Clinical Characteristics and Treatment Outcomes of Definitive versus Standard Anti-Tuberculosis Therapy in Patients with Tuberculous Lymphadenitis
Abstract
1. Introduction
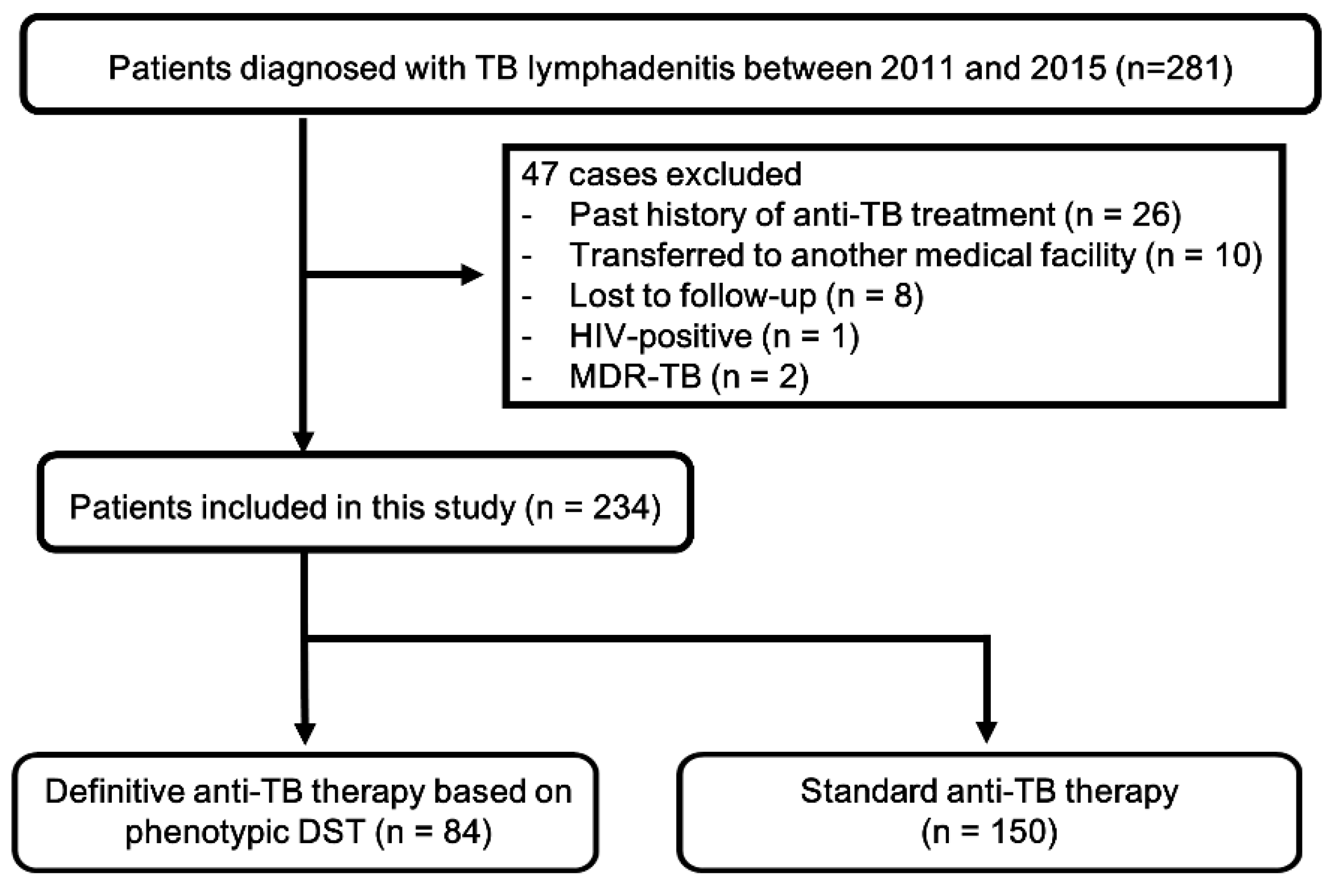
2. Materials and Methods
2.1. Study Population
2.2. Diagnosis of Tuberculous Lymphadenitis
- positive isolation of MTB in lymph node specimen.
