Initial Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy of Lung Lesions Performed by a Pulmonologist
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Biopsy Procedure
2.3. Diagnostic Performance
2.4. Statistical Analyses
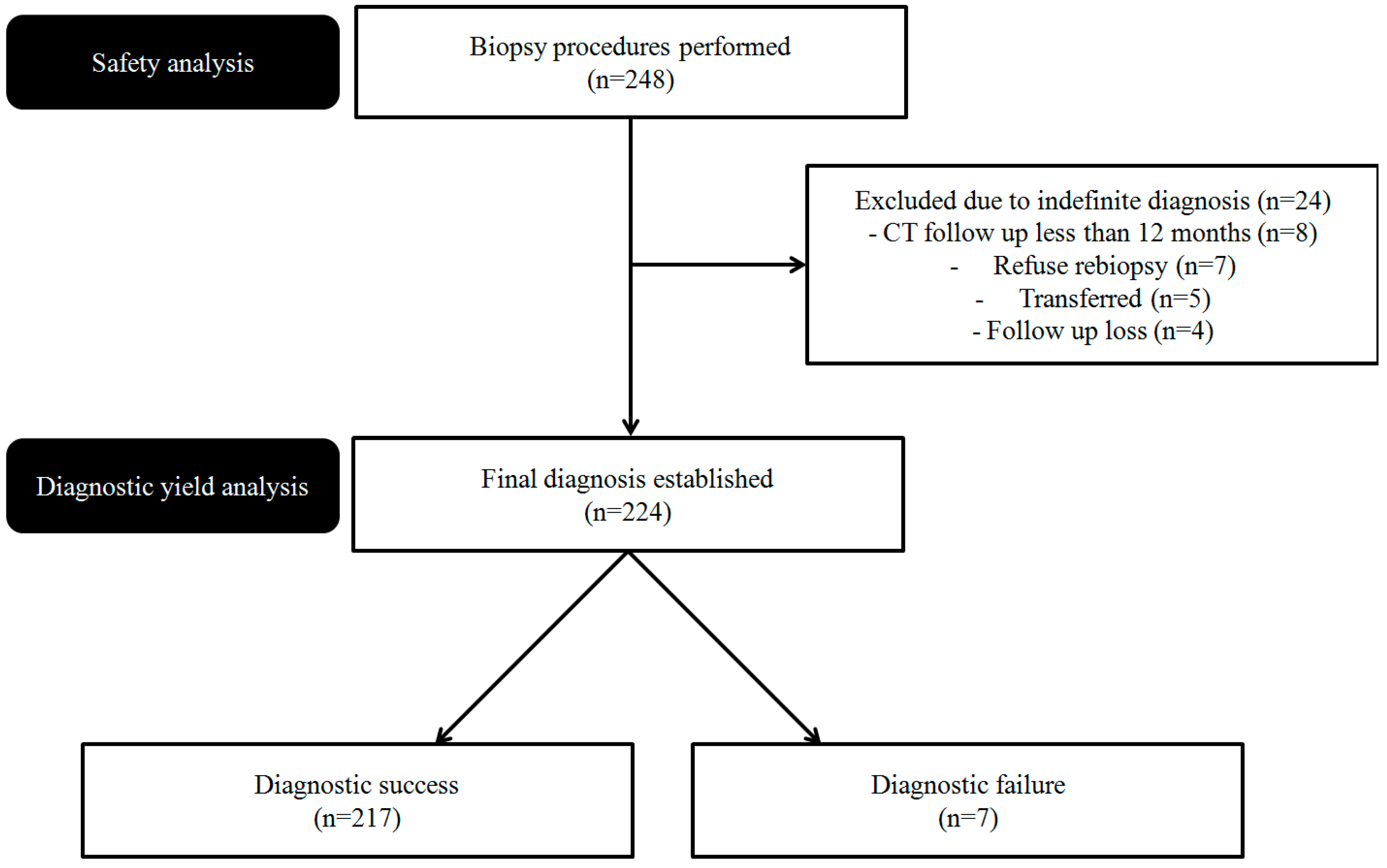
3. Results
3.1. Characteristics of the Patients, Lung Lesions, and Procedures
3.2. Pathologic Results and Diagnostic Accuracy
3.3. PTNB-Related Complications
3.4. Risk Factors for Diagnostic Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hiraki, T.; Mimura, H.; Gobara, H.; Iguchi, T.; Fujiwara, H.; Sakurai, J.; Matsui, Y.; Inoue, D.; Toyooka, S.; Sano, Y.; et al. CT fluoroscopy-guided biopsy of 1000 pulmonary lesions performed with 20-gauge coaxial cutting needles: Diagnostic yield and risk factors for diagnostic failure. Chest 2009, 136, 1612–1617. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, C.M.; Lee, K.H.; Bahn, Y.E.; Kim, J.I.; Goo, J.M. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: Clinical experience in 1108 patients. Radiology 2014, 271, 291–300. [Google Scholar] [CrossRef]
- Ohno, Y.; Hatabu, H.; Takenaka, D.; Higashino, T.; Watanabe, H.; Ohbayashi, C.; Sugimura, K. CT-guided transthoracic needle aspiration biopsy of small (≤20 mm) solitary pulmonary nodules. Am. J. Roentgenol. 2003, 180, 1665–1669. [Google Scholar] [CrossRef]
- Dhillon, S.S.; Harris, K. Bronchoscopy for the diagnosis of peripheral lung lesions. J. Thorac. Dis. 2017, 9, S1047. [Google Scholar] [CrossRef]
- Haaga, J.R.; Alfidi, R.J. Precise biopsy localization by computer tomography. Radiology 1976, 118, 603–607. [Google Scholar] [CrossRef]
- Li, H.; Boiselle, P.M.; Shepard, J.O.; Trotman-Dickenson, B.; McLoud, T.C. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: Comparison of small and large pulmonary nodules. Am. J. Roentgenol. 1996, 167, 105–109. [Google Scholar] [CrossRef]
- Tsukada, H.; Satou, T.; Iwashima, A.; Souma, T. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. Am. J. Roentgenol. 2000, 175, 239–243. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Lim, F.T.; Mikhail, A.; Martinez, F.J. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology 1996, 198, 371–375. [Google Scholar] [CrossRef]
- Laurent, F.; Michel, P.; Latrabe, V.; Tunon de Lara, M.; Marthan, R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: Incidence and risk factors. Am. J. Roentgenol. 1999, 172, 1049–1053. [Google Scholar] [CrossRef]
- Li, G.C.; Fu, Y.F.; Cao, W.; Shi, Y.B.; Wang, T. Computed tomography-guided percutaneous cutting needle biopsy for small (≤20 mm) lung nodules. Medicine 2017, 96, e8703. [Google Scholar] [CrossRef]
- Lal, H.; Neyaz, Z.; Nath, A.; Borah, S. CT-guided percutaneous biopsy of intrathoracic lesions. Korean J. Radiol. 2012, 13, 210–226. [Google Scholar] [CrossRef]
