Usefulness of the Thrombotic Microangiopathy Score as a Promising Prognostic Marker of Septic Shock for Patients in the Emergency Department
Abstract
1. Introduction
2. Experimental Section
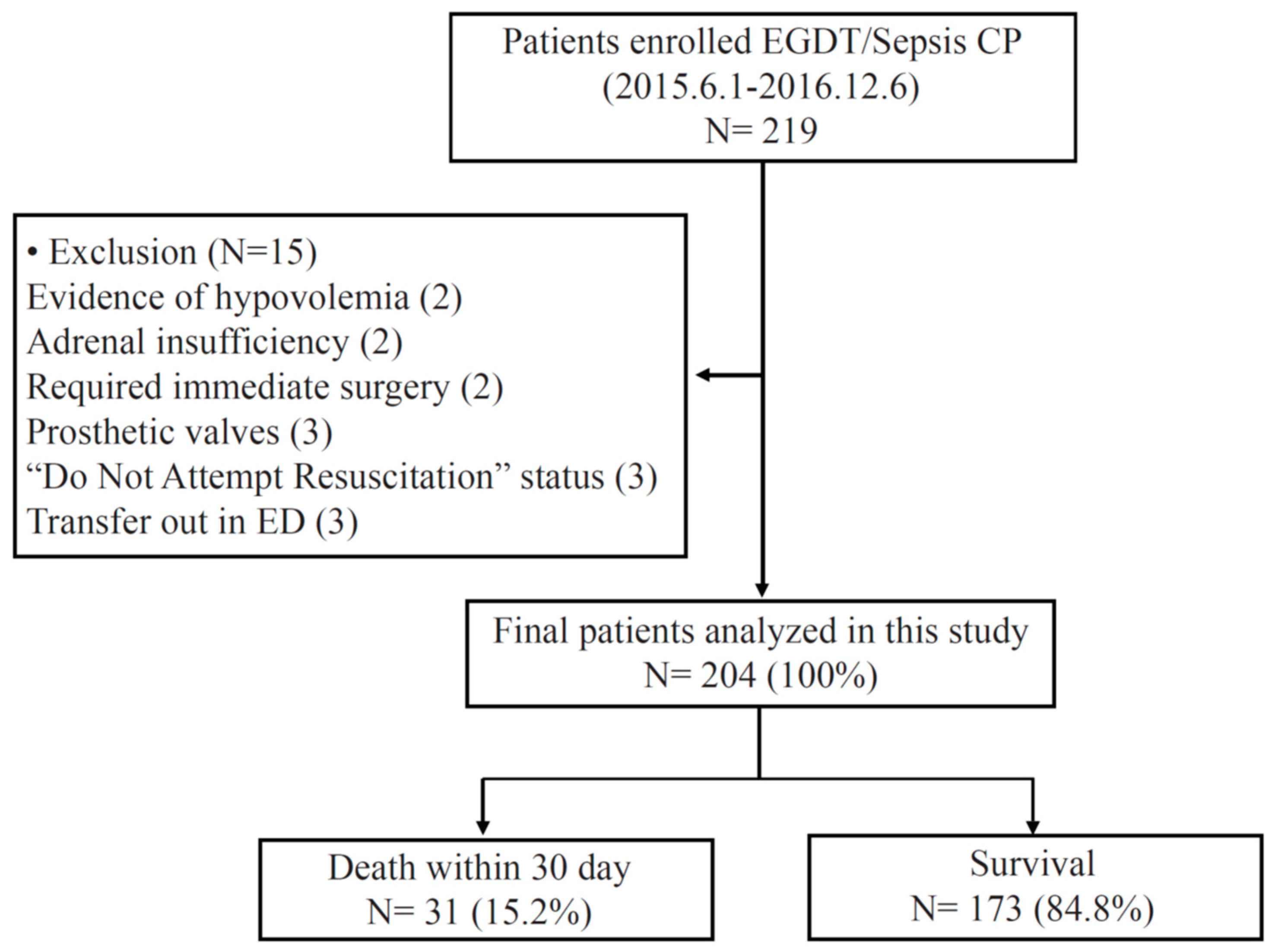
2.1. Study Population
2.2. Data Collection
2.3. Assessment of TMA Score
2.4. Clinical Endpoints
2.5. Statistical Analysis
3. Results
3.1. Study Population, Clinical Evaluation, and Treatment
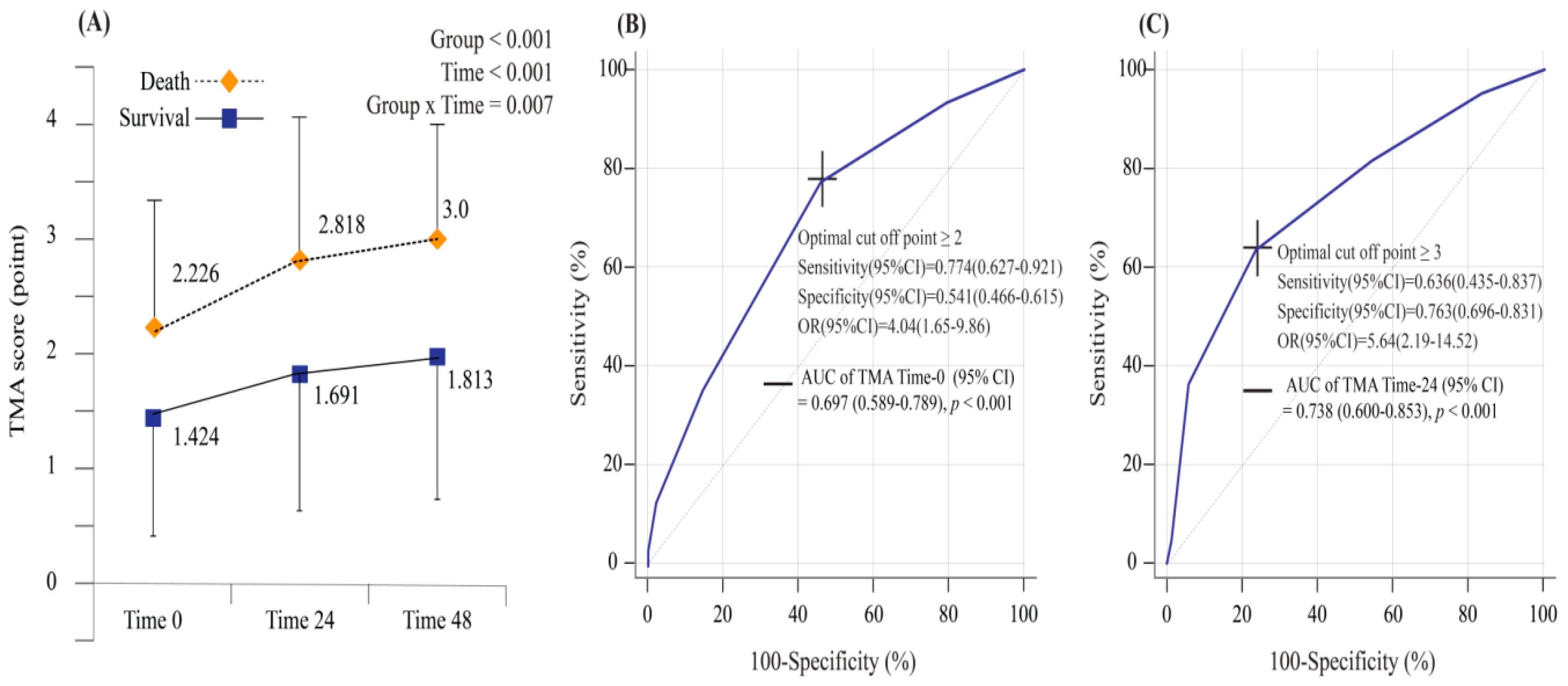
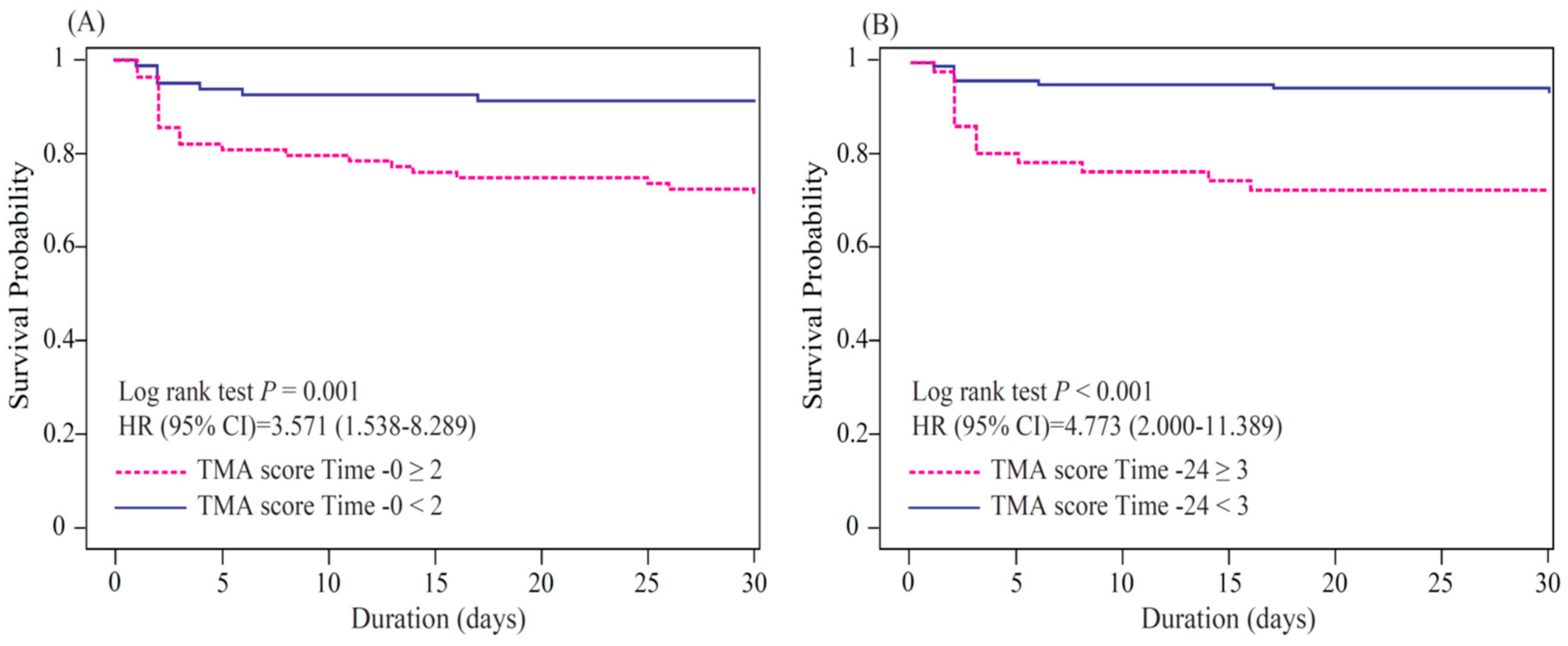
3.2. TMA Score as a Predictor of 30-Day Mortality for Sepsis
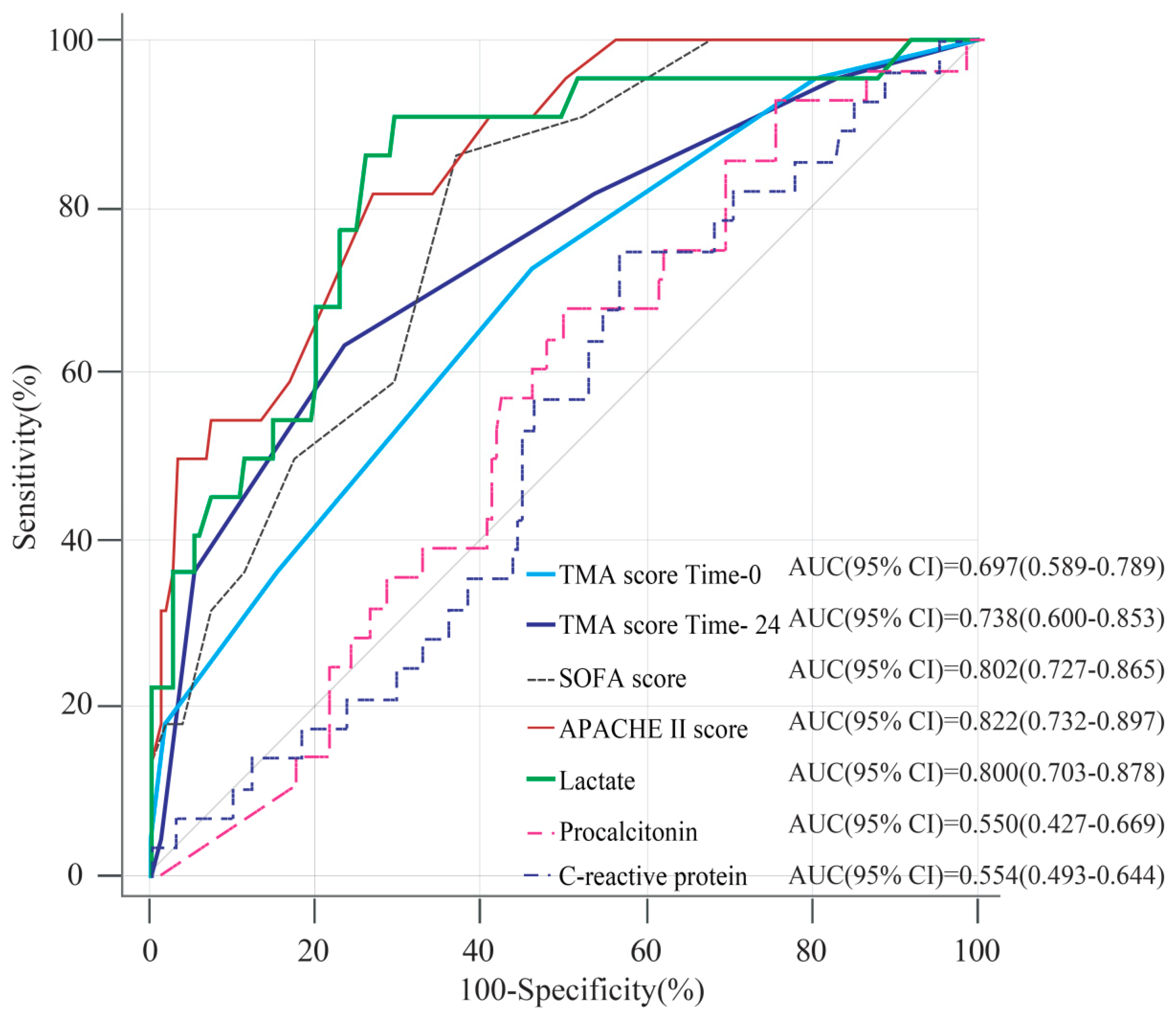
3.3. Comparison of the TMA Score and Conventional Clinical Markers as Predictors of Mortality for Patients with Sepsis
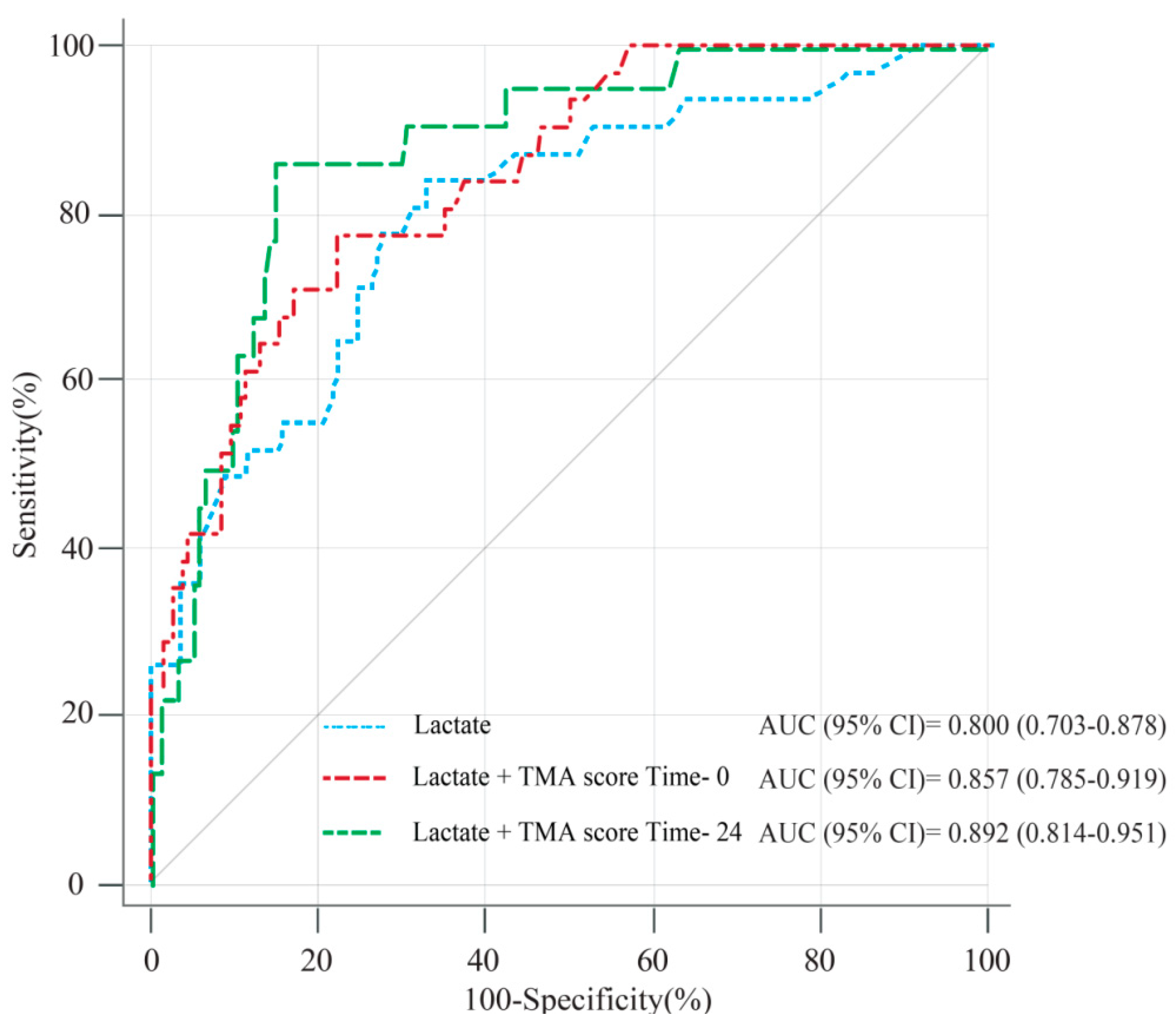
3.4. Prognostic Value of the TMA Score in Combination with Lactate on ED Admission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ Clin. Res. 2019, 364, k4891. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Angus, D.C. Enhancing recovery from sepsis: A review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, M.; Kim, Y.J.; Ryoo, S.M.; Sohn, C.H.; Ahn, S.; Kim, W.Y. Troponin testing for assessing sepsis-induced myocardial dysfunction in patients with septic shock. J. Clin. Med. 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Venkatesh, B.; Finfer, S. Sepsis and septic shock: Current approaches to management. Int. Med. J. 2019, 49, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Goag, E.K.; Lee, J.W.; Roh, Y.H.; Leem, A.Y.; Kim, S.Y.; Song, J.H.; Kim, E.Y.; Jung, J.Y.; Park, M.S.; Kim, Y.S. A simplified mortality