- positive AFB staining with positive TB-PCR.
- histological findings compatible with TB (grade I, II, and III) and positive TB-PCR.
- histological findings compatible with TB (grade I, II, and III) with positive IGRA for MTB and followed by a successful response of anti-TB treatment [8].
2.3. Treatment of Tuberculous Lymphadenitis
2.4. Definition of Recurrence after Treatment
2.5. Statistical Analysis
3. Results
3.1. Clinical, Histological, and Microbiological Differences between the Definitive Therapy and Standard Therapy Groups
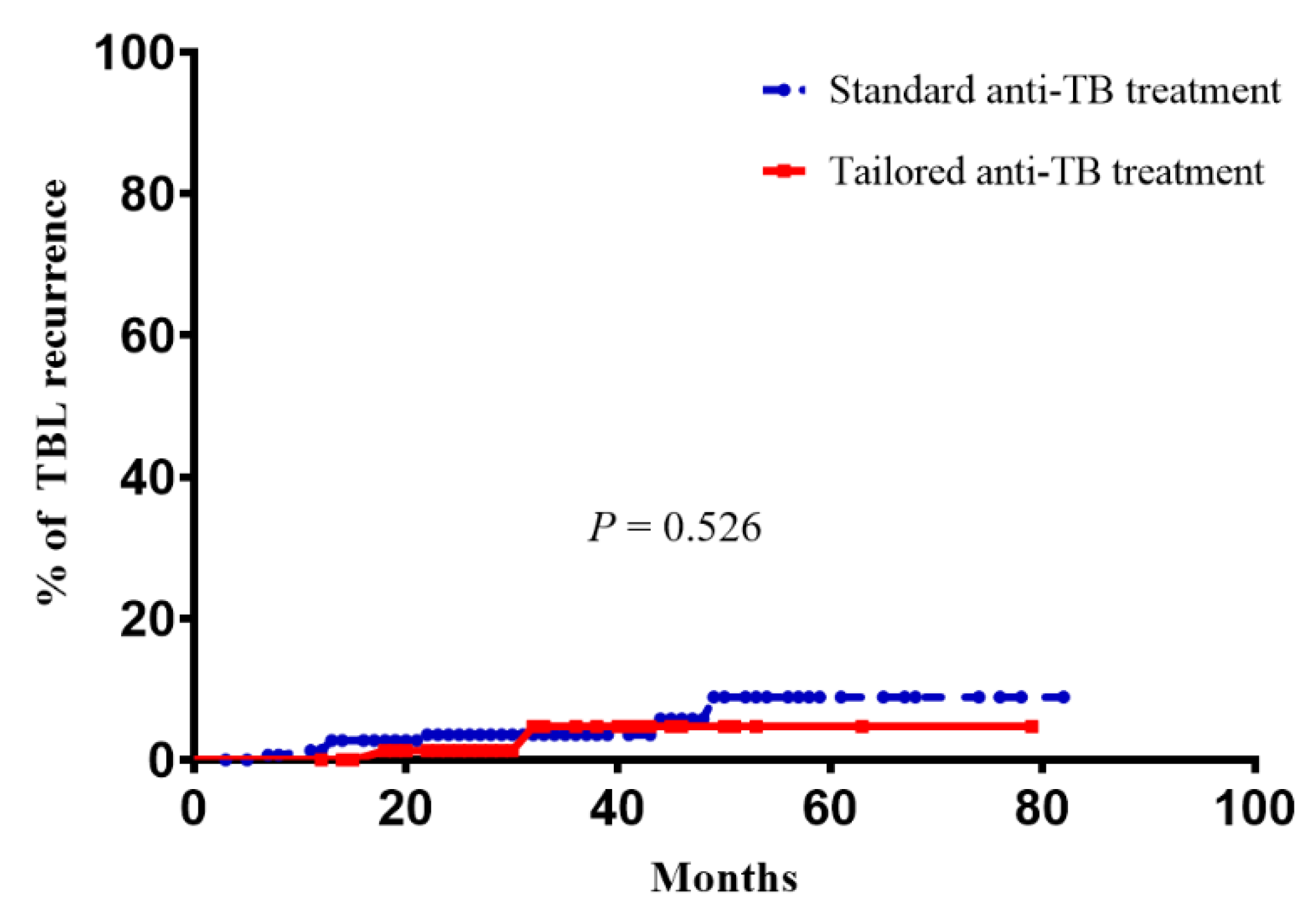
3.2. Comparison of Recurrence Rates after Treatment Completion between the Definitive Therapy and Standard Therapy Groups
4. Discussion
- It has a low incidence, and therefore is less familiar to clinicians.
