data Extraction
2.3. Statistical Analysis
2.4. Estimation of Summary Effects and Estimation of Prediction Interval
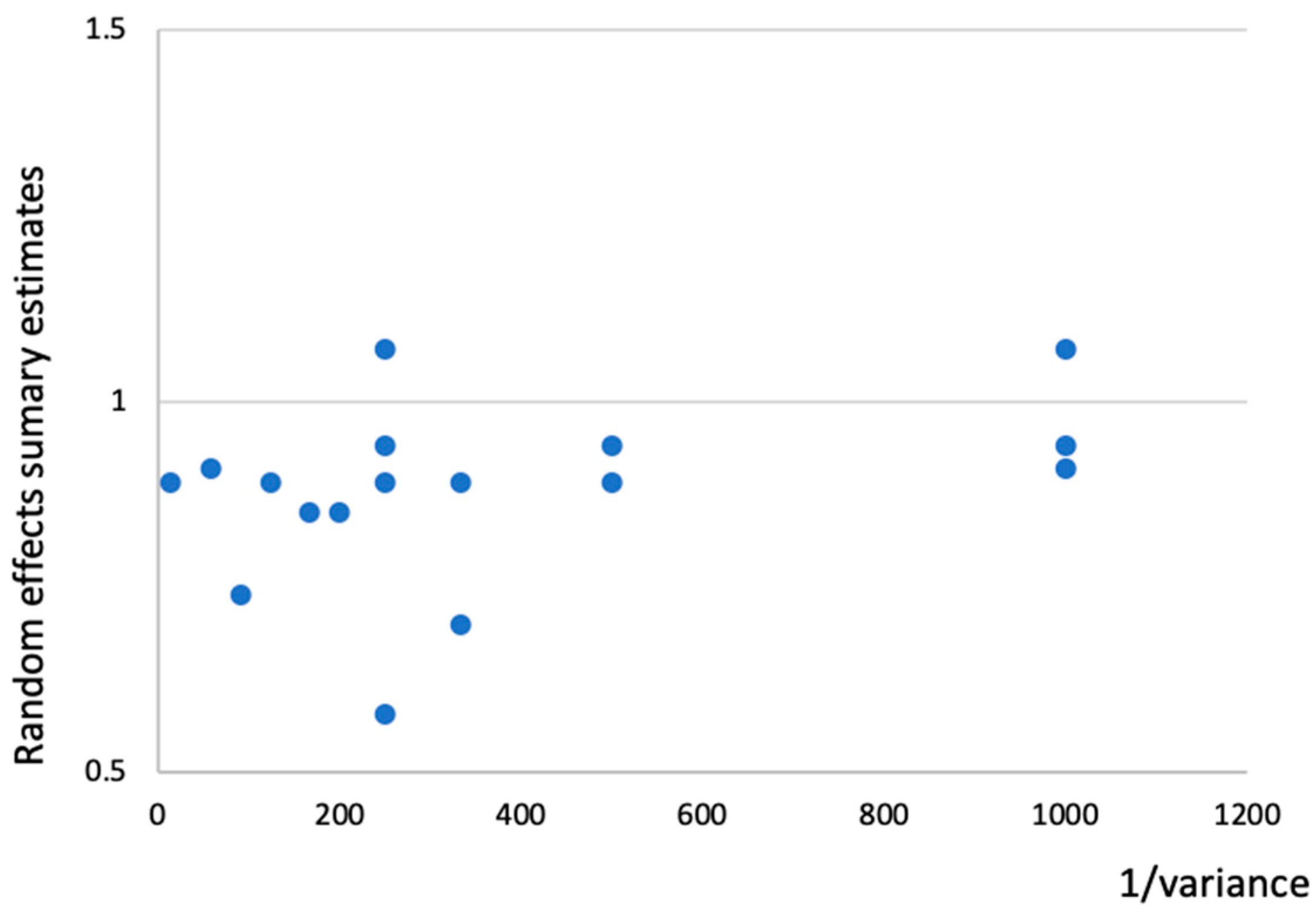
2.5. Evaluation of Between-Study Heterogeneity and Small Study Effects
2.6. Determination of the Level of Evidence
3. Results
3.1. Characteristics of Studies Included in the Final Analyses
3.2. Assessing the Effect of Statin on Cancer Incidence with Conventional Interpretation of Meta-Analyses Criteria (Random Effects p-Value < 0.05)
3.3. Assessing the Statin Effect on Cancer Incidence with Criteria by Previous Umbrella Review
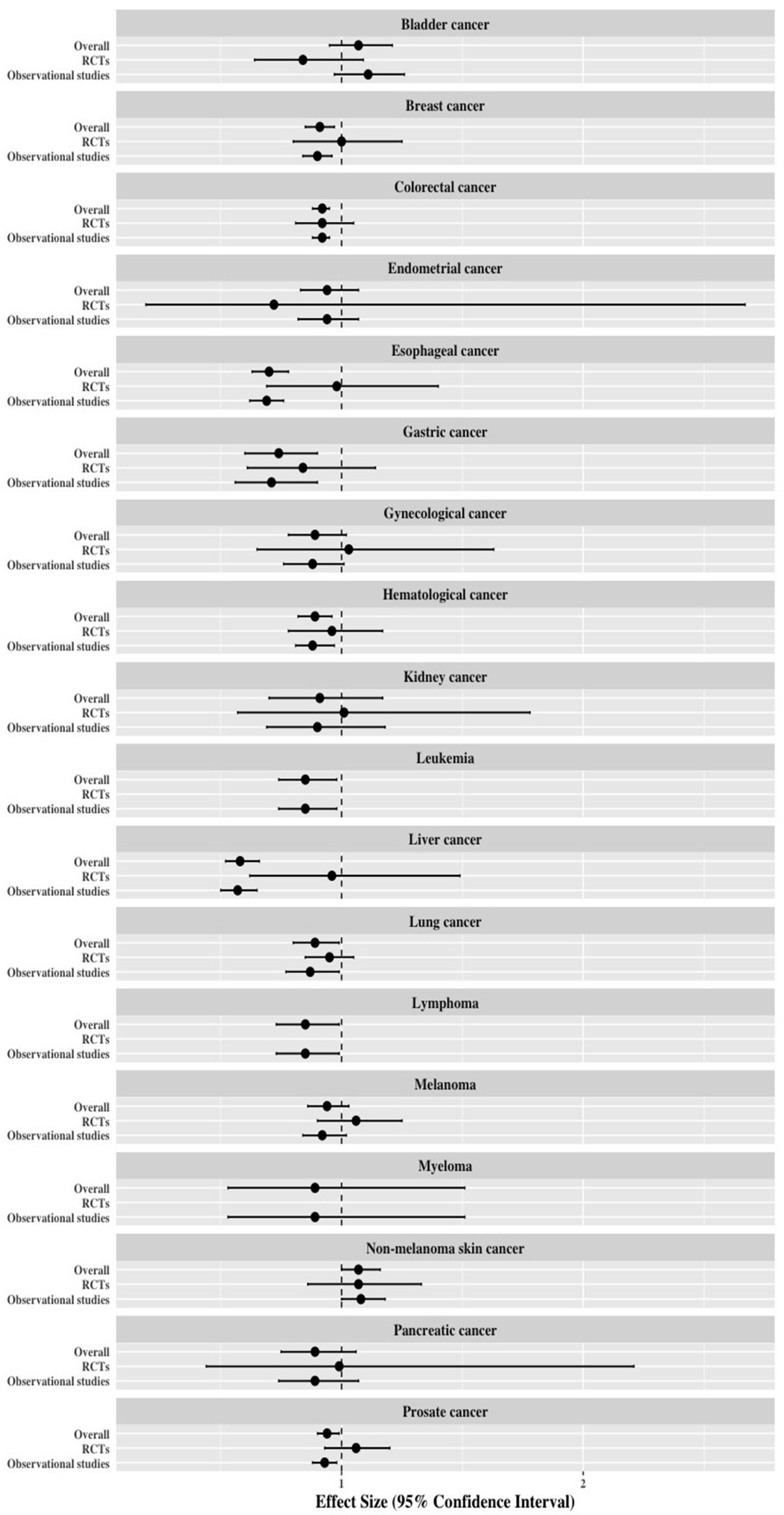
3.4. Re-Analysis of Meta-Analyses Separated by Study Design
4. Discussion
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.R.; Gaziano, J.M.; Chan, K.S.; Hennekens, C.H. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 1997, 278, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Crowe, T.; Sasiela, W.J.; Tsai, J.; Orazem, J.; Magorien, R.D.; O’Shaughnessy, C.; Ganz, P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 2005, 352, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Steinman, L.; Zamvil, S.S. Statin therapy and autoimmune disease: From protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006, 6, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.A.; Ndrepepa, G.; Schulz, S.; Neumann, F.J.; Mehilli, J.; Buttner, H.J.; Pache, J.; Seyfarth, M.; Dirschinger, J.; Kastrati, A.; et al. Statin effect on thrombin inhibitor effectiveness during percutaneous coronary intervention: A post-hoc analysis from the ISAR-REACT 3 trial. Clin. Res. Cardiol. 2011, 100, 579–585. [Google Scholar] [CrossRef]
- Jakobisiak, M.; Golab, J. Potential antitumor effects of statins (Review). Int. J. Oncol. 2003, 23, 1055–1069. [Google Scholar] [CrossRef]
- Zeichner, S.; Mihos, C.G.; Santana, O. The pleiotropic effects and therapeutic potential of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in malignancies: A comprehensive review. J. Cancer Res. Ther. 2012, 8, 176–183. [Google Scholar] [CrossRef]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Yokomichi, H.; Nagai, A.; Hirata, M.; Tamakoshi, A.; Kiyohara, Y.; Kamatani, Y.; Muto, K.; Ninomiya, T.; Matsuda, K.; Kubo, M.; et al. Statin use and all-cause and cancer mortality: BioBank Japan cohort. J. Epidemiol. 2017, 27, S84–S91. [Google Scholar] [CrossRef] [PubMed]
- Dale, K.M.; Coleman, C.I.; Henyan, N.N.; Kluger, J.; White, C.M. Statins and cancer risk: A meta-analysis. JAMA 2006, 295, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Berrier, J.; Reitman, D.; Ancona-Berk, V.A.; Chalmers, T.C. Meta-analyses of randomized controlled trials. N. Engl. J. Med. 1987, 316, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J. What is heterogeneity and is it important? BMJ 2007, 334, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef]