score using delta neutrophil index and the thrombotic microangiopathy score for prognostication in critically ill patients. Shock Inj. Inflamm. Sepsis Lab. Clin. Approaches 2018, 49, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, Y.S.; Yoon, C.Y.; Lee, H.S.; Kim, S.; Lee, J.W.; Kong, T.; You, J.S.; Park, J.W.; Chung, S.P. Delta neutrophil index for the prediction of the development of sepsis-induced acute kidney injury in the emergency department. Shock 2019. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Prowle, J.R. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 2018, 14, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Tverring, J.; Vaara, S.T.; Fisher, J.; Poukkanen, M.; Pettila, V.; Linder, A. Heparin-binding protein (hbp) improves prediction of sepsis-related acute kidney injury. Ann. Intensive Care 2017, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Lee, J.; Roh, K.H.; Kim, H.O.; Song, J.W.; Choi, J.R.; Kim, Y.K.; Lee, K.A. Rapid identification of thrombocytopenia-associated multiple organ failure using red blood cell parameters and a volume/hemoglobin concentration cytogram. Yonsei Med. J. 2011, 52, 845–850. [Google Scholar] [CrossRef]
- Schapkaitz, E.; Mezgebe, M.H. The clinical significance of schistocytes: A prospective evaluation of the international council for standardization in hematology schistocyte guidelines. Turk. J. Haematol. 2017, 34, 59–63. [Google Scholar] [CrossRef]
