Implant Fixation and Risk of Prosthetic Joint Infection Following Primary Total Hip Replacement: Meta-Analysis of Observational Cohort and Randomised Intervention Studies
Abstract
1. Introduction
2. Experimental Section
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
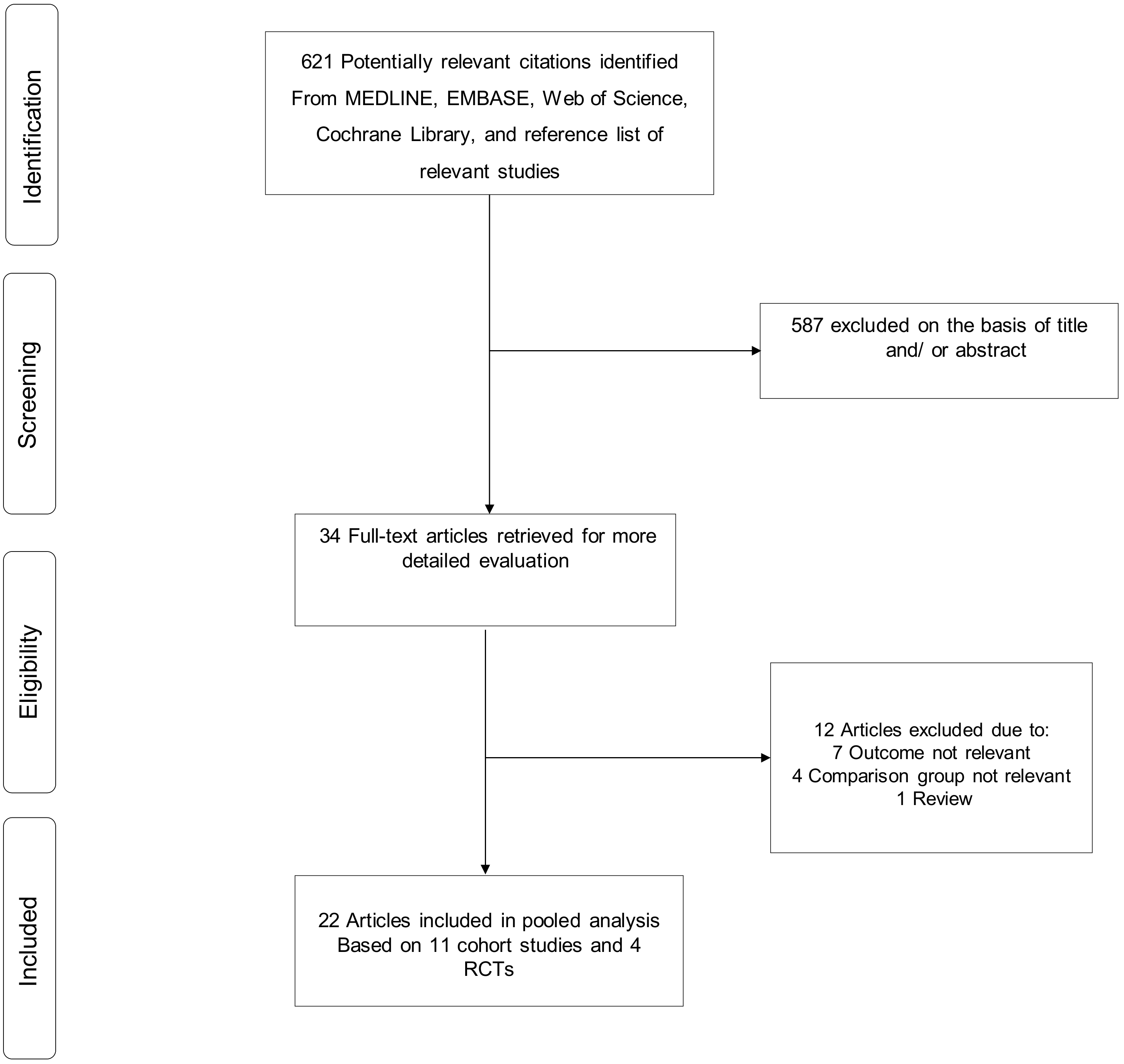
3.1. Study Identification and Selection
3.2. Study Characteristics and Study Quality
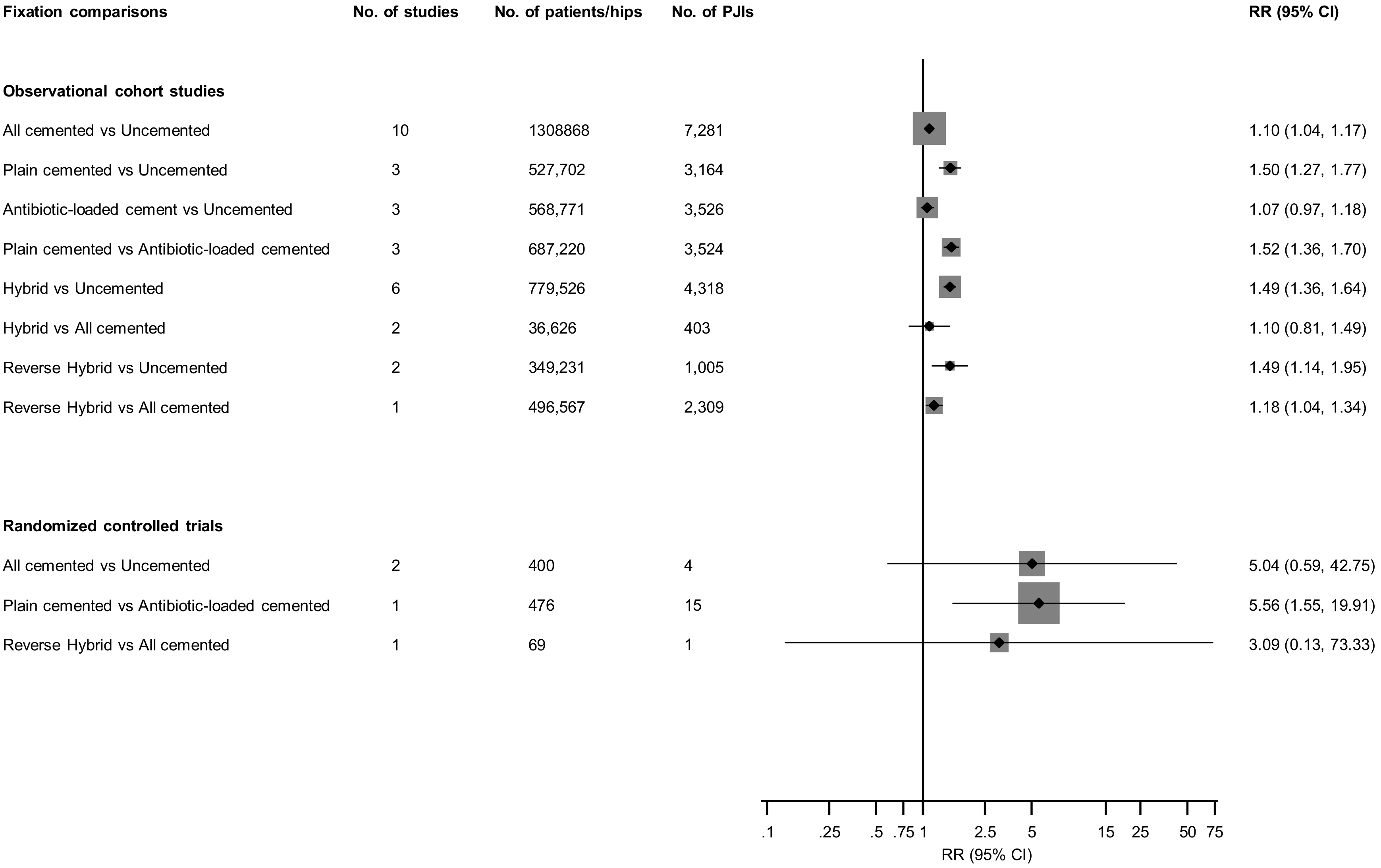
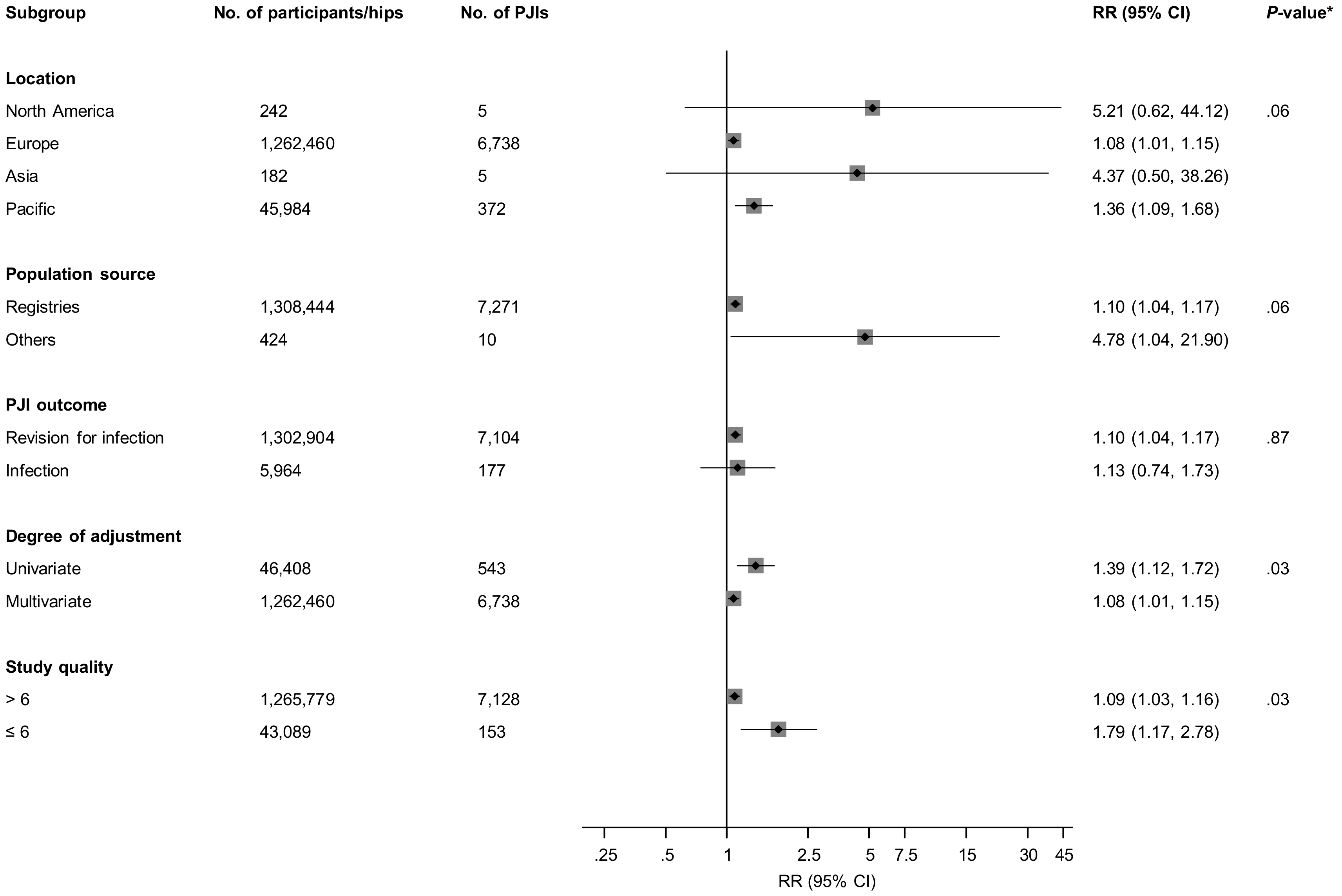
3.3. Fixation Types and Prosthetic Joint Infection (PJI) Risk
3.4. Publication Bias
4. Discussion
4.1. Key Findings
4.2. Comparison with Previous Work
4.3. Possible Explanations for Findings
4.4. Implications of Our Findings
4.5. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berry, D.J.; Harmsen, W.S.; Cabanela, M.E.; Morrey, B.F. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: Factors affecting survivorship of acetabular and femoral components. J. Bone Joint Surg. Am. 2002, 84, 171–177. [Google Scholar] [CrossRef]
- Soderman, P.; Malchau, H.; Herberts, P. Outcome after total hip arthroplasty: Part I. General health evaluation in relation to definition of failure in the Swedish National Total Hip Arthoplasty register. Acta Orthop. Scand. 2000, 71, 354–359. [Google Scholar] [CrossRef] [PubMed]
- National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Available online: http://www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2015th%20Annual%20Report%202018.pdf. (accessed on 10 May 2019).