- Choi, E.K.; Park, H.B.; Lee, K.H.; Park, J.H.; Eisenhut, M.; van der Vliet, H.J.; Kim, G.; Shin, J.I. Body mass index and 20 specific cancers: Re-analyses of dose-response meta-analyses of observational studies. Ann. Oncol. 2018, 29, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Jian-Yu, E.; Graber, J.M.; Lu, S.E.; Lin, Y.; Lu-Yao, G.; Tan, X.L. Effect of Metformin and Statin Use on Survival in Pancreatic Cancer Patients: A Systematic Literature Review and Meta-analysis. Curr. Med. Chem. 2018, 25, 2595–2607. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Mneina, A.; Johnston, J.B.; Mahmud, S.M. Associations between statin use and non-Hodgkin lymphoma (NHL) risk and survival: A meta-analysis. Hematol. Oncol. 2017, 35, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Marley, A.; Tang, H.; Song, Y.; Tang, J.Y.; Han, J. Statin use and non-melanoma skin cancer risk: A meta-analysis of randomized controlled trials and observational studies. Oncotarget 2017, 8, 75411–75417. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, Q.; Liu, Q.; Wang, Y.; Xie, W.; Hu, L. Statin use and endometrial cancer risk: A meta-analysis. Oncotarget 2017, 8, 62425–62434. [Google Scholar] [CrossRef]
- Thomas, T.; Loke, Y.; Beales, I.L.P. Systematic Review and Meta-analysis: Use of Statins Is Associated with a Reduced Incidence of Oesophageal Adenocarcinoma. J. Gastrointest. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Yang, H.C.; Nguyen, P.A.; Poly, T.N.; Huang, C.W.; Kekade, S.; Khalfan, A.M.; Debnath, T.; Li, Y.J.; Abdul, S.S. Exploring association between statin use and breast cancer risk: An updated meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 1043–1053. [Google Scholar] [CrossRef]
- Zhong, G.C.; Liu, Y.; Ye, Y.Y.; Hao, F.B.; Wang, K.; Gong, J.P. Meta-analysis of studies using statins as a reducer for primary liver cancer risk. Sci. Rep. 2016, 6, 26256. [Google Scholar] [CrossRef] [Green Version]
- Raval, A.D.; Thakker, D.; Negi, H.; Vyas, A.; Kaur, H.; Salkini, M.W. Association between statins and clinical outcomes among men with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2016, 19, 151–162. [Google Scholar] [CrossRef]
- Mansourian, M.; Haghjooy-Javanmard, S.; Eshraghi, A.; Vaseghi, G.; Hayatshahi, A.; Thomas, J. Statins Use and Risk of Breast Cancer Recurrence and Death: A Systematic Review and Meta-Analysis of Observational Studies. J. Pharm. Pharm. Sci. 2016, 19, 72–81. [Google Scholar] [CrossRef]
- Pradelli, D.; Soranna, D.; Zambon, A.; Catapano, A.; Mancia, G.; La Vecchia, C.; Corrao, G. Statins use and the risk of all and subtype hematological malignancies: A meta-analysis of observational studies. Cancer Med. 2015, 4, 770–780. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, M.; Qian, J.; Zheng, J.H.; Zhang, X.P.; Guo, C.C.; Geng, J.; Peng, B.; Che, J.P.; Wu, Y. Statin use and risk of kidney cancer: A meta-analysis of observational studies and randomized trials. Br. J. Clin. Pharmacol. 2014, 77, 458–465. [Google Scholar] [CrossRef]
- Yi, X.; Jia, W.; Jin, Y.; Zhen, S. Statin use is associated with reduced risk of haematological malignancies: Evidence from a meta-analysis. PLoS ONE 2014, 9, e87019. [Google Scholar] [CrossRef]
- Shi, M.; Zheng, H.; Nie, B.; Gong, W.; Cui, X. Statin use and risk of liver cancer: An update meta-analysis. BMJ Open 2014, 4, e005399. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, W.; Jin, G.; Chu, P.; Li, H. Effect of statins on gastric cancer incidence: A meta-analysis of case control studies. J. Cancer Res. Ther. 2014, 10, 859–865. [Google Scholar] [CrossRef]
