Isoniazid Concentration and NAT2 Genotype Predict Risk of Systemic Drug Reactions during 3HP for LTBI
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Protocol
2.4. Outcome
2.5. Statistical Analysis
3. Results
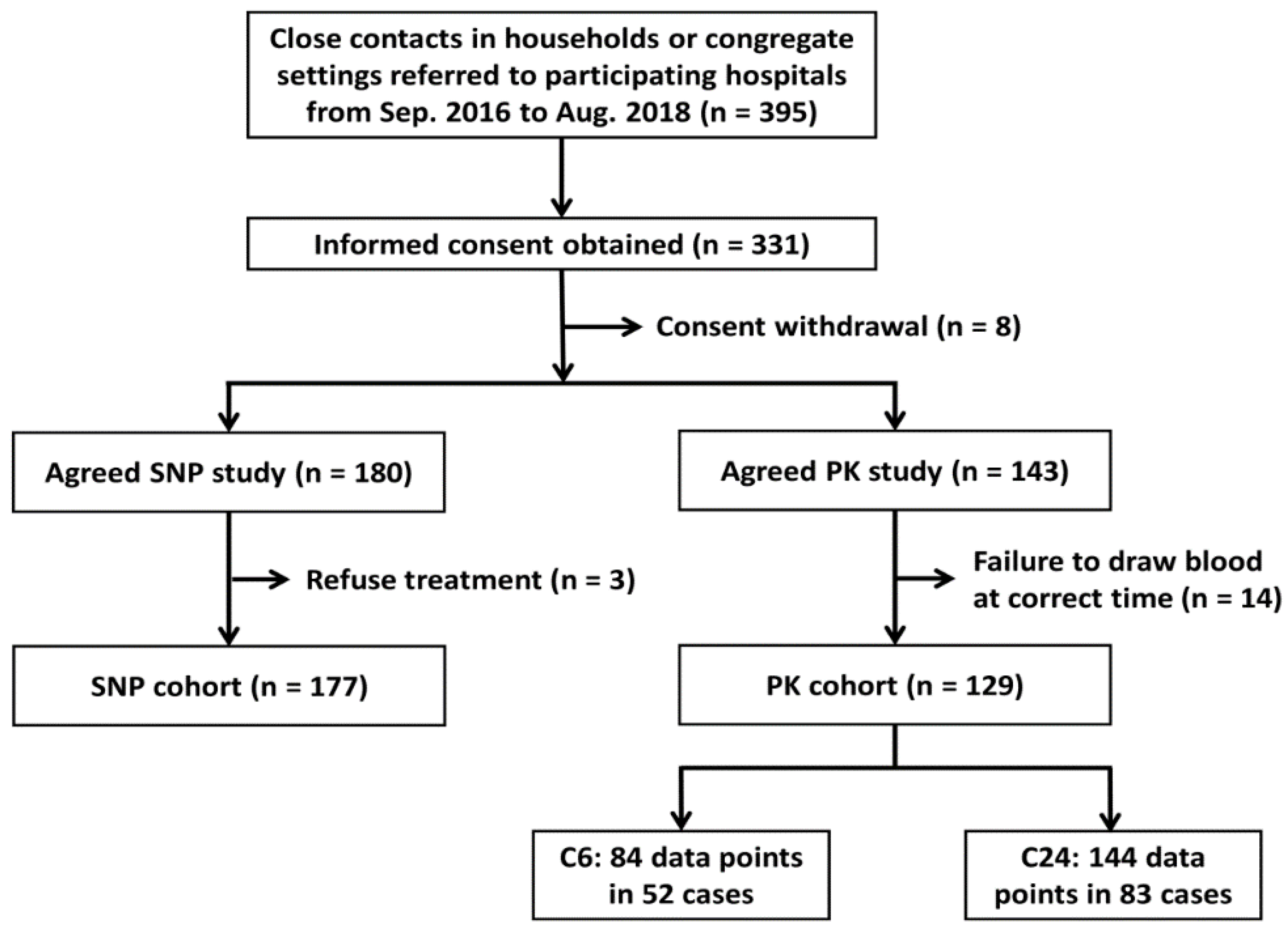
3.1. Selection of Study Participants
3.2. Clinical Characteristics of the SNP Cohort
3.3. Association of SNPs with SDRs in the SNP Cohort
3.4. Clinical Characteristics of the PK Cohort
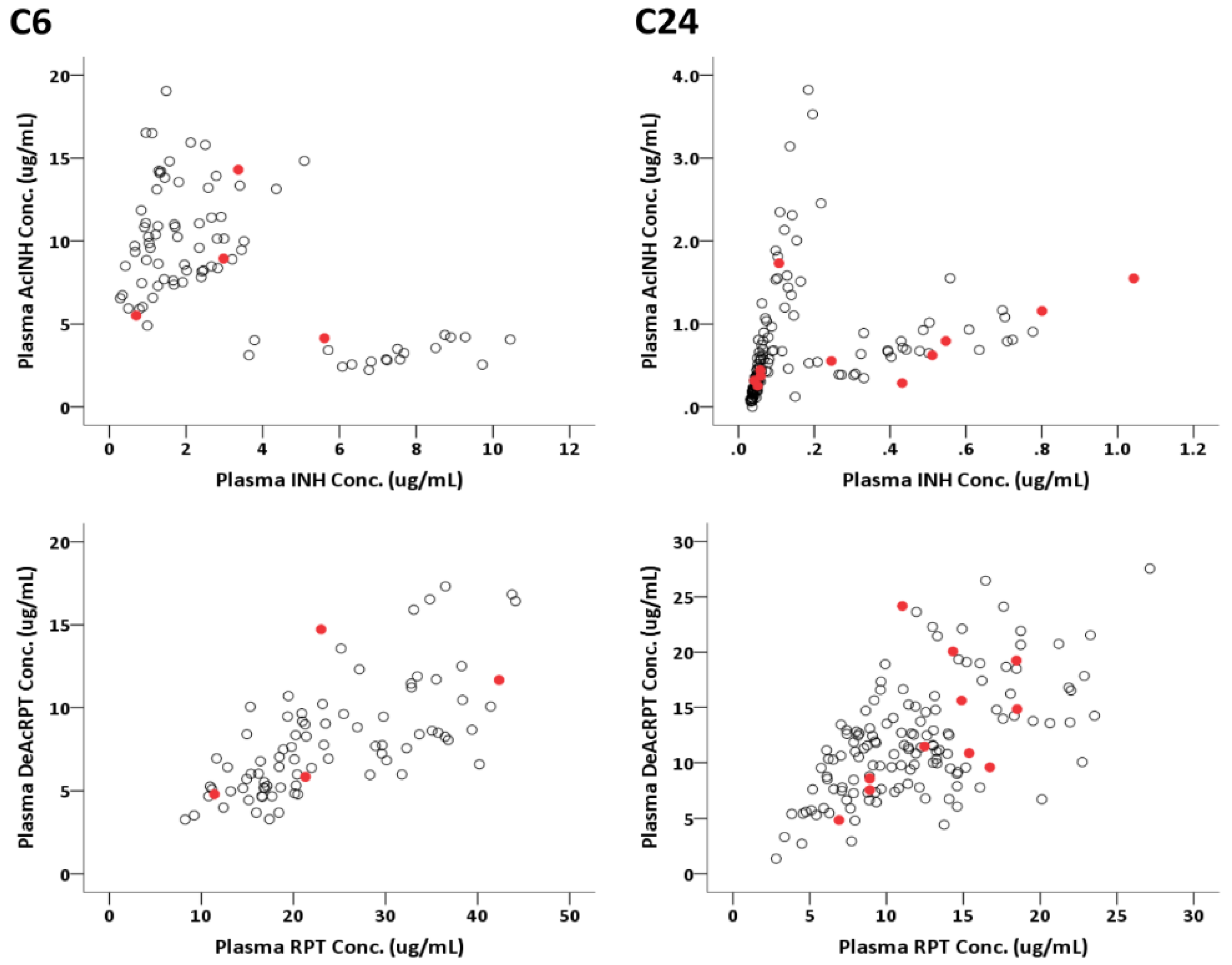
3.5. Pharmacodynamical Results of the PK Cohort
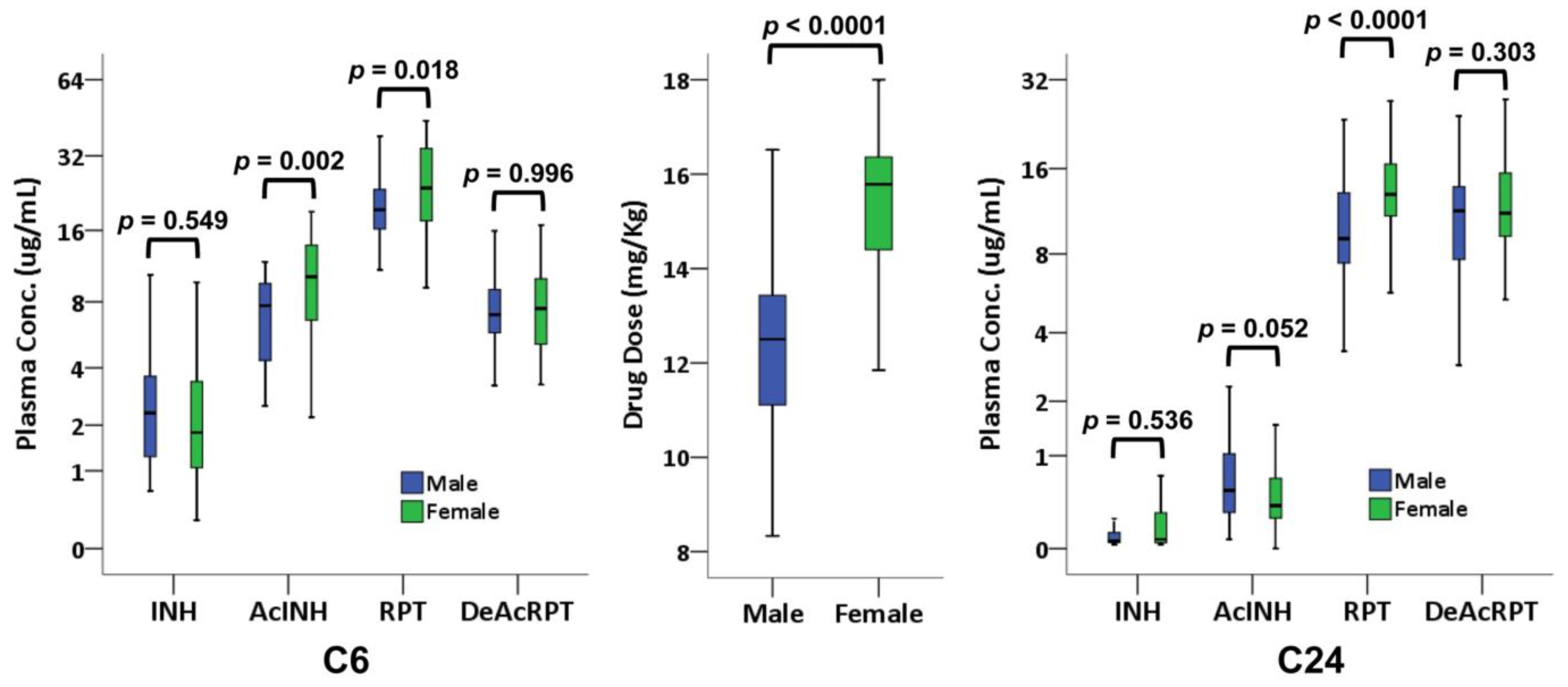
3.6. Sex-Based Discrepancy in PK Results
3.7. GEE Model in the PK Cohort
3.8. Time-Dependent Cox Proportional Hazard Model in the PK Cohort
3.9. Validation for Predicting SDR Using SNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Kasambira, T.S.; Shah, M.; Adrian, P.V.; Holshouser, M.; Madhi, S.A.; Chaisson, R.E.; Martinson, N.A.; Dorman, S.E. QuantiFERON-TB Gold In-Tube for the detection of Mycobacterium tuberculosis infection in children with household tuberculosis contact. Int. J. Tuberc. Lung Dis. 2011, 15, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Comstock, G.W.; Livesay, V.T.; Woolpert, S.F. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am. J. Epidemiol. 1974, 99, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- LoBue, P.A.; Mermin, J.H. Latent tuberculosis infection: The final frontier of tuberculosis elimination in the USA. Lancet Infect. Dis. 2017, 17, e327–e333. [Google Scholar] [CrossRef]
- Churchyard, G.J.; Swindells, S. Controlling latent TB tuberculosis infection in high-burden countries: A neglected strategy to end TB. PLoS Med. 2019, 16, e1002787. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.N.; Metzger, B.S.; Tapia, J.R.; Blumberg, H.M. A tuberculin screening and isoniazid preventive therapy program in an inner-city population. Am. J. Respir. Crit. Care Med. 1999, 159, 295–300. [Google Scholar] [CrossRef]
- Alsdurf, H.; Hill, P.C.; Matteelli, A.; Getahun, H.; Menzies, D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 1269–1278. [Google Scholar] [CrossRef]
- Sterling, T.R.; Villarino, M.E.; Borisov, A.S.; Shang, N.; Gordin, F.; Bliven-Sizemore, E.; Hackman, J.; Hamilton, C.D.; Menzies, D.; Kerrigan, A.; et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N. Engl. J. Med. 2011, 365, 2155–2166. [Google Scholar] [CrossRef]
- Villarino, M.E.; Scott, N.A.; Weis, S.E.; Weiner, M.; Conde, M.B.; Jones, B.; Nachman, S.; Oliveira, R.; Moro, R.N.; Shang, N.; et al. Treatment for preventing tuberculosis in children and adolescents: A randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015, 169, 247–255. [Google Scholar] [CrossRef]
- Sun, H.Y.; Huang, Y.W.; Huang, W.C.; Chang, L.Y.; Chan, P.C.; Chuang, Y.C.; Ruan, S.Y.; Wang, J.Y.; Wang, J.T. Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: A multicentre randomised controlled trial in Taiwan. Tuberculosis 2018, 111, 121–126. [Google Scholar] [CrossRef]
- World Health Organization. Latent TB Infection: Updated and Consolidated Guidelines for Programmatic Management. 2018. Available online: https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/ (accessed on 5 June 2019).