- The sites affected are less accessible.
- It occurs in vulnerable organs/tissues in spite of being characterized by a paucity of bacteria.
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Catano, J.C.; Robledo, J. Tuberculous Lymphadenitis and Parotitis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Gloabl Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Fontanilla, J.M.; Barnes, A.; von Reyn, C.F. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin. Infect. Dis. 2011, 53, 555–562. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar] [PubMed]
- Polesky, A.; Grove, W.; Bhatia, G. Peripheral tuberculous lymphadenitis: Epidemiology, diagnosis, treatment, and outcome. Medicine 2005, 84, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, A.; Sabnis, K.; Jeyaseelan, V.; Rupali, P. Paradoxical reaction (PR) in tuberculous lymphadenitis among HIV-negative patients: Retrospective cohort study. Postgrad. Med. J. 2016, 92, 684–685. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, J.; Lee, Y.S.; Kim, M.Y.; Lee, H.K.; Lee, Y.M.; Shin, J.H.; Ko, Y. Drug-resistance pattern of Mycobacterium tuberculosis strains from patients with pulmonary and extrapulmonary tuberculosis during 2006 to 2013 in a Korean tertiary medical center. Korean J. Intern. Med. 2015, 30, 325–334. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, M.S.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Incidence and outcomes of paradoxical lymph node enlargement after anti-tuberculosis therapy in non-HIV patients. J. Infect. 2013, 67, 408–415. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization (WHO). Definitions and Reporting Framework for Tuberculosis–2013 Revision; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Lee, J.Y. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc. Respir. Dis. 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, D.S.; Kim, Y.W.; Chung, M.P.; Uh, S.T.; Park, C.S.; Jeong, S.H.; Park, Y.B.; Lee, H.L.; Song, J.S.; et al. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: A Korean national survey. Chest 2015, 147, 465–474. [Google Scholar] [CrossRef]
- Bae, E.; Im, J.H.; Kim, S.W.; Yoon, N.S.; Sung, H.; Kim, M.N.; Shim, T.S. [Evaluation of combination of BACTEC mycobacteria growth indicator tube 960 system and Ogawa media for mycobacterial culture]. Korean J. Lab. Med. 2008, 28, 299–306. [Google Scholar] [CrossRef][Green Version]
- Cho, S.Y.; Kim, M.J.; Suh, J.T.; Lee, H.J. Comparison of diagnostic performance of three real-time PCR kits for detecting Mycobacterium species. Yonsei Med. J. 2011, 52, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Boehme, C.C.; Nabeta, P.; Hillemann, D.; Nicol, M.P.; Shenai, S.; Krapp, F.; Allen, J.; Tahirli, R.; Blakemore, R.; Rustomjee, R.; et al. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 2010, 363, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Asimacopoulos, E.P.; Berry, M.; Garfield, B.; Roesner, M.; Jepson, A.; McCarthy, J.; Kon, O.M. The diagnostic efficacy of fine-needle aspiration using cytology and culture in tuberculous lymphadenitis. Int. J. Tuberc. Lung Dis. 2010, 14, 93–98. [Google Scholar] [PubMed]
- Blumberg, H.M.; Burman, W.J.; Chaisson, R.E.; Daley, C.L.; Etkind, S.C.; Friedman, L.N.; Fujiwara, P.; Grzemska, M.; Hopewell, P.C.; Iseman, M.D.; et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 603–662. [Google Scholar] [CrossRef] [PubMed]
- Annual Report on the Notified Tuberculosis in Korea 2017; Korea Centers for Disease Control and Prevention: Cheongju, Korea, 2018.