- Lytras, T.; Nikolopoulos, G.; Bonovas, S. Statins and the risk of colorectal cancer: An updated systematic review and meta-analysis of 40 studies. World J. Gastroenterol. 2014, 20, 1858–1870. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, W.; Wang, J.; Xie, L.; Li, T.; He, Y.; Deng, Y.; Peng, Q.; Li, S.; Qin, X. Association between statin use and colorectal cancer risk: A meta-analysis of 42 studies. Cancer Causes Control 2014, 25, 237–249. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, A.; Li, T.; Qin, X.; Li, S. Effect of statin on risk of gynecologic cancers: A meta-analysis of observational studies and randomized controlled trials. Gynecol. Oncol. 2014, 133, 647–655. [Google Scholar] [CrossRef]
- Zhang, X.L.; Geng, J.; Zhang, X.P.; Peng, B.; Che, J.P.; Yan, Y.; Wang, G.C.; Xia, S.Q.; Wu, Y.; Zheng, J.H. Statin use and risk of bladder cancer: A meta-analysis. Cancer Causes Control 2013, 24, 769–776. [Google Scholar] [CrossRef]
- Wu, X.D.; Zeng, K.; Xue, F.Q.; Chen, J.H.; Chen, Y.Q. Statins are associated with reduced risk of gastric cancer: A meta-analysis. Eur. J. Clin. Pharmacol. 2013, 69, 1855–1860. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Tao, H.; Cheng, Y.; Han, L.; Li, X.; Hu, Y. Statin use and risk of lung cancer: A meta-analysis of observational studies and randomized controlled trials. PLoS ONE 2013, 8, e77950. [Google Scholar] [CrossRef]
- Tan, M.; Song, X.; Zhang, G.; Peng, A.; Li, X.; Li, M.; Liu, Y.; Wang, C. Statins and the risk of lung cancer: A meta-analysis. PLoS ONE 2013, 8, e57349. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.G.; Singh, P.P.; Murad, M.H.; Iyer, P.G. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 620–629. [Google Scholar] [CrossRef]
- Singh, P.P.; Singh, S. Statins are associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1721–1730. [Google Scholar] [CrossRef]
- Scosyrev, E.; Tobis, S.; Donsky, H.; Wu, G.; Joseph, J.; Rashid, H.; Messing, E. Statin use and the risk of biochemical recurrence of prostate cancer after definitive local therapy: A meta-analysis of eight cohort studies. BJU Int. 2013, 111, E71–E77. [Google Scholar] [CrossRef]
- Pradelli, D.; Soranna, D.; Scotti, L.; Zambon, A.; Catapano, A.; Mancia, G.; La Vecchia, C.; Corrao, G. Statins and primary liver cancer: A meta-analysis of observational studies. Eur. J. Cancer Prev. 2013, 22, 229–234. [Google Scholar] [CrossRef]
- Beales, I.L.; Hensley, A.; Loke, Y. Reduced esophageal cancer incidence in statin users, particularly with cyclo-oxygenase inhibition. World J. Gastrointest. Pharmacol. Ther. 2013, 4, 69–79. [Google Scholar] [CrossRef]
- Undela, K.; Srikanth, V.; Bansal, D. Statin use and risk of breast cancer: A meta-analysis of observational studies. Breast Cancer Res. Treat. 2012, 135, 261–269. [Google Scholar] [CrossRef]
- Mass, A.Y.; Agalliu, I.; Laze, J.; Lepor, H. Preoperative statin therapy is not associated with biochemical recurrence after radical prostatectomy: Our experience and meta-analysis. J. Urol. 2012, 188, 786–791. [Google Scholar] [CrossRef]
- Cui, X.; Xie, Y.; Chen, M.; Li, J.; Liao, X.; Shen, J.; Shi, M.; Li, W.; Zheng, H.; Jiang, B. Statin use and risk of pancreatic cancer: A meta-analysis. Cancer Causes Control 2012, 23, 1099–1111. [Google Scholar] [CrossRef]
