Value of Somatostatin Receptor Scintigraphy with 99mTc-HYNIC-TOC in Patients with Primary Sjögren Syndrome
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.1.1. Study Design
2.1.2. Radiopharmaceutical Details
2.1.3. Imaging Procedures
99mTc-HYNIC-TOC Scintigraphy
99mTcO4− Sialoscintigraphy
2.1.4. Image Analysis
2.1.5. Statistical Analysis
3. Results
3.1. Patient Groups
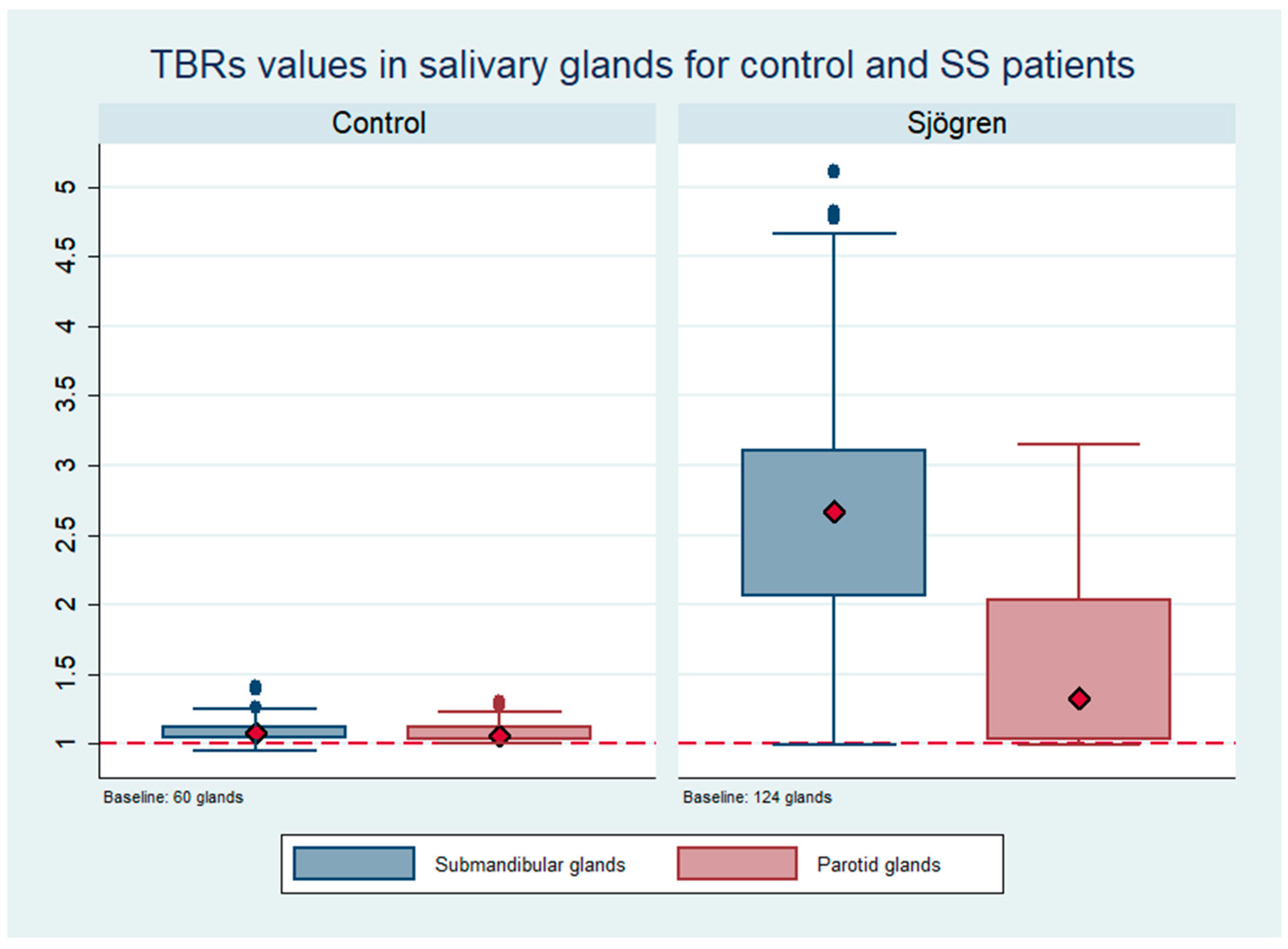
3.2. Salivary Gland Uptake of 99mTc-HYNIC-TOC
3.3. Comparison between 99mTc-HYNIC-TOC in Salivary Glands and 99mTc-Sialoscintigraphy
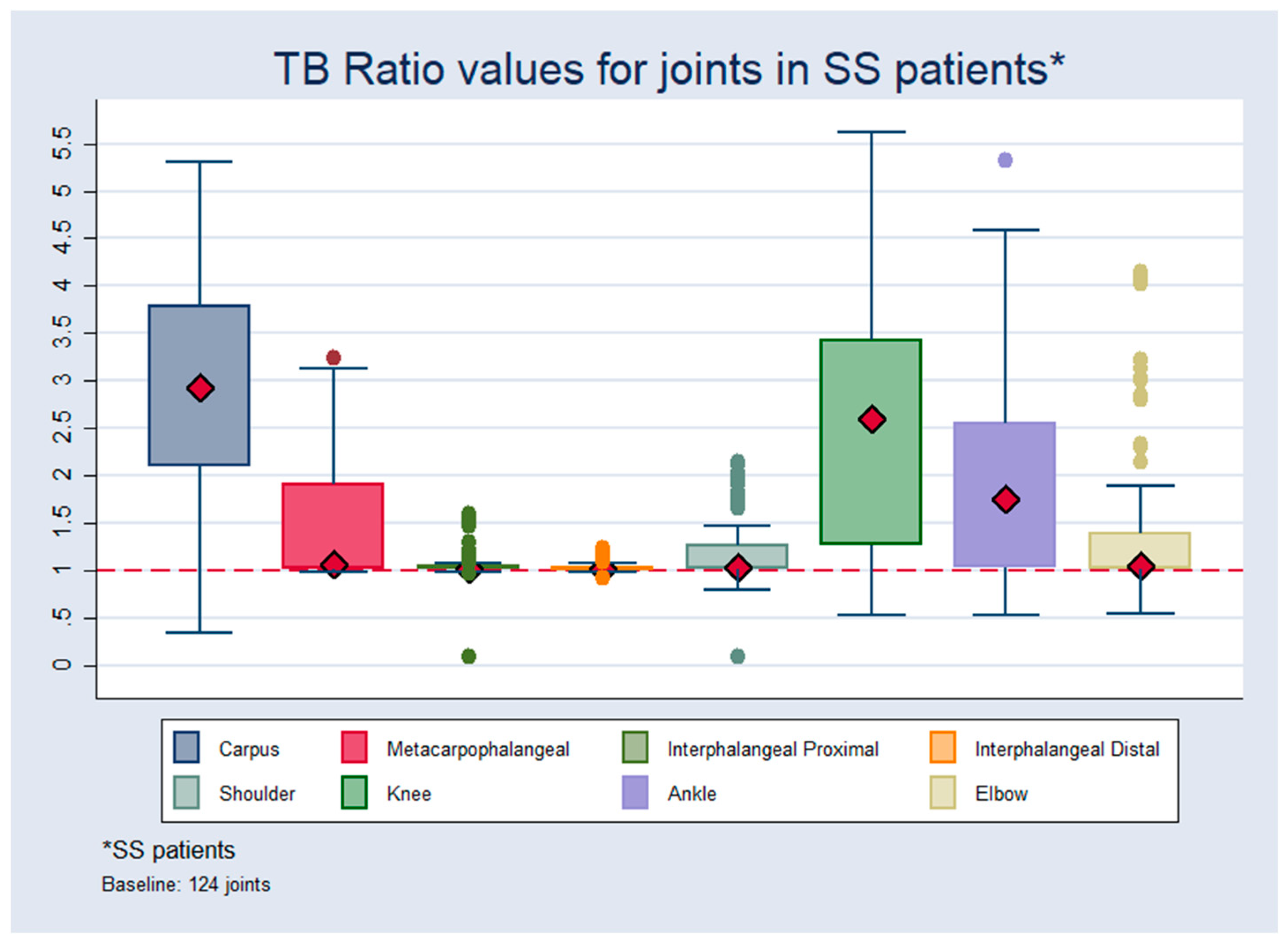
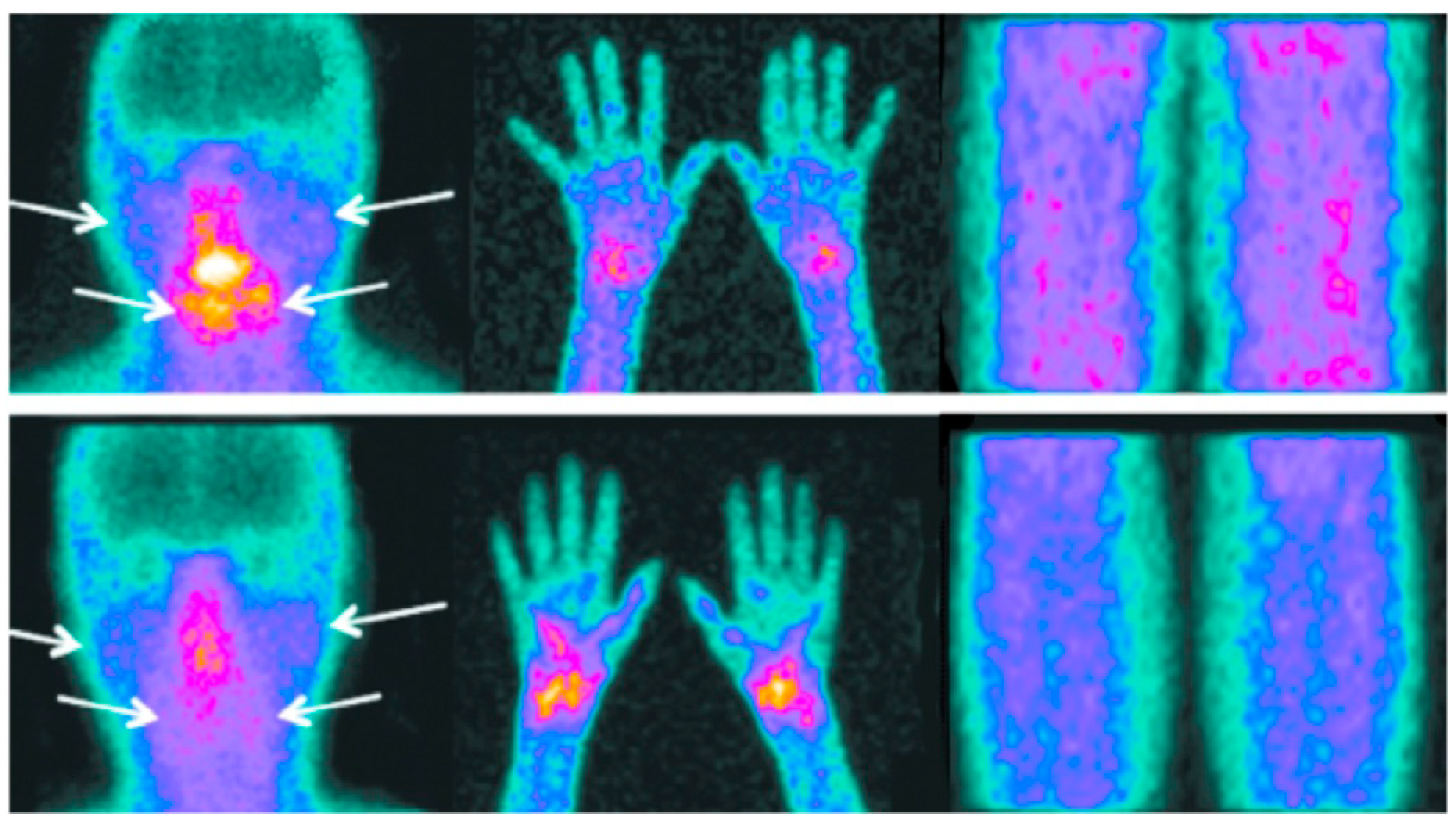
3.4. Joint Uptake of 99mTc-HYNIC-TOC
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Ethical approval Code
References
- Fox, R.J. Sjogren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Binard, A.; Devauchelle-Pensec, V.; Fautrel, B.; Jousse, S.; Youinou, P.; Saraux, A. Epidemiology of Sjogren’s syndrome: Where are we now? Clin. Exp. Rheumatol. 2007, 25, 1–4. [Google Scholar] [PubMed]
- Daridon, C.; Guerrier, T.; Devauchelle, V.; Saraux, A.; Pers, J.O.; Youinou, P. Polarization of B effector cell in Sjogren’s syndrome. Autoimmun. Rev. 2007, 6, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Baldini, C.; Bombardieri, S. Current concepts on classification criteria and disease status indexes in Sjrogen’s síndrome. In Sjrogen’s Sydrome Practical Guidelines to Diagnosis and Therapy; Fox, R.I., Fox, C., Eds.; Springer: New York, NY, USA, 2011; pp. 59–71. [Google Scholar]