- Sterling, T.R.; Moro, R.N.; Borisov, A.S.; Phillips, E.; Shepherd, G.; Adkinson, N.F.; Weis, S.; Ho, C.; Villarino, M.E.; Tuberculosis Trials Consortium. Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT tuberculosis study. Clin. Infect. Dis. 2015, 61, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.; Bock, N.; Peloquin, C.A.; Burman, W.J.; Khan, A.; Vernon, A.; Zhao, Z.; Weis, S.; Sterling, T.R.; Hayden, K.; et al. Pharmacokinetics of rifapentine at 600, 900, and 1,200 mg during once-weekly tuberculosis therapy. Am. J. Respir. Crit. Care Med. 2004, 169, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.; Savic, R.M.; Kenzie, W.R.; Wing, D.; Peloquin, C.A.; Engle, M.; Bliven, E.; Prihoda, T.J.; Gelfond, J.A.; Scott, N.A.; et al. Rifapentine pharmacokinetics and tolerability in children and adults treated once weekly with rifapentine and isoniazid for latent tuberculosis infection. J. Pediatric Infect. Dis. Soc. 2014, 3, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0; NCI, NIH, DHHS: Washington, DC, USA, 2009.
- Borisov, A.S.; Bamrah Morris, S.; Njie, G.J.; Winston, C.A.; Burton, D.; Goldberg, S.; Yelk Woodruff, R.; Allen, L.; LoBue, P.; Vernon, A. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent mycobacterium tuberculosis Infection. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Singapore Tuberculosis Service/British Medical Reseach Council. Controlled trial of intermittent regimens of rifampicin plus isoniazid for pulmonary tuberculosis in Singapore. Lancet 1975, 2, 1105–1109. [Google Scholar]
- Hong Kong Tuberculosis Treatment Services/Brompton Hospital/British Medical Research Council; Reseach Council. A controlled trial of daily and intermittent rifampicin plus ethambutol in the retreatment of patients with pulmonary tuberculosis: Results up to 30 months. Tubercle 1975, 56, 179–189. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Mitchison, D.A.; Lee, S.K.; Ong, Y.Y.; O’Mahoney, M.G.; Girling, D.J.; Nunn, A.J. Serum rifampicin concentration related to dose size and to the incidence of the ‘flu’ syndrome during intermittent rifampicin administration. J. Antimicrob. Chemother. 1977, 3, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Jindani, A.; Harrison, T.S.; Nunn, A.J.; Phillips, P.P.; Churchyard, G.J.; Charalambous, S.; Hatherill, M.; Geldenhuys, H.; McIlleron, H.M.; Zvada, S.P.; et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N. Engl. J. Med. 2014, 371, 1599–1608. [Google Scholar] [CrossRef]
- Dorman, S.E.; Savic, R.M.; Goldberg, S.; Stout, J.E.; Schluger, N.; Muzanyi, G.; Johnson, J.L.; Nahid, P.; Hecker, E.J.; Heilig, C.M.; et al. Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial. Am. J. Respir. Crit. Care Med. 2015, 191, 333–343. [Google Scholar] [CrossRef]