- Bansal, D.; Undela, K.; D’Cruz, S.; Schifano, F. Statin use and risk of prostate cancer: A meta-analysis of observational studies. PLoS ONE 2012, 7, e46691. [Google Scholar] [CrossRef]
- Alexandre, L.; Clark, A.B.; Cheong, E.; Lewis, M.P.; Hart, A.R. Systematic review: Potential preventive effects of statins against oesophageal adenocarcinoma. Aliment. Pharmacol. Ther. 2012, 36, 301–311. [Google Scholar] [CrossRef]
- Bonovas, S.; Nikolopoulos, G.; Filioussi, K.; Peponi, E.; Bagos, P.; Sitaras, N.M. Can statin therapy reduce the risk of melanoma? A meta-analysis of randomized controlled trials. Eur. J. Epidemiol. 2010, 25, 29–35. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.; Martel, M. Effect of statin therapy on colorectal cancer. Gut 2010, 59, 1572–1585. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.L.; Wells, B.J.; Smolak, M.J. Statins and cancer: A meta-analysis of case-control studies. Eur. J. Cancer Prev. 2008, 17, 259–268. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Sitaras, N.M. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int. J. Cancer 2008, 123, 899–904. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Sitaras, N.M. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: Evidence from a meta-analysis of 12 studies. Am. J. Gastroenterol. 2008, 103, 2646–2651. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Tsantes, A.; Sitaras, N.M. Use of statins and risk of haematological malignancies: A meta-analysis of six randomized clinical trials and eight observational studies. Br. J. Clin. Pharmacol. 2007, 64, 255–262. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Flordellis, C.S.; Sitaras, N.M. Statins and the risk of colorectal cancer: A meta-analysis of 18 studies involving more than 1.5 million patients. J. Clin. Oncol. 2007, 25, 3462–3468. [Google Scholar] [CrossRef]
- Freeman, S.R.; Drake, A.L.; Heilig, L.F.; Graber, M.; McNealy, K.; Schilling, L.M.; Dellavalle, R.P. Statins, fibrates, and melanoma risk: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2006, 98, 1538–1546. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Tsavaris, N.; Sitaras, N.M. Statins and cancer risk: A literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J. Clin. Oncol. 2006, 24, 4808–4817. [Google Scholar] [CrossRef]
- Dellavalle, R.P.; Drake, A.; Graber, M.; Heilig, L.F.; Hester, E.J.; Johnson, K.R.; McNealy, K.; Schilling, L. Statins and fibrates for preventing melanoma. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Tsavaris, N.; Sitaras, N.M. Use of statins and breast cancer: A meta-analysis of seven randomized clinical trials and nine observational studies. J. Clin. Oncol. 2005, 23, 8606–8612. [Google Scholar] [CrossRef]
- Berman, N.G.; Parker, R.A. Meta-analysis: Neither quick nor easy. BMC Med. Res. Methodol. 2002, 2, 10. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Gasevic, D.; Brunt, E.; McLachlan, F.; Millenson, M.; Timofeeva, M.; Ioannidis, J.P.A.; Campbell, H.; Theodoratou, E. Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann. Intern. Med. 2018, 169, 543–553. [Google Scholar] [CrossRef]
- Markozannes, G.; Tzoulaki, I.; Karli, D.; Evangelou, E.; Ntzani, E.; Gunter, M.J.; Norat, T.; Ioannidis, J.P.; Tsilidis, K.K. Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence. Eur. J. Cancer 2016, 69, 61–69. [Google Scholar] [CrossRef]