- Saigo, K.; Jiang, M.; Tanaka, C.; Fujimoto, K.; Kobayashi, A.; Nozu, K.; Iijima, K.; Ryo, R.; Sugimoto, T.; Imoto, S. Usefulness of automatic detection of fragmented red cells using a hematology analyzer for diagnosis of thrombotic microangiopathy. Clin. Lab. Haematol. 2002, 24, 347–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moake, J.L. Thrombotic microangiopathies. N. Engl. J. Med. 2002, 347, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Labelle, C.A.; Kitchens, C.S. Disseminated intravascular coagulation: Treat the cause, not the lab values. Clevel. Clin. J. Med. 2005, 72, 377–378. [Google Scholar] [CrossRef]
- Gomez, H.; Kellum, J.A. Lactate in sepsis. JAMA 2015, 313, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the surviving sepsis campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Chung, S.P.; Park, Y.S.; Chung, H.S.; Lee, H.S.; Park, J.W.; Lee, J.W.; Hong, J.H.; You, J.S.; Park, I. Newly designed delta neutrophil index-to-serum albumin ratio prognosis of early mortality in severe sepsis. Am. J. Emerg. Med. 2015, 33, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Umbro, I.; Gentile, G.; Tinti, F.; Muiesan, P.; Mitterhofer, A.P. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. J. Infect. 2016, 72, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Gustot, T. Multiple organ failure in sepsis: Prognosis and role of systemic inflammatory response. Curr. Opin. Crit. Care 2011, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Benz, K.; Amann, K. Thrombotic microangiopathy: New insights. Curr. Opin. Nephrol. Hypertens. 2010, 19, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Zini, G.; d’Onofrio, G.; Briggs, C.; Erber, W.; Jou, J.M.; Lee, S.H.; McFadden, S.; Vives-Corrons, J.L.; Yutaka, N.; Lesesve, J.F. Icsh recommendations for identification, diagnostic value, and quantitation of schistocytes. Int. J. Lab. Hematol. 