- Lenguerrand, E.; Wylde, V.; Gooberman-Hill, R.; Sayers, A.; Brunton, L.; Beswick, A.D.; Dieppe, P.; Blom, A.W. Trajectories of Pain and Function after Primary Hip and Knee Arthroplasty: The ADAPT Cohort Study. PLoS ONE 2016, 11, e0149306. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J.; Berry, D.; Parvizi, J. Prosthetic joint infection risk after TKA in the Medicare population. Clin. Orthop. Relat. Res. 2010, 468, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.J.; Blom, A.W.; Whitehouse, M.R.; Gooberman-Hill, R. Deep prosthetic joint infection: A qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open 2015, 5, e009495. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.J.; Katz, P.; Cisternas, M.; Ono, L.; Ries, M.D.; Showstack, J. Hospital resource utilization for primary and revision total hip arthroplasty. J. Bone Joint Surg. Am. 2005, 87, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; INFORM Team. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0150866. [Google Scholar] [CrossRef] [PubMed]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Kunutsor, S.K.; Burston, B.; Porter, M.; Blom, A.W. Risk factors associated with revision for prosthetic joint infection after hip replacement: A prospective observational cohort study. Lancet Infect. Dis. 2018, 1004–1014. [Google Scholar] [CrossRef]
- Hosman, A.H.; van der Mei, H.C.; Bulstra, S.K.; Busscher, H.J.; Neut, D. Effects of metal-on-metal wear on the host immune system and infection in hip arthroplasty. Acta Orthop. 2010, 81, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.; Hallan, G.; Espehaug, B.; Havelin, L.I.; Engesaeter, L.B. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop. 2009, 80, 639–645. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Svendsson, J.E.; Johnsen, S.P.; Riis, A.; Overgaard, S. Risk factors for revision due to infection after primary total hip arthroplasty. A population-based study of 80,756 primary procedures in the Danish Hip Arthroplasty Registry. Acta Orthop. 2010, 81, 542–547. [Google Scholar] [CrossRef]
- Dale, H.; Fenstad, A.M.; Hallan, G.; Havelin, L.I.; Furnes, O.; Overgaard, S.; Pedersen, A.B.; Karrholm, J.; Garellick, G.; Pulkkinen, P.; et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012, 83, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.J.; Lau, E.C.; Ong, K.L.; Vail, T.P.; Rubash, H.E.; Berry, D.J. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J. Arthroplast. 2012, 27, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 August 2018).