- Schroll, J.B.; Moustgaard, R.; Gotzsche, P.C. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med. Res. Methodol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.; Tzoulaki, I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015, 14, 263–273. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Siontis, K.C.; Hernandez-Boussard, T.; Ioannidis, J.P. Overlapping meta-analyses on the same topic: Survey of published studies. BMJ 2013, 347, f4501. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009, 181, 488–493. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005, 294, 218–228. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Why most discovered true associations are inflated. Epidemiology 2008, 19, 640–648. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
| Cancer Type | No of Studies | No of Total Participants | Random Effects (RR, 95%CI) | P (Random) | Fixed Effects (RR, 95%CI) | P (Fixed) | Largest Effect§ (RR, 95%CI) | D/N/I | Egger | I2 (P) † | 95% PI (Random Effects) | 95% PI (Fixed Effects) | Small Study Effects | Concordant Direction | Evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bladder cancer | 13 | 1,266,218 | 1.07 (0.95–1.21) | 0.282 | 1.12 (1.07–1.19) | <0.001 | 1.08 (0.99–1.19) | 0/11/2 | 0.851 | 62.6 (0.001) | 0.76–1.51 | 0.81–1.56 | No | Yes | Non-significant |
| Breast cancer | 62 | 3,884,629 | 0.91 (0.85–0.97) | 0.004 | 1.00 (0.97–1.02) | 0.724 | 1.04 (0.98–1.11) | 12/44/3 | 0.023 | 79.6 (<0.001) | 0.63–1.32 | 0.69–1.44 | Yes | No | Weak |
| Colorectal cancer | 59 | 13,855,147 | 0.92 (0.88–0.95) | <0.001 | 0.94 (0.93–0.96) | <0.001 | 0.88 (0.81–0.95) | 15/33/3 | 0.106 | 71.5 (<0.001) | 0.76–1.11 | 0.78–1.14 | No | Yes | Weak |
| Endometrial cancer | 15 | 878,885 | 0.94 (0.82–1.07) | 0.349 | 1.02 (0.97–1.08) | 0.423 | 1.05 (0.95–1.15) | 4/11/0 | 0.043 | 54.9 (<0.001) | 0.66–1.34 | 0.73–1.43 | Yes | Yes | Non-significant |
| Esophageal cancer | 27 | 3,158,414 | 0.70 (0.63–0.78) | <0.001 | 0.85 (0.71–0.89) | <0.001 | 0.68 (0.52–0.88) | 15/12/0 | 0.115 | 60.7 (<0.001) | 0.46–1.05 | 0.50–1.11 | No | Yes | Suggestive * |
| Gastric cancer | 16 | 5,396,224 | 0.74 (0.60–0.90) | 0.004 | 0.84 (0.79–0.88) | <0.001 | 0.97 (0.74–1.26) | 5/11/0 | 0.325 | 90.8 (<0.001) | 0.33–1.62 | 0.39–1.78 | No | No | Weak |
| Gynecological cancer | 23 | 928,721 | 0.89 (0.78–1.02) | 0.087 | 1.00 (0.93–1.06) | 0.899 | 1.05 (0.95–1.15) | 4/19/0 | 0.003 | 43.7 (0.014) | 0.62–1.29 | 0.70–1.41 | Yes | Yes | Non-significant |
| Hematological cancer | 34 | NA | 0.89 (0.82–0.96) | 0.005 | 0.86 (0.81–0.90) | <0.001 | NA ** | 7/26/1 | 0.161 | 46.7 (0.002) | 0.60–1.20 | 0.64–1.15 | No | - | Suggestive |
| Kidney cancer | 11 | 4,052,120 | 0.91 (0.70–1.17) | 0.457 | 0.94 (0.88–1.00) | 0.034 | 1.08 (0.99–1.18) | 2/9/0 | 0.722 | 88.7 (<0.001) | 0.39–2.09 | 0.43–2.05 | No | Yes | Non-significant |
| Leukemia | 9 | 1174 | 0.85 (0.74–0.98) | 0.031 | 0.83 (0.74–0.92) | 0.001 | 0.74 (0.62–0.87) | 2/7/0 | 0.120 | 25.0 (0.220) | 0.63–1.16 | 0.62–1.10 | No | Yes | Suggestive |
| Liver cancer | 27 | 2,622,626 | 0.58 (0.52–0.66) | <0.001 | 0.65 (0.62–0.68) | <0.001 | 0.52 (0.41–0.66) | 22/5/0 | 0.117 | 83.8 (<0.001) | 0.33–1.03 | 0.38–1.13 | No | Yes | Suggestive * |