- Kassan, S.S.; Mutsopoulos, H.M. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch. Intern. Med. 2004, 164, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C. Classification criteria for Sjogren’s syndrome. Ann. Rheum. Dis. 2003, 62, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Moutsopoulos, H.M.; Balestrieri, G.; Bencivelli, W.; Bernstein, R.M.; Bjerrum, K.B.; Braga, S.; Coll, J.; De Vita, S.; et al. Preliminary criteria for the classification of Sjogren’s syndrome. Results of a prospective concerted action supported by the European community. Arthritis Rheum. 1993, 36, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Ice, J.A.; Li, H.; Grundahl, K.; Kelly, J.A.; Radfar, L.; Stone, D.U.; Hefner, K.S.; Anaya, J.M.; Rohrer, M.; et al. Comparison of the American-European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann. Rheum. Dis. 2014, 73, 31–38. [Google Scholar] [CrossRef]
- Vitali, C.; Del Papa, N. Classification criteria for Sjögren Syndrome. In Sjögren’S Syndrome; Gerli, R., Bartoloni, E., Alunno, A., Eds.; Elsevier: London, UK, 2016; pp. 47–60. [Google Scholar]
- Chen, K.S.; Jiang, M.C.; Li, C.J.; Liu, O.K.; Tsai, C.S. Discrimination Between Sjogren’s and Non-Sjogren’s Sicca Syndrome by Sialoscintigraphy and Antibodies Against α-Fodrin and Ro/La Autoantigens. J. Int. Med. Res. 2009, 37, 1088–1096. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carson, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American–European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Ferone, D.; van Hagen, P.M.; Semino, C.; Dalm, V.A.; Barreca, A.; Colao, A.; Lamberts, S.W.; Minuto, F.; Hofland, L.J. Somatostatin receptor distribution and function in immune system. Dig. Liver Dis. 2004, 36 (Suppl. 1), S68–S77. [Google Scholar] [CrossRef]
- Hoyer, D.; Bell, G.I.; Berelowitz, M.; Epelbaum, J.; Feniuk, W.; Humphrey, P.P.; O’Carroll, A.-M.; Patel, Y.C.; Schonbrunn, A.; Taylor, J.E.; et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol. Sci. 1995, 16, 86–88. [Google Scholar] [CrossRef]