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Cornfield, J. A method of estimating comparative rates from clinical data; applications to cancer of the lung, breast, and cervix. J. Natl. Cancer Inst. 1951, 11, 1269–1275. [Google Scholar] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Sharp, S.J. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat. Med. 1999, 18, 2693–2708. [Google Scholar] [CrossRef]
- Wykman, A.; Olsson, E.; Axdorph, G.; Goldie, I. Total hip arthroplasty. A comparison between cemented and press-fit noncemented fixation. J. Arthroplast. 1991, 6, 19–29. [Google Scholar] [CrossRef]
- Katz, R.L.; Bourne, R.B.; Rorabeck, C.H.; McGee, H. Total hip arthroplasty in patients with avascular necrosis of the hip. Follow-up observations on cementless and cemented operations. Clin. Orthop. Relat. Res. 1992, 145–151. [Google Scholar]
- Laupacis, A.; Bourne, R.; Rorabeck, C.; Feeny, D.; Tugwell, P.; Wong, C. Comparison of total hip arthroplasty performed with and without cement: A randomized trial. J. Bone Joint Surg. Am. 2002, 84-A, 1823–1828. [Google Scholar] [CrossRef]
- Engesaeter, L.B.; Espehaug, B.; Lie, S.A.; Furnes, O.; Havelin, L.I. Does cement increase the risk of infection in primary total hip arthroplasty? Revision rates in 56,275 cemented and uncemented primary THAs followed for 0–16 years in the Norwegian Arthroplasty Register. Acta Orthop. 2006, 77, 351–358. [Google Scholar] [CrossRef]
- Pospula, W.; Abu Noor, T.; Roshdy, T.; Al Mukaimi, A. Cemented and cementless total hip replacement. Critical analysis and comparison of clinical and radiological results of 182 cases operated in Al Razi Hospital, Kuwait. Med. Princ. Pract. 2008, 17, 239–243. [Google Scholar] [CrossRef]
- Hooper, G.J.; Rothwell, A.G.; Stringer, M.; Frampton, C. Revision following cemented and uncemented primary total hip replacement: A seven-year analysis from the New Zealand Joint Registry. J. Bone Joint Surg. Br. 2009, 91, 451–458. [Google Scholar] [CrossRef]
- Dale, H.; Skramm, I.; Lower, H.L.; Eriksen, H.M.; Espehaug, B.; Furnes, O.; Skjeldestad, F.E.; Havelin, L.I.; Engesaeter, L.B. Infection after primary hip arthroplasty: A comparison of 3 Norwegian health registers. Acta Orthop. 2011, 82, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Hailer, N.P.; Garellick, G.; Karrholm, J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010, 81, 34–41. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.S.; Park, J.W.; Joo, J.H. Comparison of total hip replacement with and without cement in patients younger than 50 years of age: The results at 18 years. J. Bone Joint Surg. Br. 2011, 93, 449–455. [Google Scholar] [CrossRef]
- Takenaga, R.K.; Callaghan, J.J.; Bedard, N.A.; Liu, S.S.; Klaassen, A.L.; Pedersen, D.R. Cementless total hip arthroplasty in patients fifty years of age or younger: A minimum ten-year follow-up. J. Bone Joint Surg. Am. 2012, 94, 2153–2159. [Google Scholar] [CrossRef]
- Bolland, B.J.; Whitehouse, S.L.; Timperley, A.J. Indications for early hip revision surgery in the UK—A re-analysis of NJR data. Hip. Int. 2012, 22, 145–152. [Google Scholar] [CrossRef]
- Makela, K.T.; Matilainen, M.; Pulkkinen, P.; Fenstad, A.M.; Havelin, L.; Engesaeter, L.; Furnes, O.; Pedersen, A.B.; Overgaard, S.; Karrholm, J.; et al. Failure rate of cemented and uncemented total hip replacements: Register study of combined Nordic database of four nations. BMJ 2014, 348, f7592. [Google Scholar] [CrossRef]
- Wyatt, M.; Hooper, G.; Frampton, C.; Rothwell, A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J. Orthop. 2014, 5, 591–596. [Google Scholar] [CrossRef]