- Dutt, A.K.; Moers, D.; Stead, W.W. Undesirable side effects of isoniazid and rifampin in largely twice-weekly short-course chemotherapy for tuberculosis. Am. Rev. Respir. Dis. 1983, 128, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Eule, H.; Werner, E.; Winsel, K.; Iwainsky, H. Intermittent chemotherapy of pulmonary tuberculosis using rifampicin and isoniazid for primary treatment: The influence of various factors on the frequency of side-effects. Tubercle 1974, 55, 81–89. [Google Scholar] [CrossRef]
- Metcalfe, C.; Macdonald, I.K.; Murphy, E.J.; Brown, K.A.; Raven, E.L.; Moody, P.C. The tuberculosis prodrug isoniazid bound to activating peroxidases. J. Biol. Chem. 2008, 283, 6193–6200. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.M.; George, J.M.; Pau, A.K.; Rupert, A.; Mehaffy, C.; De, P.; Dobos, K.M.; Kellogg, A.; McLaughlin, M.; McManus, M.; et al. Cytokine-mediated systemic adverse drug reactions in a drug-drug interaction study of dolutegravir with once-weekly isoniazid and rifapentine. Clin. Infect. Dis. 2018, 67, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Gupta, D.; Birbian, N.; Singh, J. Association of NAT2, GST and CYP2E1 polymorphisms and anti-tuberculosis drug-induced hepatotoxicity. Tuberculosis 2014, 94, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Fukami, T.; Kobayashi, Y.; Watanabe, A.; Nakajima, M.; Yokoi, T. Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: Rifampicin, rifabutin, and rifapentine. Biochem. Pharmacol. 2011, 82, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Ohno, M.; Kubota, R.; Yokota, S.; Nagai, T.; Tsuyuguchi, K.; Okuda, Y.; Takashima, T.; Kamimura, S.; Fujio, Y.; et al. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: A randomized controlled trial for pharmacogenetics-based therapy. Eur. J. Clin. Pharmacol. 2013, 69, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Adjobimey, M.; Ruslami, R.; Trajman, A.; Sow, O.; Kim, H.; Obeng Baah, J.; Marks, G.B.; Long, R.; Hoeppner, V.; et al. Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. N. Engl. J. Med. 2018, 379, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Swindells, S.; Ramchandani, R.; Gupta, A.; Benson, C.A.; Leon-Cruz, J.; Mwelase, N.; Jean Juste, M.A.; Lama, J.R.; Valencia, J.; Omoz-Oarhe, A. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N. Engl. J. Med. 2019, 380, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Liu, C.H.; Hu, F.C.; Chang, H.C.; Liu, J.L.; Chen, J.M.; Yu, C.J.; Lee, L.N.; Kao, J.H.; Yang, P.C. Risk factors of hepatitis during anti-tuberculous treatment and implications of hepatitis virus load. J. Infect. 2011, 62, 448–455. [Google Scholar] [CrossRef] [PubMed]

| SNP Group (n = 177) | SDR (n = 14) | No SDR (n = 163) | p Value | PK Group (n = 129) | SDR (n = 13) | No SDR (n = 116) | p Value | |
|---|---|---|---|---|---|---|---|---|