2012, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lesesve, J.F.; Martin, M.; Banasiak, C.; Andre-Kerneis, E.; Bardet, V.; Lusina, D.; Kharbach, A.; Genevieve, F.; Lecompte, T. Schistocytes in disseminated intravascular coagulation. Int. J. Lab. Hematol. 2014, 36, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Visudhiphan, S.; Piankijagum, A.; Sathayapraseart, P.; Mitrchai, N. Erythrocyte fragmentation in disseminated intravascular coagulation and other diseases. N. Engl. J. Med. 1983, 309, 113. [Google Scholar] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The effect of sepsis on the erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef] [PubMed]
- Langenfeld, J.E.; Livingston, D.H.; Machiedo, G.W. Red cell deformability is an early indicator of infection. Surgery 1991, 110, 398–403. [Google Scholar] [PubMed]
- April, M.D.; Donaldson, C.; Tannenbaum, L.I.; Moore, T.; Aguirre, J.; Pingree, A.; Lantry, J.H. Emergency department septic shock patient mortality with refractory hypotension vs hyperlactatemia: A retrospective cohort study. Am. J. Emerg. Med. 2017, 35, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Gotmaker, R.; Peake, S.L.; Forbes, A.; Bellomo, R. Mortality is greater in septic patients with hyperlactatemia than with refractory hypotension. Shock Inj. Inflamm. Sepsis Lab. Clin. Approaches 2017, 48, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.J.; Ryoo, S.M.; Sohn, C.H.; Ahn, S.; Seo, D.W.; Lim, K.S.; Kim, W.Y. Risk factors for same pathogen sepsis readmission following hospitalization for septic shock. J. Clin. Med. 2019, 8, 181. [Google Scholar] [CrossRef]