- Diacon, A.H.; Schuurmans, M.M.; Theron, J.; Schubert, P.T.; Wright, C.A.; Bolliger, C.T. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respir. Int. Rev. Thorac. Dis. 2004, 71, 519–522. [Google Scholar] [CrossRef]
- Laursen, C.B.; Naur, T.M.; Bodtger, U.; Colella, S.; Naqibullah, M.; Minddal, V.; Konge, L.; Davidsen, J.R.; Hansen, N.C.; Graumann, O.; et al. Ultrasound-guided Lung Biopsy in the Hands of Respiratory Physicians: Diagnostic Yield and Complications in 215 Consecutive Patients in 3 Centers. J. Bronchol. Interv. Pulmonol. 2016, 23, 220–228. [Google Scholar] [CrossRef]
- Garcia-Ortega, A.; Briones-Gomez, A.; Fabregat, S.; Martinez-Tomas, R.; Martinez-Garcia, M.A.; Cases, E. Benefit of Chest Ultrasonography in the Diagnosis of Peripheral Thoracic Lesions in an Interventional Pulmonology Unit. Arch. Bronconeumol. 2016, 52, 244–249. [Google Scholar] [CrossRef]
- Meena, N.; Bartter, T. Ultrasound-Guided Percutaneous Needle Aspiration by Pulmonologists: A Study of Factors with Impact on Procedural Yield and Complications. J. Bronchol. Interv. Pulmonol. 2015, 22, 204–208. [Google Scholar] [CrossRef]
- Wu, R.H.; Tzeng, W.S.; Lee, W.J.; Chang, S.C.; Chen, C.H.; Fung, J.L.; Wang, Y.J.; Mak, C.W. CT-guided transthoracic cutting needle biopsy of intrathoracic lesions: Comparison between coaxial and single needle technique. Eur. J. Radiol. 2012, 81, e712–e716. [Google Scholar] [CrossRef]
- Geraghty, P.R.; Kee, S.T.; McFarlane, G.; Razavi, M.K.; Sze, D.Y.; Dake, M.D. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: Needle size and pneumothorax rate. Radiology 2003, 229, 475–481. [Google Scholar] [CrossRef]
- Boskovic, T.; Stanic, J.; Pena-Karan, S.; Zarogoulidis, P.; Drevelegas, K.; Katsikogiannis, N.; Machairiotis, N.; Mpakas, A.; Tsakiridis, K.; Kesisis, G.; et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J. Thorac. Dis. 2014, 6 (Suppl. 1), S99–S107. [Google Scholar]
- Jeon, M.C.; Kim, J.O.; Jung, S.S.; Park, H.S.; Lee, J.E.; Moon, J.Y.; Chung, C.U.; Kang, D.H.; Park, D.I. CT-Guided Percutaneous Transthoracic Needle Biopsy Using the Additional Laser Guidance System by a Pulmonologist with 2 Years of Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy. Tuberc. Respir. Dis. 2018, 81, 330–338. [Google Scholar] [CrossRef]
- Yang, W.; Sun, W.; Li, Q.; Yao, Y.; Lv, T.; Zeng, J.; Liang, W.; Zhou, X.; Song, Y. Diagnostic Accuracy of CT-Guided Transthoracic Needle Biopsy for Solitary Pulmonary Nodules. PLoS ONE 2015, 10, e0131373. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Tan, X.; Tian, P. CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: Diagnostic yield and complications in 1484 patients. Medicine 2016, 95, e4460. [Google Scholar] [CrossRef]