- Virgolini, I.; Pangerl, T.; Bischof, C.; Smith-Jones, P.; Peck-Radosavljevic, M. Somatostatin receptor subtype expression in human tissues: A prediction for diagnosis and treatment of cancer? Eur. J. Clin. Investig. 1997, 27, 645–647. [Google Scholar] [CrossRef]
- Cascini, G.L.; Cuccurullo, V.; Mansi, L. The non tumour uptake of (111)In-octreotide creates new clinical indications in benign diseases, but also in oncology. Q. J. Nucl. Med. Mol. Imaging 2010, 54, 24–36. [Google Scholar] [PubMed]
- Lebtahi, R.; Moreau, S.; Marchand-Adam, S.; Debray, M.P.; Brauner, M.; Soler, P.; Marchal, J.; Raguin, O.; Gruaz-Guyon, A.; Reubi, J.C.; et al. Increased uptake of 111In-octreotide in idiopathic pulmonary fibrosis. J. Nucl. Med. 2006, 47, 1281–1287. [Google Scholar] [PubMed]
- Krenning, E.P.; de Jong, M.; Kooij, P.P.; Breeman, W.A.; Bakker, W.H.; de Herder, W.W.; Van Eijck, C.H.; Kwekkeboom, D.J.; Jamar, F.; Pauwels, S.; et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann. Oncol. 1999, 10 (Suppl. 2), S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; Krenning, E.P. Somatostatin receptor imaging. Semin. Nucl. Med. 2002, 32, 84–91. [Google Scholar] [CrossRef]
- Duet, M.; Liote, F. Somatostatin and somatostatin analog scintigraphy: Any benefits for rheumatology patients? Joint Bone Spine 2004, 71, 530–535. [Google Scholar] [CrossRef]
- Migliore, A.; Signore, A.; Capuano, A.; Bizzi, E.; Massafra, U.; Vacca, T.V.; Chianelli, M. Relevance of 99mTc-HYNIC-tir-octreotide scintigraphy in a patient affected by sarcoidosis with lung and joints involvement and secondary Sjogren’s syndrome treated with infliximab: Case report. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 127–130. [Google Scholar]