| Lung cancer | 33 | 8,833,965 | 0.89 (0.80–0.99) | 0.036 | 0.82 (0.80–0.84) | <0.001 | 1.03 (0.94–1.21) | 5/28/0 | 0.265 | 94.9 (<0.001) | 0.51–1.57 | 0.47–1.42 | No | No | Weak |
| Lymphoma | 16 | 8863 | 0.85 (0.73–0.99) | 0.042 | 0.86 (0.80–0.92) | <0.001 | 0.96 (0.83–1.11) | 6/9/1 | 0.850 | 69.1 (<0.001) | 0.52–1.40 | 0.54–1.39 | No | No | Weak |
| Melanoma | 24 | 434,680 | 0.94 (0.86–1.03) | 0.204 | 0.94 (0.88–1.00) | 0.063 | 0.94 (0.88–1.00) | 3/21/0 | 0.836 | 26.0 (0.121) | 0.74–1.19 | 0.60–1.46 | No | No | Non-significant |
| Myeloma | 5 | 609 | 0.89 (0.53–1.51) | 0.674 | 0.89 (0.73–1.09) | 0.251 | 0.83 (0.61–1.12) | 2/2/1 | 0.983 | 81.0 (<0.001) | 0.14–5.73 | 0.17–4.78 | No | Yes | Non-significant |
| Pancreatic cancer | 20 | 2,832,052 | 0.89 (0.75–1.06) | 0.207 | 0.91 (0.86–0.97) | 0.003 | 1.10 (0.81–1.49) | 1/18/1 | 0.927 | 79.0 (<0.001) | 0.46–1.71 | 0.49–1.71 | No | Yes | Non-significant |
| Prostate cancer | 44 | NA | 0.94 (0.90–0.99) | 0.017 | 1.02 (1.00–1.04) | 0.056 | NA ** | 18/42/4 | 0.002 | 74.5 (<0.001) | 0.71–1.24 | 0.78–1.33 | Yes | - | Weak |
| Non-melanoma skin cancer | 17 | 1,240,281 | 1.07 (1.00–1.16) | 0.063 | 1.09 (1.06–1.13) | <0.001 | 1.09 (1.06–1.13) | 1/11/5 | 0.768 | 58.5 (0.001) | 0.88–1.31 | 0.90–1.32 | No | No | Non-significant |
| Cancer Type | Overall | Randomized Controlled Studies | Observational Studies * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Random Effects (RR, 95%CI) | p-Value | Evidence | No. of Studies | Random Effects (RR, 95%CI) | p-Value | Evidence | No. of Studies | Random Effects (RR, 95%CI) | p-Value | Evidence | |
| Bladder cancer | 13 | 1.07 (0.95–1.21) | 0.282 | Non-significant | 3 | 0.84 (0.64–1.09) | 0.180 | Non-significant | 10 | 1.11 (0.97–1.26) | 0.118 | Non-significant |
| Breast cancer | 62 | 0.91 (0.85–0.97) | 0.004 | Weak | 12 | 1.00 (0.80–1.25) | 0.661 | Non-significant | 50 | 0.90 (0.84–0.96) | 0.003 | Weak |
| Colorectal cancer | 59 | 0.92 (0.88–0.95) | <0.001 | Weak | 13 | 0.92 (0.81–1.05) | 0.214 | Non-significant | 46 | 0.92 (0.88–0.95) | <0.001 | Weak |
| Endometrial cancer | 15 | 0.94 (0.83–1.07) | 0.349 | Non-significant | 2 | 0.72 (0.19–2.67) | 0.621 | Non-significant | 13 | 0.94 (0.82–1.07) | 0.361 | Non-significant |
| Esophageal cancer | 27 | 0.70 (0.63–0.78) | <0.001 | Suggestive | 1 | 0.98 (0.69–1.40) | NR | Non-significant | 26 | 0.69 (0.62–0.76) | <0.001 | Suggestive |
| Gastric cancer | 16 | 0.74 (0.60–0.90) | 0.004 | Weak | 3 | 0.84 (0.61–1.14) | 0.259 | Non-significant | 13 | 0.71 (0.56–0.90) | 0.004 | Weak |
| Gynecological cancer | 23 | 0.89 (0.78–1.02) | 0.087 | Non-significant | 6 | 1.03 (0.65–1.63) | 0.902 | Non-significant | 17 | 0.88 (0.76–1.01) | 0.069 | Non-significant |
| Hematological cancer | 34 | 0.89 (0.82–0.96) | 0.005 | Suggestive | 8 | 0.96 (0.78–1.17) | 0.667 | Non-significant | 26 | 0.88 (0.81–0.97) | 0.006 | Suggestive |
| Kidney cancer | 11 | 0.91 (0.70–1.17) | 0.457 | Non-significant | 2 | 1.01 (0.57–1.78) | 0.985 | Non-significant | 9 | 0.90 (0.69–1.18) | 0.455 | Non-significant |
| Leukemia | 9 | 0.85 (0.74–0.98) | 0.031 | Suggestive | - | - | - | - | 9 | 0.85 (0.74–0.98) | 0.031 | Suggestive |