| Variables | Total | Survival | Death | p |
|---|---|---|---|---|
| N = 204 (100%) | N = 173 (84.8%) | N = 31 (15.2%) | ||
| Age (years) | 67 ± 15 | 66 ± 15 | 72 ± 11 | 0.016 * |
| Male sex (n (%)) | 91 (44.61) | 78 (45.09) | 13 (41.94) | 0.745 |
| BMI (kg/m2) | 21.47 ± 3.43 | 21.41 ± 3.36 | 21.82 ± 3.83 | 0.542 |
| SOFA score (points) | 7.61 ± 2.84 | 7.11 ± 2.53 | 10.39 ± 2.93 | <0.001 * |
| Initial Vital Sign | ||||
| Systolic blood pressure (mmHg) | 85.46 ± 24.03 | 86.73 ± 23.39 | 78.32 ± 26.61 | 0.073 |
| Diastolic blood pressure (mmHg) | 52.93 ± 15.10 | 53.83 ± 15.2 | 47.90 ± 13.72 | 0.044 * |
| Heart rate (bpm) | 104.46 ± 24.72 | 104.10 ± 25.70 | 106.45 ± 18.55 | 0.545 |
| Respiratory rate (bpm) | 18.59 ± 3.72 | 18.57 ± 3.82 | 18.71 ± 3.19 | 0.844 |
| Body temperature (°C) | 37.51 ± 3.03 | 37.83 ± 1.32 | 35.719 ± 6.94 | 0.102 |
| Comorbidity (n (%)) | ||||
| Hypertension | 121 (59.31) | 102 (58.96) | 19 (61.29) | 0.808 |
| Diabetes mellitus | 66 (32.35) | 52 (30.06) | 14 (45.16) | 0.098 |
| Cardiovascular disease | 33 (16.18) | 24 (13.87) | 9 (29.03) | 0.035 * |
| Heart failure | 7 (3.43) | 6 (3.47) | 1 (3.23) | >0.999 |
| Chronic kidney disease | 23 (11.27) | 21 (12.14) | 2 (6.45) | 0.54 |
| Liver disease | 23 (11.27) | 19 (10.98) | 4 (12.90) | 0.759 |
| Malignancy | 52 (25.49) | 43 (24.86) | 9 (29.03) | 0.623 |
| Treatment | ||||
| Admission to antibiotics time (hours) | 3.64 ± 4.54 | 3.81 ± 4.88 | 2.70 ± 1.44 | 0.016 * |