- Zhao, R.; Wang, J.; Deng, J.; Yang, W.; Wang, J. Efficacy of (99m)Tc-EDDA/HYNIC-TOC Spect/CT scintigraphy in Graves’ ophthalmopathy. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 242–247. [Google Scholar]
- Anzola, L.K.; Chianelli, M.; Galli, F.; Glaudemans, A.W.J.M.; Martin, L.; Migliore, A.; Signore, A. Somatostatine receptor scintigraphy in patients with rheumatoid arthritis and secondary Sjogrens syndrome treated with Infliximab: A pilot study. EJNMMI Res. 2016, 6, 49. [Google Scholar] [CrossRef][Green Version]
- Bangard, M.; Béhé, M.; Guhlke, S.; Otte, R.; Bender, H.R.; Maecke, H.; Biersack, H.J. Detection of somatostatin receptor-positive tumours using the new 99mTc-tricine-HYNIC-d-Phe1-Tyr3-octreotide: First results in patients and comparison with 111In-DTPA-d-Phe1-octreotide. Eur. J. Nucl. Med. 2000, 27, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Schall, G.L.; Anderson, L.G.; Wolf, R.O.; Herdt, J.R.; Tarpley, T.M., Jr.; Cummings, N.A.; Zeiger, L.S.; Talal, N. Xerostomia in Sjogren’s syndrome. Evaluation by sequential salivary scintigraphy. JAMA 1971, 216, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Youinou, P.; Pers, J.O. Latest update on the primary Sjögren’s syndrome. Press Med. 2012, 41, e437–e439. [Google Scholar] [CrossRef] [PubMed]
- Sumida, T.; Tsuboi, H.; Iizuka, M.; Nakamura, Y.; Matsumoto, I. Functional role of M3 muscarinic acetylcholine receptor (M3R) reactive T cells and anti M3R auntoantibodies in patients with Sjögren’s syndrome. Autoimmun. Rev. 2010, 9, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Bacman, S.; Berro, A.; Sherin-Borda, L.; Borda, E. Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjogren’s syndrome. Investig. Opthalmol. Vis. Sci. 2001, 42, 321–327. [Google Scholar]