| Liver cancer | 27 | 0.58 (0.52–0.66) | <0.001 | Suggestive | 3 | 0.96 (0.62–1.49) | 0.867 | Non-significant | 24 | 0.57 (0.50–0.65) | <0.001 | Suggestive |
| Lung cancer | 33 | 0.89 (0.80–0.99) | 0.036 | Weak | 9 | 0.95 (0.85–1.05) | 0.324 | Non-significant | 24 | 0.87 (0.77–0.99) | 0.034 | Weak |
| Lymphoma | 16 | 0.85 (0.73–0.99) | 0.042 | Weak | - | - | - | - | 16 | 0.85 (0.73–0.99) | 0.042 | Weak |
| Melanoma | 24 | 0.94 (0.86–1.03) | 0.204 | Non-significant | 13 | 1.06 (0.90–1.25) | 0.474 | Non-significant | 11 | 0.92 (0.84–1.02) | 0.105 | Non-significant |
| Myeloma | 5 | 0.89 (0.53–1.51) | 0.674 | Non-significant | - | - | - | - | 5 | 0.89 (0.53–1.51) | 0.674 | Non-significant |
| Pancreatic cancer | 20 | 0.89 (0.75–1.06) | 0.207 | Non-significant | 3 | 0.99 (0.44–2.21) | 0.982 | Non-significant | 17 | 0.89 (0.74–1.07) | 0.202 | Non-significant |
| Prostate cancer | 64 | 0.94 (0.90–0.99) | 0.017 | Weak | 7 | 1.06 (0.93–1.20) | 0.386 | Non-significant | 57 | 0.93 (0.88–0.98) | 0.005 | Weak |
| Non-melanoma skin cancer | 17 | 1.07 (1.00–1.16) | 0.063 | Non-significant | 8 | 1.07 (0.86–1.33) | 0.519 | Non-significant | 9 | 1.08 (1.00–1.18) | 0.048 | Weak |
| Cancer Type | Overall | Randomized Controlled Trials | Observational Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Meta-Analyses | D/N/I | C/S/W | Number of Meta-Analyses | D/N/I | C/S/W | Number of Meta-Analyses | D/N/I | C/S/W | ||||
| Bladder cancer | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | |||
| Breast cancer | 2 | 0/2/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | 3 | 1/2/0 | 0/1/0 | |||
| Colorectal cancer | 4 | 4/0/0 | 1/0/3 | 4 | 0/4/0 | 0/0/0 | 5 | 5/0/0 | 1/0/4 | |||
| Endometrial cancer | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | |||
| Esophageal cancer | 2 | 2/0/0 | 1/0/1 | 0 | 0/0/0 | 0/0/0 | 6 | 6/0/0 | 2/4/0 | |||
| Gastric cancer | 2 | 2/0/0 | 0/0/2 | 1 | 0/1/0 | 0/0/0 | 2 | 2/0/0 | 0/0/2 | |||
| Gynecological cancer | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | |||
| Hematological cancer | 2 | 1/1/0 | 0/0/1 | 2 | 0/2/0 | 0/0/0 | 2 | 1/1/0 | 0/0/1 | |||
| Kidney cancer | 1 | 0/0/1 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | |||
| Leukemia | 0 | 0/0/0 | 0/0/0 | 0 | 0/0/0 | 0/0/0 | 1 | 1/0/0 | 0/1/0 | |||
| Liver cancer | 3 | 3/0/0 | 1/1/1 | 0 | 0/0/0 | 0/0/0 | 1 | 1/0/0 | 0/1/0 | |||
| Lung cancer | 2 | 0/2/0 | 0/0/0 | 3 | 0/3/0 | 0/0/0 | 3 | 0/3/0 | 0/0/0 | |||
| Lymphoma | 0 | 0/0/0 | 0/0/0 | 0 | 0/0/0 | 0/0/0 | 2 | 1/1/0 | 0/0/1 | |||
| Melanoma | 1 | 0/1/0 | 0/0/0 | 3 | 0/3/0 | 0/0/0 | 0 | 0/0/0 | 0/0/0 | |||
| Myeloma | 0 | 0/0/0 | 0/0/0 | 0 | 0/0/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | |||
| Pancreatic cancer | 2 | 0/2/0 | 0/0/0 | 2 | 0/2/0 | 0/0/0 | 2 | 0/2/0 | 0/0/0 | |||
| Prostate cancer | 1 | 0/1/0 | 0/0/0 | 1 | 0/1/0 | 0/0/0 | 6 | 1/5/0 | 0/0/1 | |||
| Non-melanoma skin cancer | 2 | 0/1/1 | 0/0/1 | 1 | 0/1/0 | 0/0/0 | 1 | 0/0/1 | 0/0/1 | |||
| Type of Cancer | Randomized Controlled Trials | Observational Studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Our Study | Largest Meta-Analysis * | Our Study | Largest Meta-Analysis * | |||||||||
| No. of Study | Random Effects (RR 95% CI) | No. of Study | Random Effects (RR 95% CI) | No. of Study | Random Effects (RR 95% CI) | No. of Study | Random Effects (RR 95% CI) | |||||