- Mason, R.; Broaddus, V.C.; Martin, T.; King, T.; Schraufnagel, D.; Murray, J.; Nadel, J. Murray and Nadel’s Textbook of Respiratory Medicine, 5th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2010. [Google Scholar]
- Ye, W.; Zhang, R.; Xu, X.; Liu, Y.; Ying, K. Diagnostic Efficacy and Safety of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Intrathoracic Tuberculosis: A Meta-analysis. J. Ultrasound Med. 2015, 34, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Zinicola, R.; Watson, D.; Bajwa, A.; McDonald, P.J. Intra-abdominal and gastrointestinal tuberculosis. Colorectal Dis. 2007, 9, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.N.; Cho, O.H.; Park, K.H.; Jung, J.; Kim, Y.K.; Lee, J.Y.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; et al. Late paradoxical lymph node enlargement during and after anti-tuberculosis treatment in non-HIV-infected patients. Int. J. Tuberc. Lung Dis. 2015, 19, 1388–1394. [Google Scholar] [CrossRef]
- Kirwan, D.E.; Ugarte-Gil, C.; Gilman, R.H.; Caviedes, L.; Rizvi, H.; Ticona, E.; Chavez, G.; Cabrera, J.L.; Matos, E.D.; Evans, C.A.; et al. Microscopic Observation Drug Susceptibility Assay for Rapid Diagnosis of Lymph Node Tuberculosis and Detection of Drug Resistance. J. Clin. Microbiol. 2016, 54, 185–189. [Google Scholar] [CrossRef]
- Rageade, F.; Picot, N.; Blanc-Michaud, A.; Chatellier, S.; Mirande, C.; Fortin, E.; van Belkum, A. Performance of solid and liquid culture media for the detection of Mycobacterium tuberculosis in clinical materials: Meta-analysis of recent studies. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 867–870. [Google Scholar] [CrossRef]
- Van Loenhout-Rooyackers, J.H.; Laheij, R.J.; Richter, C.; Verbeek, A.L. Shortening the duration of treatment for cervical tuberculous lymphadenitis. Eur. Respir. J. 2000, 15, 192–195. [Google Scholar] [CrossRef]
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; Chaisson, L.H.; Chaisson, R.E.; Daley, C.L.; Grzemska, M.; et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis. 2016, 63, e147–e195. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lim, J.K.; Lee, D.H.; Yoo, S.S.; Lee, S.Y.; Cha, S.I.; Park, J.Y.; Lee, J. Outcomes of standard and tailored anti-tuberculosis regimens in patients with tuberculous pleural effusion. Int. J. Tuberc. Lung Dis. 2016, 20, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Dutt, A.K.; Moers, D.; Stead, W.W. Smear- and culture-negative pulmonary tuberculosis: Four-month short-course chemotherapy. Am. Rev. Respir. Dis. 1989, 139, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.L.; Chen, C.H.; Yeh, C.Y.; Sheu, J.R.; Chang, S.C. Early effective drainage in the treatment of loculated tuberculous pleurisy. Eur. Respir. J. 2008, 31, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Conde, M.B.; Loivos, A.C.; Rezende, V.M.; Soares, S.L.; Mello, F.C.; Reingold, A.L.; Daley, C.L.; Kritski, A.L. Yield of sputum induction in the diagnosis of pleural tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Combs, D.L.; O’Brien, R.J.; Geiter, L.J. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: Effectiveness, toxicity, and acceptability. The report of final results. Ann. Intern. Med. 1990, 112, 397–406. [Google Scholar] [CrossRef] [PubMed]