- Sumida, T.; Tsuboi, H.; Iizuka, M.; Hirota, T.; Asashima, H.; Matsumoto, I. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjogren’s syndrome: A critical review. J. Autoimmun. 2014, 51, 44e50. [Google Scholar] [CrossRef]
- Halla, J.T.; Hardin, J.G. Clinical features of the arthritis of mixed connective tissue disease. Arthritis Rheum. 1978, 21, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Youinou, P.; Devauchelle-Pensec, V.; Pers, J.O. Siginificance of B cells and B cell clonality in Sjögren syndrome. Arthritis Rheum. 2010, 62, 2605–2610. [Google Scholar] [CrossRef]
- Youinou, P.; Pers, J.O. The late news on BAFF in autoimmune diseases. Autoimmun. Rev. 2010, 9, 804–806. [Google Scholar] [CrossRef]
- Cornec, D.; Devauchelle-Pensec, V.; Tobón, G.J.; Pers, J.O.; Jousse-Joulin, S.; Saraux, A. B cells in Sjögren’s syndrome: From pathophysiology to diagnosis and treatment. J. Autoimmun. 2012, 39, 161–167. [Google Scholar] [CrossRef]
- Makula, E.; Pokorny, G.; Kiss, M.; Vörös, E.; Kovács, L.; Kovács, A.; Csernay, L.; Palkó, A. The place of magnetic resonance and ultrasonographic examination of the parotid gland in the diagnosis and follow–up of primary Sjögren’s syndrome. Rheumatology 2000, 39, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Milic, V.D.; Petrovic, R.R.; Boricic, I.V.; Marinkovic-Eric, J.; Radunovic, G.L.; Jeremic, P.D.; Damjanov, N. Diagnostic Value of salivary gland ultrasonographic scoring system in primary Sjögren Syndrome: A comparison with scontigraphy and biopsy. J. Rheumatol. 2009, 36, 1495–1500. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Markusse, H.M.; Krenning, E.P.; VanHagen, M.; Laissue, J.A. Vascular somatostatin receptors in synovium from patients with rheumatoid arthritis. Eur. J. Pharmacol. 1994, 271, 371–378. [Google Scholar] [CrossRef]