| Bladder cancer | 3 | 0.84 (0.64–1.09) | 3 | 0.83 (0.64–1.09) | 10 | 1.11 (0.97–1.26) | 10 | 1.11 (0.97–1.26) | ||||
| Breast cancer | 12 | 1.00 (0.80–1.25) | 7 | 1.19 (0.81–1.73) | 50 | 0.90 (0.84–0.96) | 21 | 0.99 (0.94–1.04) | ||||
| Colorectal cancer | 13 | 0.92 (0.81–1.05) | 11 | 0.96 (0.85–1.08) | 46 | 0.92 (0.88–0.95) | 32 | 0.92 (0.87–0.96) | ||||
| Endometrial cancer | 2 | 0.72 (0.19–2.67) | 2 | 0.72 (0.19–2.67) | 13 | 0.94 (0.82–1.07) | 13 | 0.94 (0.82–1.07) | ||||
| Esophageal cancer | 1 | 0.98 (0.69–1.40) | - | - | 26 | 0.69 (0.62–0.76) | 10 | 0.59 (0.50–0.68) | ||||
| Gastric cancer | 3 | 0.84 (0.61–1.14) | 3 | 0.84 (0.61–1.14) | 13 | 0.71 (0.56–0.90) | 9 | 0.70 (0.53–0.93) | ||||
| Gynecological cancer | 6 | 1.03 (0.65–1.63) | 6 | 1.03 (0.65–1.63) | 17 | 0.88 (0.76–1.01) | 17 | 0.88 (0.76–1.01) | ||||
| Hematological cancer | 8 | 0.96 (0.78–1.17) | 6 | 0.92 (0.78–1.09) | 26 | 0.88 (0.81–0.97) | 22 | 0.88 (0.80–0.98) | ||||
| Kidney cancer | 2 | 1.01 (0.57–1.78) | 2 | 1.01 (0.57–1.78) | 9 | 0.90 (0.69–1.18) | 9 | 0.90 (0.69–1.18) | ||||
| Leukemia | - | - | - | - | 9 | 0.85 (0.74–0.98) | 9 | 0.85 (0.74–0.98) | ||||
| Liver cancer | 3 | 0.96 (0.62–1.49) | - | - | 24 | 0.57 (0.50–0.65) | 6 | 0.58 (0.46–0.74) | ||||
| Lung cancer | 9 | 0.95 (0.85–1.05) | 7 | 0.95 (0.84–1.09) | 24 | 0.87 (0.77–0.99) | 15 | 0.88 (0.75–1.03) | ||||
| Lymphoma | - | - | - | - | 16 | 0.85 (0.73–0.99) | 13 | 0.83 (0.69–0.99) | ||||
| Melanoma | 13 | 1.06 (0.90–1.25) | 9 | 0.92 (0.62–1.36) | 11 | 0.92 (0.84–1.02) | - | - | ||||
| Myeloma | - | - | - | - | 5 | 0.89 (0.53–1.51) | 5 | 0.89 (0.53–1.51) | ||||
| Pancreatic cancer | 3 | 0.99 (0.44–2.21) | 3 | 0.99 (0.44–2.21) | 17 | 0.89 (0.74–1.07) | 15 | 0.88 (0.73–1.07) | ||||
| Prostate cancer | 7 | 1.06 (0.93–1.20) | 6 | 1.06 (0.93–1.20) | 57 | 0.93 (0.88–0.98) | 27 | 0.90 (0.80–1.01) | ||||
| Non-melanoma skin cancer | 8 | 1.07 (0.86–1.33) | 7 | 1.09 (0.85–1.39) | 9 | 1.08 (1.00–1.18) | 5 | 1.11 (1.02–1.22) | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, G.H.; Lee, K.H.; Kim, J.Y.; Eisenhut, M.; Kronbichler, A.; van der Vliet, H.J.; Hong, S.H.; Shin, J.I.; Gamerith, G. Effect of Statin on Cancer Incidence: An Umbrella Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 819. https://doi.org/10.3390/jcm8060819
Jeong GH, Lee KH, Kim JY, Eisenhut M, Kronbichler A, van der Vliet HJ, Hong SH, Shin JI, Gamerith G. Effect of Statin on Cancer Incidence: An Umbrella Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(6):819. https://doi.org/10.3390/jcm8060819
Chicago/Turabian StyleJeong, Gwang Hun, Keum Hwa Lee, Jong Yeob Kim, Michael Eisenhut, Andreas Kronbichler, Hans J. van der Vliet, Sung Hwi Hong, Jae Il Shin, and Gabriele Gamerith. 2019. "Effect of Statin on Cancer Incidence: An Umbrella Systematic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 6: 819. https://doi.org/10.3390/jcm8060819
APA StyleJeong, G. H., Lee, K. H., Kim, J. Y., Eisenhut, M., Kronbichler, A., van der Vliet, H. J., Hong, S. H., Shin, J. I., & Gamerith, G. (2019). Effect of Statin on Cancer Incidence: An Umbrella Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 8(6), 819. https://doi.org/10.3390/jcm8060819