- Tian, P.; Wang, Y.; Li, L.; Zhou, Y.; Luo, W.; Li, W. CT-guided transthoracic core needle biopsy for small pulmonary lesions: Diagnostic performance and adequacy for molecular testing. J. Thorac. Dis. 2017, 9, 333–343. [Google Scholar] [CrossRef]
- Choi, S.H.; Chae, E.J.; Kim, J.E.; Kim, E.Y.; Oh, S.Y.; Hwang, H.J.; Lee, H.J. Percutaneous CT-guided aspiration and core biopsy of pulmonary nodules smaller than 1 cm: Analysis of outcomes of 305 procedures from a tertiary referral center. Am. J. Roentgenol. 2013, 201, 964–970. [Google Scholar] [CrossRef]
- Kim, G.R.; Hur, J.; Lee, S.M.; Lee, H.J.; Hong, Y.J.; Nam, J.E.; Kim, H.S.; Kim, Y.J.; Choi, B.W.; Kim, T.H.; et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: A prospective controlled study to assess radiation doses and diagnostic performance. Eur. Radiol. 2011, 21, 232–239. [Google Scholar] [CrossRef]
- Hirose, T.; Mori, K.; Machida, S.; Tominaga, K.; Yokoi, K.; Adachi, M. Computed tomographic fluoroscopy-guided transthoracic needle biopsy for diagnosis of pulmonary nodules. Jpn. J. Clin. Oncol. 2000, 30, 259–262. [Google Scholar] [CrossRef]
- Choi, C.M.; Um, S.W.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Shim, Y.S.; Lee, C.T. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest 2004, 126, 1516–1521. [Google Scholar] [CrossRef]
- Freund, M.C.; Petersen, J.; Goder, K.C.; Bunse, T.; Wiedermann, F.; Glodny, B. Systemic air embolism during percutaneous core needle biopsy of the lung: Frequency and risk factors. BMC Pulm. Med. 2012, 12, 2. [Google Scholar] [CrossRef]
- Collings, C.L.; Westcott, J.L.; Banson, N.L.; Lange, R.C. Pneumothorax and dependent versus nondependent patient position after needle biopsy of the lung. Radiology 1999, 210, 59–64. [Google Scholar] [CrossRef]
- Fish, G.D.; Stanley, J.H.; Miller, K.S.; Schabel, S.I.; Sutherland, S.E. Postbiopsy pneumothorax: Estimating the risk by chest radiography and pulmonary function tests. Am. J. Roentgenol. 1988, 150, 71–74. [Google Scholar] [CrossRef]
- Heck, S.L.; Blom, P.; Berstad, A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur. Radiol. 2006, 16, 1387–1392. [Google Scholar] [CrossRef]
- Han, Y.; Kim, H.J.; Kong, K.A.; Kim, S.J.; Lee, S.H.; Ryu, Y.J.; Lee, J.H.; Kim, Y.; Shim, S.S.; Chang, J.H. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191590. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Patient | |
| Age, years | 68.2 ± 12.5 |
| Male | 174 (70.2%) |
| Pulmonary function test (n = 241), % | |
| FEV1 | 80.7 ± 20.1 |
| FVC | 79.8 ± 16.6 |
| FEV1/FVC | 71.9 ± 12.4 |
| Lung lesions | |
| Location | |
| Upper lobe | 147 (59.3%) |
| Middle lobe | 9 (3.6%) |
| Lower lobe | 92 (37.1%) |
| Computed tomography findings | |
| Solid | 228 (91.9%) |
| Subsolid | 17 (6.9%) |