- Sharma, P.; Arora, S.; Karunanithi, S.; Khadgawat, R.; Durgapal, P.; Sharma, R.; Kandasamy, D.; Bal, C.; Kumar, R. Somatostatin receptor based PET/CT imaging with 68Ga-DOTA-NaI3octreotide for localization of clinically and biochemically suspected insulinoma. Q. J. Nucl. Med. Mol. Imaging. 2016, 60, 69–76. [Google Scholar] [PubMed]
- Hjelmervik, T.O.; Petersen, K.; Jonassen, I.; Jonsson, R.; Bolstad, A.I. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005, 52, 1534–1544. [Google Scholar] [CrossRef]
| Control Patients n = 30 | SS Patients n = 62 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Gender, female | 22 | 73.3 | 52 | 83.87 |
| Age, median (range) | 58.5 | 64 (16–80) | 48.5 | 48 (15–71) |
| 12–18 years | 1 | 3.33 | 1 | 1.61 |
| 19–40 years | 1 | 3.33 | 15 | 24.19 |
| 41–60 years | 9 | 30.00 | 34 | 54.84 |
| >60 years | 19 | 63.34 | 12 | 19.35 |
| Dry eye * | - | - | 60 | 96.77 |
| Dry mouth * | - | - | 60 | 96.77 |
| Schirmer test * | - | - | 49 | 79.03 |
| Msg histopathology * | - | - | 47 | 75.80 |
| Sialoscintigraphy * | - | - | 17 | 27.42 |
| Joint pain | - | - | 54 | 87.10 |
| TBR Values for Joints in Control Patients | TBR Values for Joints in SS Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | SD | Min | Max | n | Median | SD | Min | Max | |
| Carpus | 60 | 1.02 | 0.16 | 0.30 | 1.70 | 124 | 2.92 | 1.09 | 0.33 | 5.50 |
| Metcp | 60 | 1.10 | 0.13 | 0.13 | 1.70 | 124 | 1.06 | 0.57 | 0.90 | 3.20 |
| Intphpr | 60 | 1.00 | 0.13 | 0.98 | 1.70 | 124 | 1.02 | 0.14 | 0.90 | 1.60 |