| Admission to vasopressor time (hours) | 2.63 ± 3.92 | 2.76 ± 4.20 | 1.92 ± 1.59 | 0.052 |
| Antibiotics administration <3 h (n (%)) | 135 (66.18) | 111 (64.16) | 24 (77.42) | 0.151 |
| Laboratory Data | ||||
| White blood cell count (103/μL) | 13.88 ± 9.25 | 14.16 ± 8.65 | 12.33 ± 12.15 | 0.429 |
| Hematocrit (%) | 35.88 ± 6.52 | 36.10 ± 6.04 | 34.62 ± 8.73 | 0.37 |
| Platelet count (103/μL) | 185 ± 106 | 188 ± 98 | 169 ± 144 | 0.486 |
| Neutrophil count (103/μL) | 12.40 ± 8.77 | 12.70 ± 8.20 | 10.72 ± 11.49 | 0.363 |
| Prothrombin time (INR) | 1.25 ± 0.39 | 1.23 ± 0.40 | 1.37 ± 0.34 | 0.069 |
| Creatinine (mg/dL) | 2.07 ± 1.65 | 1.99 ± 1.62 | 2.54 ± 1.78 | 0.088 |
| C-reactive protein (mg/L) | 160 ± 117 | 158 ± 116 | 175 ± 121 | 0.459 |
| Procalcitonin (ng/mL) | 28.60 ± 36.26 | 28.25 ± 36.71 | 30.67 ± 34.08 | 0.745 |
| Albumin (g/dL) | 3.14 ± 0.66 | 3.21 ± 0.61 | 2.73 ± 0.77 | <0.001 * |
| Lactate (mmol/L) | 3.53 ± 3.22 | 2.87 ± 2.23 | 7.14 ± 5.02 | <0.001 * |
| Total CO2 (mmol/L) | 17.45 ± 4.39 | 18.18 ± 3.86 | 13.23 ± 4.95 | <0.001 * |
| Bacteremia (n (%)) | 102 (50.00) | 83 (47.98) | 19 (61.29) | 0.172 |
| TMA score Time 0 (points) | 1.55 ± 1.08 | 1.42 ± 1.02 | 2.23 ± 1.15 | <0.001 * |
| TMA score Time 24 (points) | 1.83 ± 1.23 | 1.69 ± 1.16 | 2.82 ± 1.30 | <0.001 * |
| Variable | Enter Method | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| SOFA score (per 1 point) | 1.237 (1.012–1.513) | 0.038 * | 1.184(0.949–1.476) | 0.134 |
| History of cardiovascular disease | 2.730 (0.818–9.108) | 0.102 | 2.831(0.687–11.671) | 0.15 |
| Albumin (g/dL) | 0.515(0.225–1.179) | 0.116 | 0.484(0.198–1.182) | 0.111 |