| Ground-glass opacity | 3 (1.2%) |
| Longest diameter of the lesion, mm | 39.5 ± 19.3 |
| Cavitary lesion | 31 (12.5%) |
| Procedure | |
| Patient position during biopsy | |
| Supine | 64 (25.8%) |
| Prone | 141 (56.9%) |
| Decubitus | 43 (17.3%) |
| Needle diameter (gauge) | |
| 20 | 248 (100%) |
| Number of specimens (n = 244) | 1.7 ± 0.7 |
| Length of aerated lung traversed by needle, mm | 14.5 ± 15.3 |
| Emphysema along the needle pathway | 30 (12.1%) |
| Poor cooperation | 26 (10.5%) |
| Transfissural approach | 13 (5.2%) |
| Initial Pathologic Results (n = 244) | Final Diagnosis (n = 248) | |
|---|---|---|
| Malignant | 171 | 178 |
| Primary lung cancer | 165 | 172 |
| Adenocarcinoma | 90 | 93 a |
| Squamous cell carcinoma | 51 | 53 b |
| NSCLC, NOS | 7 | 8 c |
| SCLC | 11 | 12 d |
| Large cell neuroendocrine carcinoma | 1 | 1 |
| Pleomorphic carcinoma | 2 | 2 |
| Malignant spindle cell tumor | 3 | 2 |
| Leiomyosarcoma | 0 | 1 e |
| Metastasis | 6 | 6 |
| Breast | 2 | 2 |
| Colon | 1 | 1 |
| Melanoma | 1 | 1 |
| Trachea | 1 | 1 |
| Salivary gland | 1 | 1 |
| Benign | 69 | 46 |
| Definite benign features | 31 | 33 |
| Pulmonary tuberculosis | 13 | 14 f |
| Organizing pneumonia | 7 | 7 |
| Tuberculous granuloma | 6 | 6 |
| Cryptococcosis | 2 | 3 g |
| PMF | 1 | 1 |
| Pulmonary infarction | 1 | 1 |
| GPA | 1 | 1 |
| Non-specific benign features | 38 | 13 |
| Indeterminate | 4 | 24 h |
| Parameter | ≤20 mm (n = 26) | 21–39 mm (n = 99) | ≥40 mm (n = 99) | Overall (n = 224) |
|---|---|---|---|---|
| True-positive, n | 20 | 73 | 78 | 171 |
| True-negative, n | 5 | 22 | 19 | 46 |
| False-positive, n | 0 | 0 | 0 | 0 |
| False-negative, n | 0 | 2 | 1 | 3 |
| Technical failure, n | 1 | 2 | 1 | 4 a |
| Sensitivity, % | 95.2 | 94.8 | 97.5 | 96.1 |
| Specificity, % | 100.0 | 100.0 | 100.0 | 100 |
| PPV, % | 100.0 | 100.0 | 100.0 | 100 |
| NPV, % | 83.3 | 84.6 | 90.5 | 86.8 |
| Diagnostic accuracy, % | 96.2 | 96.0 | 98.0 | 96.9 |
| Complication Type | n |
|---|---|
| Pneumothorax | 67 (27.0%) |
| Oxygen therapy and close observation | 51 (20.6%) |
| Chest tube insertion | 16 (6.5%) |
| Hemoptysis | 13 (5.2%) |
| Oxygen therapy and close observation | 13 (5.2%) |
| Hemothorax | 2 (0.8%) |
| Chest tube insertion | 2 (0.8%) |
| Characteristic | Diagnostic Success a (n = 217) | Diagnostic Failure b (n = 7) | p Value |
|---|---|---|---|
| Patient | |||
| Age, years | 68.2 ± 12.5 | 70.6 ± 10.9 | 0.623 |
| Male | 154 (96.9%) | 5 (3.1%) | 1.000 |
| Pulmonary function test (n = 241), % | |||
| FEV1 | 81.2 ± 20.0 | 79.0 ± 19.0 | 0.779 |
| FVC | 80.0 ± 16.8 | 81.3 ± 14.2 | 0.840 |
| FEV1/FVC | 72.2 ± 12.0 | 69.7 ± 18.2 | 0.604 |
| Lung lesions | |||
| Final diagnosis | |||
| Malignancy | 171 (96.1%) | 7 (3.9%) | 0.349 |