| No. of Patients (%) or Median (IQR) | |
|---|---|
| Age, years, median (IQR) | 46.0 (30.0–56.8) |
| Female | 153 (65.4) |
| Comorbidity | |
| COPD and BA | 4 (1.7) |
| Thyroid disease | 7 (3.0) |
| Chronic liver disease | 10 (4.3) |
| Diabetes | 14 (6.0) |
| Cerebrovascular disease | 7 (3.0) |
| Cardiovascular disease | 5 (2.1) |
| Chronic kidney disease | 8 (3.4) |
| Rheumatic disease | 3 (1.3) |
| Malignancy | 24 (10.3) |
| Hematologic disease | 1 (0.4) |
| Neurologic disease | 2 (0.9) |
| Combined TB in another site | |
| PTB | 36 (15.4) |
| TB colitis | 3 (1.3) |
| TB pleuritis | 1 (0.4) |
| TB pericarditis | 1 (0.4) |
| Location of affected lymph node | |
| Cervical | 210 (89.7) |
| Mediastinal | 11 (4.7) |
| Axillary | 11 (4.7) |
| Abdominal | 8 (3.4) |
| Submandibular | 3 (1.3) |
| Periauricular | 2 (0.9) |
| Inguinal | 1 (0.4) |
| All Patients | Definitive | Standard | p-Value | |
|---|---|---|---|---|
| (n = 234) | (n = 84) | (n = 150) | ||
| Age, years, median (IQR) | 46.0 (30.0–56.8) | 41.0 (30.0–54.3) | 47.0 (31.8–58.0) | 0.067 |
| Female | 153 (65.4) | 50 (59.5) | 103 (68.7) | 0.158 |
| Lymph node size | 2.5 (2.0–3.6) | 2.7 (2.1–3.8) | 2.5 (2.0–3.6) | 0.166 |
| Diagnostic procedure | 0.308 | |||
| Excisional biopsy | 118 (50.4) | 37 (44.0) | 81 (54.0) | |
| Core needle biopsy | 44 (18.3) | 19 (22.6) | 25 (16.7) | |
| Fine needle aspiration biopsy | 72 (30.8) | 28 (33.3) | 44(29.3) | |
| Histology | 0.056 | |||
| Gr I, epithelioid granuloma reaction with caseation | 132 (56.4) | 40 (47.6) | 92 (61.3) | |
| Gr II, epithelioid granulomatous reaction without caseation | 78 (33.3) | 31 (36.9) | 47 (31.3) | |
| Gr III, non-granulomatous reaction with necrosis | 24 (10.3) | 13 (15.5) | 11 (7.3) | |
| Gr IV, non-specific | ||||
| Gr V, inadequate sample | ||||
| Microbiological examination | ||||
| MTB-PCR positive | 202/218 (92.7) | 75/84 (89.3) | 127/134 (94.8) | 0.003 |
| AFB stain positive | 36/180 (20.0) | 23/80 (28.8) | 13/100 (13.0) | <0.001 |
| AFB culture positive | 84/137 (61.3) | 84/84 (100) | 0/53 (0) | <0.001 |
| IGRA positive | 54/63 (85.7) | 25/27 (92.6) | 29/36 (80.6) | 0.345 |
| Treatment duration, months | 8.6 (6.3–9.7) | 7.7 (6.1–9.5) | 8.8 (6.4–9.8) | 0.207 |
| After treatment completion | ||||
| Follow-up duration, months | 28.0 (24.0–43.0) | 27.0 (20.0–36.0) | 29.0 (24.0–45.3) | 0.079 |
| Recurrence | ||||
| Microbiological recurrence | 0 | 0 | 0 | |