| Intphd | 60 | 1.00 | 0.13 | 0.96 | 1.70 | 124 | 1.02 | 0.10 | 0.90 | 1.20 |
| Shoul | 60 | 1.25 | 0.25 | 0.95 | 1.90 | 124 | 1.03 | 0.40 | 0.90 | 2.10 |
| Knee | 60 | 1.10 | 0.15 | 0.98 | 1.70 | 124 | 2.60 | 1.30 | 0.90 | 5.60 |
| Ankle | 60 | 1.02 | 015 | 0.97 | 1.70 | 124 | 1.00 | 1.00 | 0.90 | 5.30 |
| Elbow | 60 | 1.02 | 0.15 | 0.94 | 1.70 | 124 | 1.10 | 0.70 | 0.90 | 4.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzola, L.K.; Rivera, J.N.; Dierckx, R.A.; Lauri, C.; Valabrega, S.; Galli, F.; Moreno Lopez, S.; Glaudemans, A.W.J.M.; Signore, A. Value of Somatostatin Receptor Scintigraphy with 99mTc-HYNIC-TOC in Patients with Primary Sjögren Syndrome. J. Clin. Med. 2019, 8, 763. https://doi.org/10.3390/jcm8060763
Anzola LK, Rivera JN, Dierckx RA, Lauri C, Valabrega S, Galli F, Moreno Lopez S, Glaudemans AWJM, Signore A. Value of Somatostatin Receptor Scintigraphy with 99mTc-HYNIC-TOC in Patients with Primary Sjögren Syndrome. Journal of Clinical Medicine. 2019; 8(6):763. https://doi.org/10.3390/jcm8060763
Chicago/Turabian StyleAnzola, Luz Kelly, Josè Nelson Rivera, Rudi A. Dierckx, Chiara Lauri, Stefano Valabrega, Filippo Galli, Sergio Moreno Lopez, Andor W. J. M. Glaudemans, and Alberto Signore. 2019. "Value of Somatostatin Receptor Scintigraphy with 99mTc-HYNIC-TOC in Patients with Primary Sjögren Syndrome" Journal of Clinical Medicine 8, no. 6: 763. https://doi.org/10.3390/jcm8060763
APA StyleAnzola, L. K., Rivera, J. N., Dierckx, R. A., Lauri, C., Valabrega, S., Galli, F., Moreno Lopez, S., Glaudemans, A. W. J. M., & Signore, A. (2019). Value of Somatostatin Receptor Scintigraphy with 99mTc-HYNIC-TOC in Patients with Primary Sjögren Syndrome. Journal of Clinical Medicine, 8(6), 763. https://doi.org/10.3390/jcm8060763