| Benign | 46 (100.0%) | 0 (0%) | |
| Locations | |||
| Upper lobe | 127 (96.9%) | 4 (3.1%) | 0.851 |
| Middle lobe | 9 (100.0%) | 0 (0%) | |
| Lower lobe | 81 (96.4%) | 3 (3.6%) | |
| CT findings | |||
| Solid | 201 (96.6%) | 7 (3.4%) | 0.496 |
| Subsolid | 13 (100.0%) | 0 (0%) | |
| GGO | 3 (100.0%) | 0 (0%) | |
| Longest diameter of the lesion, mm | 40.3 ± 19.5 | 31.6 ± 11.3 | 0.244 |
| Cavitary lesion | 27 (100.0%) | 0 (0%) | 1.000 |
| Procedure | |||
| Patient position during biopsy | |||
| Supine | 57 (98.3%) | 1 (1.7%) | 0.142 |
| Prone | 122 (97.6%) | 3 (2.4%) | |
| Decubitus | 38 (92.7%) | 3 (7.3%) | |
| Number of specimens (n = 244) | 1.7 ± 0.7 | 1.3 ± 0.6 | 0.402 |
| Length of aerated lung traversed by needle, mm | 14.7 ± 15.7 | 21.9 ± 16.9 | 0.236 |
| Emphysema along the needle pathway | 25 (92.6%) | 2 (7.4%) | 0.201 |
| Poor cooperation | 23 (92.0%) | 2 (8.0%) | 0.177 |
| Transfissural approach | 12 (100.0%) | 0 (0%) | 1.000 |
| Aerated lung traversed by needle | 141 (95.9%) | 6 (4.1%) | 0.427 |
| Complications | |||
| Pneumothorax | 57 (93.4%) | 4 (6.6%) | 0.09 |
| Pneumothorax, requiring chest tube insertion | 11 (73.3%) | 4 (26.7) | <0.001 |
| Hemoptysis | 12 (100.0%) | 0 (0%) | 1.000 |
| Studies | Country | No. of Nodules | Biopsy Methods | Sensitivity (%) | Specificity (%) | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|
| Hiraki 2009 [1] | Japan | 1000 | Core biopsy | 94.2 | 99.1 | 95.2 |
| Yang 2015 [20] | China | 311 | Core biopsy | 95.3 | 95.7 | 92.9 |
| Wang 2016 [21] | China | 1484 | Core biopsy | 94.4 | 100 | 94.8 |
| Tian 2017 [22] | China | 560 | Core biopsy | 92.0 | 98.6 | 94.6 |
| Kim 2011 [24] | Korea | 72 | Aspiration | 97.8 | 100 | 98.4 |
| Choi 2013 [23] | Korea | 153 | Core biopsy | 93.6 | 100 | 95.2 |
| Ahn (present study) | Korea | 224 | Core biopsy | 96.1 | 100 | 96.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.H.; Jang, J.G. Initial Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy of Lung Lesions Performed by a Pulmonologist. J. Clin. Med. 2019, 8, 821. https://doi.org/10.3390/jcm8060821
Ahn JH, Jang JG. Initial Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy of Lung Lesions Performed by a Pulmonologist. Journal of Clinical Medicine. 2019; 8(6):821. https://doi.org/10.3390/jcm8060821
Chicago/Turabian StyleAhn, June Hong, and Jong Geol Jang. 2019. "Initial Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy of Lung Lesions Performed by a Pulmonologist" Journal of Clinical Medicine 8, no. 6: 821. https://doi.org/10.3390/jcm8060821
APA StyleAhn, J. H., & Jang, J. G. (2019). Initial Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy of Lung Lesions Performed by a Pulmonologist. Journal of Clinical Medicine, 8(6), 821. https://doi.org/10.3390/jcm8060821