| Total CO2 (per 1 mmol/L) | 0.898(0.767–1.052) | 0.184 | 0.918(0.762–1.106) | 0.368 |
| Lactate (per 1 mmol/L) | 1.247(1.039–1.497) | 0.018 * | 1.298(1.047–1.609) | 0.018 * |
| TMA score Time 0 (per 1 point) | 1.928(1.187–3.130) | 0.008 * | ||
| TMA score Time 24 (per 1 point) | 1.683 (0.996–2.844) | 0.052 | ||
| Variable | Stepwise Selection | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| SOFA score (per 1 point) | 1.264 (1.062–1.505) | 0.008 * | ||
| History of cardiovascular disease | ||||
| Albumin (g/dL) | 0.410 (0.178–0.945) | 0.036 * | ||
| Total CO2 (per 1 mmol/L) | ||||
| Lactate (per 1 mmol/L) | 1.338 (1.156–1.548) | <0.001 * | 1.430 (1.207–1.693) | <0.001 * |
| TMA score Time 0 (per 1 point) | 1.972 (1.253–3.106) | 0.003 * | ||
| TMA score Time 24 (per 1 point) | 1.863 (1.132–3.066) | 0.014 * | ||
| Variable | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR (95% CI) |
|---|---|---|---|---|---|
| TMA score 0 ≥ 2 | 77.4 (62.7–92.1) | 54.1 (46.6–61.5) | 23.3 (15.1–31.5) | 93.0 (88.0–98.0) | 4.035 (1.651–9.863) |
| TMA score 24 ≥ 3 | 63.6 (43.5–83.7) | 76.3 (69.6–83.1) | 28.0 (15.6–40.4) | 93.5 (89.2–97.9) | 5.639 (2.190–14.519) |
| Lactate ≥ 4.0 | 64.5 (47.7–81.4) | 75.4 (69.0–81.9) | 32.3 (20.6–43.9) | 92.1 (87.7–96.6) | 5.584 (2.474–12.603) |
| TMA score 0 ≥ 2 and Lactate ≥ 4.0 | 45.2 (27.6–62.7) | 88.8 (84.1–93.6) | 42.4 (25.6–59.3) | 89.9 (85.3–94.4) | 6.545 (2.788–15.362) |
| TMA score 24 ≥ 3 and Lactate ≥ 4.0 | 40.9 (20.4–61.5) | 92.7 (88.5–96.8) | 45.0 (23.2–66.8) | 91.4 (87.0–95.9) | 8.748 (3.066–24.960) |