| Age (year) | 37.1 ± 17.8 | 46.6 ± 14.5 | 36.3 ± 17.9 | 0.038 | 48.8 ± 17.2 | 51.6 ± 12.7 | 48.5 ± 17.6 | 0.533 |
| ≤35 | 94 (53%) | 2 (14%) | 92 (56%) | 0.002 | 30 (23%) | 2 (15%) | 28 (24%) | 0.868 |
| 35–55 | 44 (25%) | 8 (57%) | 36 (22%) | 46 (36%) | 5 (38%) | 41 (35%) | ||
| >55 | 39 (22%) | 4 (29%) | 35 (21%) | 53 (41%) | 6 (46%) | 47 (41%) | ||
| Female sex | 83 (47%) | 6 (43%) | 77 (47%) | 0.753 | 67 (52%) | 6 (46%) | 61 (53%) | 0.660 |
| Household contact | 38 (21%) | 2 (13%) | 36 (22%) | 0.737 | 53 (41%) | 5 (38%) | 48 (41%) | >0.999 |
| Height (cm) | 165.8 ± 8.3 | 165.1 ± 8.8 | 165.9 ± 8.2 | 0.729 | 164.3 ± 9.0 | 163.7 ± 7.5 | 164.4 ± 9.1 | 0.788 |
| Weight (kg) | 64.0 ± 11.9 | 65.7 ± 12.0 | 63.8 ± 10.9 | 0.568 | 65.5 ± 12.1 | 63.1 ± 9.4 | 65.8 ± 12.4 | 0.444 |
| Body-mass index (kg/m2) | 23.2 ± 3.52 | 24.1 ± 3.22 | 23.1 ± 3.55 | 0.334 | 24.2 ± 3.36 | 23.45 ± 2.23 | 24.26 ± 3.46 | 0.413 |
| Current smoker | 20 (11%) | 4 (29%) | 16 (80%) | 0.057 | 28 (22%) | 5 (38%) | 23 (20%) | 0.154 |
| eGFR (mL/min/1.73 m2) | 0.005 | 0.670 | ||||||
| <60 | 14 (8%) | 0 | 14 (9%) | 6 (5%) | 1 (8%) | 5 (4%) | ||
| 60–90 | 54 (31%) | 10 (71%) | 44 (27%) | 47 (36%) | 4 (31%) | 43 (37%) | ||
| ≥90 | 109 (62%) | 4 (29%) | 105 (66%) | 77 (59%) | 8 (62%) | 68 (59%) | ||
| Comorbidity | ||||||||
| HBV infection | 3 (2%) | 0 | 3 (2%) | >0.999 | 7 (5%) | 1 (8%) | 6 (5%) | 0.534 |
| HCV infection | 2 (1%) | 0 | 2 (1%) | >0.999 | 3 (2%) | 0 | 3 (3%) | >0.999 |
| Diabetes mellitus | 3 (2%) | 0 | 3 (2%) | >0.999 | 11 (9%) | 2 (15%) | 9 (8%) | 0.306 |
| Malignancy | 1 (1%) | 1 (7%) | 0 | 0.079 | 6 (5%) | 2 (15%) | 4 (3%) | 0.112 |
| Autoimmune | 1 (1%) | 0 | 1 (1%) | >0.999 | 1 (1%) | 1 (8%) | 0 | 0.100 |
| Asthma | 0 | 0 | 0 | 1 (1%) | 0 | 1 (1%) | >0.999 | |
| Hypertension | 5 (3%) | 2 (14%) | 3 (2%) | 0.051 | 25 (19%) | 5 (38%) | 20 (17%) | 0.130 |
| Anti-hypertensive medication | 5 (3%) | 2 (14%) | 3 (2%) | 0.051 | 19 (15%) | 4 (31%) | 15 (13%) | 0.101 |
| Isoniazid dose (mg/kg) | 14.2 ± 2.1 | 13.8 ± 2.0 | 14.3 ± 2.1 | 0.483 | 14.0 ± 2.2 | 14.3 ± 1.9 | 13.9 ± 2.2 | 0.512 |
| Rifapentine dose (mg/kg) | 14.2 ± 2.1 | 13.8 ± 2.0 | 14.3 ± 2.1 | 0.454 | 14.0 ± 2.2 | 14.3 ± 1.9 | 13.9 ± 2.2 | 0.512 |
| Hemoglobin (g/dL) | 14.0 ± 1.6 | 14.2 ± 1.5 | 14.0 ± 1.6 | 0.643 | 14.0 ± 1.5 | 13.8 ± 1.6 | 14.1 ± 1.5 | 0.560 |
| Leukocyte (K/µL) | 6.44 ± 1.77 | 6.78 ± 1.42 | 6.41 ± 1.80 | 0.448 | 6.81 ± 1.85 | 6.98 ± 1.44 | 6.78 ± 1.90 | 0.732 |
| Platelet (K/µL) | 258 ± 56 | 253 ± 57 | 259 ± 56 | 0.705 | 270 ± 58 | 280 ± 45 | 269 ± 59 | 0.511 |
| AST (U/L) | 23.4 ± 17.0 | 28.0 ± 19.6 | 23.0 ± 16.8 | 0.291 | 23.3 ± 10.0 | 25.5 ± 5.6 | 23.0 ± 10.4 | 0.201 |
| ALT (U/L) | 23.0 ± 28.0 | 27.6 ± 30.8 | 22.6 ± 27.9 | 0.526 | 23.7 ± 18.9 | 27.2 ± 11.1 | 23.3 ± 19.6 | 0.290 |