| Clinical recurrence | 9 (3.8) | 2 (2.4) | 7 (4.7) | 0.526 |
| Paradoxical response | ||||
| Paradoxical response during treatment | 18 (7.7) | 3 (3.6) | 15 (10.0) | 0.122 |
| Time to paradoxical response, months | 2.0 (1.6–3.3) | 1.7 (0.5–4.0) | 2.0 (1.6–3.0) | 0.441 |
| Paradoxical response after treatment | 3 (1.3) | 2 (2.4) | 1 (0.7) | 0.263 |
| Time to paradoxical response, months | 3.3 (3.3–15.4) | 3.3 (2.5–3.5) | 0.157 |
| All Patients | |
|---|---|
| (n = 9) | |
| Time of recurrence after completion of treatment | 21.6 (12.2–37.9) |
| Age | 49.0 (24.0–54.5) |
| Female | 7 (77.8) |
| Lymph node size at recurrence | 2.3 (1.9–3.0) |
| Nature of recurrence of TBL | |
| New node | 9 (100) |
| Enlargement of node at previous site(s) | 1 (11.1) |
| New draining sinus | 1 (11.1) |
| Histology | |
| Gr I, epithelioid granuloma reaction with caseation | 8 (88.9) |
| Gr II, epithelioid granulomatous reaction without caseation | |
| Gr III, non-granulomatous reaction with necrosis | 1 (11.1) |
| Gr IV, non-specific | |
| Gr V, inadequate sample | |
| Microbiological results of rebiopsy of affected lymph node | |
| MTB-PCR positive | 9/9 (100) |
| AFB stain positive | 0/9 (0) |
| AFB culture positive | 0/9 (0) |
| Treatment duration, months | 9.3 (9.1–15.5) |
| After treatment completion | |
| Follow-up duration, months | 29.5 (21.3–39.3) |
| Recurrence | |
| Microbiological recurrence | 0 |
| Clinical recurrence | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.; Kim, C.; Park, Y.B.; Mo, E.-K.; Moon, J.-W.; Park, S.; Sim, Y.S.; Hong, J.Y.; Baek, M.S. Clinical Characteristics and Treatment Outcomes of Definitive versus Standard Anti-Tuberculosis Therapy in Patients with Tuberculous Lymphadenitis. J. Clin. Med. 2019, 8, 813. https://doi.org/10.3390/jcm8060813
Ko Y, Kim C, Park YB, Mo E-K, Moon J-W, Park S, Sim YS, Hong JY, Baek MS. Clinical Characteristics and Treatment Outcomes of Definitive versus Standard Anti-Tuberculosis Therapy in Patients with Tuberculous Lymphadenitis. Journal of Clinical Medicine. 2019; 8(6):813. https://doi.org/10.3390/jcm8060813
Chicago/Turabian StyleKo, Yousang, Changwhan Kim, Yong Bum Park, Eun-Kyung Mo, Jin-Wook Moon, Sunghoon Park, Yun Su Sim, Ji Young Hong, and Moon Seong Baek. 2019. "Clinical Characteristics and Treatment Outcomes of Definitive versus Standard Anti-Tuberculosis Therapy in Patients with Tuberculous Lymphadenitis" Journal of Clinical Medicine 8, no. 6: 813. https://doi.org/10.3390/jcm8060813
APA StyleKo, Y., Kim, C., Park, Y. B., Mo, E.-K., Moon, J.-W., Park, S., Sim, Y. S., Hong, J. Y., & Baek, M. S. (2019). Clinical Characteristics and Treatment Outcomes of Definitive versus Standard Anti-Tuberculosis Therapy in Patients with Tuberculous Lymphadenitis. Journal of Clinical Medicine, 8(6), 813. https://doi.org/10.3390/jcm8060813