| TMA score 0 ≥ 2 or Lactate ≥ 4.0 | 96.8 (90.6–100.0) | 41.2 (33.8–48.6) | 23.1 (15.8–30.3) | 98.6 (95.9–100.0) | 20.998 (2.798–157.588) |
| TMA score 24 ≥ 3 or Lactate ≥ 4.0 | 90.9 (78.9–100.0) | 61.3 (53.5–69.1) | 25.6 (16–35.3) | 97.9 (95.0–100.0) | 15.862 (3.574–70.398) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, D.R.; Kong, T.; Lee, H.S.; Kim, S.; Lee, J.W.; Chung, H.S.; Chung, S.P.; You, J.S.; Park, J.W. Usefulness of the Thrombotic Microangiopathy Score as a Promising Prognostic Marker of Septic Shock for Patients in the Emergency Department. J. Clin. Med. 2019, 8, 808. https://doi.org/10.3390/jcm8060808
Ko DR, Kong T, Lee HS, Kim S, Lee JW, Chung HS, Chung SP, You JS, Park JW. Usefulness of the Thrombotic Microangiopathy Score as a Promising Prognostic Marker of Septic Shock for Patients in the Emergency Department. Journal of Clinical Medicine. 2019; 8(6):808. https://doi.org/10.3390/jcm8060808
Chicago/Turabian StyleKo, Dong Ryul, Taeyoung Kong, Hye Sun Lee, Sinae Kim, Jong Wook Lee, Hyun Soo Chung, Sung Phil Chung, Je Sung You, and Jong Woo Park. 2019. "Usefulness of the Thrombotic Microangiopathy Score as a Promising Prognostic Marker of Septic Shock for Patients in the Emergency Department" Journal of Clinical Medicine 8, no. 6: 808. https://doi.org/10.3390/jcm8060808
APA StyleKo, D. R., Kong, T., Lee, H. S., Kim, S., Lee, J. W., Chung, H. S., Chung, S. P., You, J. S., & Park, J. W. (2019). Usefulness of the Thrombotic Microangiopathy Score as a Promising Prognostic Marker of Septic Shock for Patients in the Emergency Department. Journal of Clinical Medicine, 8(6), 808. https://doi.org/10.3390/jcm8060808