| Total bilirubin (mg/dL) | 0.65 ± 0.28 | 0.63 ± 0.38 | 0.66 ± 0.27 | 0.823 | 0.63 ± 0.22 | 0.70 ± 0.25 | 0.62 ± 0.22 | 0.215 |
| Creatinine (mg/dL) | 0.82 ± 0.20 | 0.83 ± 0.16 | 0.82 ± 0.20 | 0.754 | 0.84 ± 0.29 | 0.83 ± 0.18 | 0.84 ± 0.30 | 0.876 |
| Treatment completion | 159 (90%) | 4 (29%) | 155 (95%) | <0.0001 | 107 (83%) | 4 (31%) | 103 (89%) | <0.0001 |
| Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) * | p Value | ||
|---|---|---|---|---|---|
| Additive model | |||||
| NAT2 rs1041983 | CC | Ref | Ref | ||
| CT | 0.85 (0.14–5.29) | 0.101 | 0.87 (0.14–5.46) | 0.132 | |
| TT | 7.67 (1.51–39.0) | 0.0006 | 5.82 (1.08–35.1) | 0.003 | |
| CYP2E1 rs2070673 | TT | Ref | Ref | ||
| TA | 0.84 (0.20–3.52) | 0.815 | 0.89 (0.21–3.80) | 0.871 | |
| AA | 3.21 (0.79–15.0) | 0.103 | 3.28 (0.78–13.9) | 0.106 | |
| Dominant model | |||||
| NAT2 rs1041983 | CC | Ref | Ref | ||
| CT+TT | 2.41 (0.51–11.3) | 0.265 | 2.01 (0.41–9.96) | 0.394 | |
| CYP2E1 rs2070673 | TT | Ref | Ref | ||
| TA+AA | 1.43 (0.42–4.84) | 0.568 | 1.49 (0.43–5.20) | 0.532 | |
| Recessive model | |||||
| NAT2 rs1041983 | CC+CT | Ref | Ref | ||
| TT | 8.47 (2.55–28.1) | 0.0005 | 7.00 (2.03–24.1) | 0.002 | |
| CYP2E1 rs2070673 | TT+TA | Ref | Ref | ||
| AA | 3.51 (1.05–11.7) | 0.041 | 3.50 (1.02–12.0) | 0.047 |
| Age/Sex | BW (kg)/BH (cm) | Adverse Reactions | Severity (Grade) | Comorbidity & Medication | Risk Allele in NAT2/CYP2E1 * | INH/RPT Conc. # [Sampling Week] | Onset (Week) | Time of Onset/Duration (h) | Outcome of 3HP |
|---|---|---|---|---|---|---|---|---|---|
| 66.3/F | 50.0/149 | fever, chills, malaise, myalgia, headache | 2 | Lung adenocarcinoma under gefitinib | 1/2 | C24: 0.11/18.5 [3] | 3 | 7/29 | Stop |
| 64.4/M | 62.5/170 | fever, myalgia, chills, weakness, sweating | 2 | HTN under amlodipine & olmesartan | 2/1 | C6: 5.61/21.3 [4] | 3 | 6/18 | Stop |
| 59.8/F | 59.5/154 | fever, chills, dyspnea, angioedema, malaise | 3 | Breast cancer, cured | 2/2 | C24: 0.51/16.7 [3] | 3 | 4/>100 | Stop |
| 56.7/M | 73.0/175 | shock (BP 90/60 mmHg), fever, flush, myalgia, dyspnea, rash | 3 | HTN under lercanidipine | 2/2 | C24: 1.04/8.9 [3] | 3 | 5/47 | Stop |
| 53.4/F | 63.0/160 | fever, chills, dizziness, myalgia, dizziness | 2 | Nil | 1/1 | C6: 2.98/11.4 [4] | 3 | 9/15 | Stop |
| 51.4/M | 74.0/167 | shock (BP 85/67 mmHg), dizziness, vomiting | 2 | DM, HTN | 2/1 | C24: 0.80/8.9 [3] | 3 | 3/47 | Stop |
| 50.5/M | 72.0/168 | shock (BP 88/63 mmHg), fever, nausea, vomiting, dizziness, sweating | 2 | HTN under bisoprolol & olmesartan | 2/0 | C24: 0.25/6.9 [7] | 7 | 1/8 | Stop |
| 33.9/F | 47.0/158 | shock (BP 82/57 mmHg), fever, headache, nausea, vomiting, malaise | 3 | Nil | 2/0 | C24: 0.43/15.4 [3] | 3 | 1/88 | Stop |
| 20.6/F | 53.0/162 | shock, fever, chills, headache, myalgia, nausea | 3 | Nil | 1/1 | C6: 3.36/42.3 [4] C24: 0.06/18.4 [4] | 3 | 2/30 | Stop |
| 60.9/M | 62.0/161 | fever, myalgia, nausea, vomiting dizziness | 2 | DM, HTN amlodipine & valsartan | 0/1 | C24: 0.06/11.0 [3] | 3 | 6/28 | Complete |
| 55.9/F | 60.0/163 | fever, chills, myalgia, malaise, headache | 3 | AS under celecoxib | 0/0 | C24: 0.04/14.3 [4] | 3 | 5/76 | Complete |
| 53.7/M | 76.5/174 | fever, chills, dizziness, malaise, nausea | 2 | HBV carrier not Tx | 2/1 | C24: 0.55/14.9 [6] | 6 | 3/16 | Complete |
| 43.8/M | 67.5/167 | fever, myalgia, dizziness, tachypnea, malaise | 2 | Nil | 1/0 | C6: 0.70/23.0 [4] | 4 | 1/27 | Complete |
| Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) * | p Value | ||
|---|---|---|---|---|---|
| Additive model | |||||
| NAT2 rs1041983 | CC | Ref | Ref | ||
| CT | 1.09 (0.19–6.28) | 0.925 | 1.06 (0.18–6.34) | 0.948 | |
| TT | 4.52 (0.86–23.8) | 0.075 | 4.61 (0.82–25.8) | 0.082 | |
| CYP2E1 rs2070673 | TT | Ref | Ref | ||
| TA | 1.84 (0.49–6.94) | 0.807 | 2.10 (0.54–8.20) | 0.285 | |
| AA | 2.53 (0.51–12.5) | 0.383 | 2.80 (0.55–14.3) | 0.216 | |
| Dominant model | |||||
| NAT2 rs1041983 | CC | Ref | Ref | ||
| CT+TT | 2.13 (0.45–10.2) | 0.343 | 2.04 (0.41–10.1) | 0.384 | |
| CYP2E1 rs2070673 | TT | Ref | Ref | ||
| TA+AA | 2.03 (0.59–6.96) | 0.262 | 2.30 (0.65–8.15) | 0.199 | |
| Recessive model | |||||
| NAT2 rs1041983 | CC+CT | Ref | Ref | ||
| TT | 4.23 (1.30–13.8) | 0.017 | 4.43 (1.30–15.1) | 0.017 | |
| CYP2E1 rs2070673 | TT+TA | Ref | Ref | ||
| AA | 1.84 (0.46–7.41) | 0.392 | 1.90 (0.46–7.80) | 0.375 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-R.; Huang, H.-L.; Lin, S.-W.; Cheng, M.-H.; Lin, Y.-T.; Chang, S.-Y.; Yan, B.-S.; Kuo, C.-H.; Lu, P.-L.; Wang, J.-Y.; et al. Isoniazid Concentration and NAT2 Genotype Predict Risk of Systemic Drug Reactions during 3HP for LTBI. J. Clin. Med. 2019, 8, 812. https://doi.org/10.3390/jcm8060812
Lee M-R, Huang H-L, Lin S-W, Cheng M-H, Lin Y-T, Chang S-Y, Yan B-S, Kuo C-H, Lu P-L, Wang J-Y, et al. Isoniazid Concentration and NAT2 Genotype Predict Risk of Systemic Drug Reactions during 3HP for LTBI. Journal of Clinical Medicine. 2019; 8(6):812. https://doi.org/10.3390/jcm8060812
Chicago/Turabian StyleLee, Meng-Rui, Hung-Ling Huang, Shu-Wen Lin, Meng-Hsuan Cheng, Ya-Ting Lin, So-Yi Chang, Bo-Shiun Yan, Ching-Hua Kuo, Po-Liang Lu, Jann-Yuan Wang, and et al. 2019. "Isoniazid Concentration and NAT2 Genotype Predict Risk of Systemic Drug Reactions during 3HP for LTBI" Journal of Clinical Medicine 8, no. 6: 812. https://doi.org/10.3390/jcm8060812
APA StyleLee, M.-R., Huang, H.-L., Lin, S.-W., Cheng, M.-H., Lin, Y.-T., Chang, S.-Y., Yan, B.-S., Kuo, C.-H., Lu, P.-L., Wang, J.-Y., & Chong, I.-W. (2019). Isoniazid Concentration and NAT2 Genotype Predict Risk of Systemic Drug Reactions during 3HP for LTBI. Journal of Clinical Medicine, 8(6), 812. https://doi.org/10.3390/jcm8060812