- Wangen, H.; Havelin, L.I.; Fenstad, A.M.; Hallan, G.; Furnes, O.; Pedersen, A.B.; Overgaard, S.; Karrholm, J.; Garellick, G.; Makela, K.; et al. Reverse hybrid total hip arthroplasty. Acta Orthop. 2017, 88, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chammout, G.; Muren, O.; Laurencikas, E.; Boden, H.; Kelly-Pettersson, P.; Sjoo, H.; Stark, A.; Skoldenberg, O. More complications with uncemented than cemented femoral stems in total hip replacement for displaced femoral neck fractures in the elderly. Acta Orthop. 2017, 88, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Schrama, J.C.; Fenstad, A.M.; Dale, H.; Havelin, L.; Hallan, G.; Overgaard, S.; Pedersen, A.B.; Karrholm, J.; Garellick, G.; Pulkkinen, P.; et al. Increased risk of revision for infection in rheumatoid arthritis patients with total hip replacements. Acta Orthop. 2015, 86, 469–476. [Google Scholar] [CrossRef]
- Trela-Larsen, L.; Sayers, A.; Blom, A.W.; Webb, J.C.J.; Whitehouse, M.R. The association between cement type and the subsequent risk of revision surgery in primary total hip replacement. Acta Orthop. 2018, 89, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wannske, M.; Tscherne, H. Results of prophylactic use of Refobacin-Palacos in implantation of endoprostheses of the hip joint in Hannover. Aktuelle Probl. Chir. Orthop. 1979, 201–205. [Google Scholar]
- Yoon, B.H.; Ha, Y.C.; Lee, Y.K.; Koo, K.H. Postoperative Deep Infection After Cemented Versus Cementless Total Hip Arthroplasty: A Meta-Analysis. J. Arthroplast. 2015, 30, 1823–1827. [Google Scholar] [CrossRef]
- Abdulkarim, A.; Ellanti, P.; Motterlini, N.; Fahey, T.; O’Byrne, J.M. Cemented versus uncemented fixation in total hip replacement: A systematic review and meta-analysis of randomized controlled trials. Orthop. Rev. (Pavia) 2013, 5, e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, C.; Cheng, T.; Peng, X.; Zhang, W.; Qin, H.; Zhang, X. A systematic review and meta-analysis of antibiotic-impregnated bone cement use in primary total hip or knee arthroplasty. PLoS ONE 2013, 8, e82745. [Google Scholar] [CrossRef]
- Whitehouse, M.R.; Atwal, N.S.; Pabbruwe, M.; Blom, A.W.; Bannister, G.C. Osteonecrosis with the use of polymethylmethacrylate cement for hip replacement: Thermal-induced damage evidenced in vivo by decreased osteocyte viability. Eur. Cell Mater. 2014, 27, 50–62. [Google Scholar] [CrossRef]
- Van Jonbergen, J.P.; Anderson, P.G.; Faber, F.W. Total hip arthroplasty with Boneloc cement: Unsatisfactory results in 163 hips after 9 to 11 years. Hip. Int. 2004, 14, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.G.; Neut, D.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Bacterial survival in the interfacial gap in gentamicin-loaded acrylic bone cements. J. Bone Joint Surg. Br. 2005, 87, 272–276. [Google Scholar] [CrossRef]
- Elson, R.A.; Jephcott, A.E.; McGechie, D.B.; Verettas, D. Antibiotic-loaded acrylic cement. J. Bone Joint Surg. Br. 1977, 59, 200–205. [Google Scholar] [CrossRef]
- Stevens, C.M.; Tetsworth, K.D.; Calhoun, J.H.; Mader, J.T. An articulated antibiotic spacer used for infected total knee arthroplasty: A comparative in vitro elution study of Simplex and Palacos bone cements. J. Orthop. Res. 2005, 23, 27–33. [Google Scholar] [CrossRef]
- Penner, M.J.; Duncan, C.P.; Masri, B.A. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J. Arthroplast. 1999, 14, 209–214. [Google Scholar] [CrossRef]
- Penner, M.J.; Masri, B.A.; Duncan, C.P. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J. Arthroplast. 1996, 11, 939–944. [Google Scholar] [CrossRef]
- Lawson, K.J.; Marks, K.E.; Brems, J.; Rehm, S. Vancomycin vs tobramycin elution from polymethylmethacrylate: An in vitro study. Orthopedics 1990, 13, 521–524. [Google Scholar]
- Baker, A.S.; Greenham, L.W. Release of gentamicin from acrylic bone cement. Elution and diffusion studies. J. Bone Joint. Surg. Am. 1988, 70, 1551–1557. [Google Scholar] [CrossRef]
- DeLuise, M.; Scott, C.P. Addition of hand-blended generic tobramycin in bone cement: Effect on mechanical strength. Orthopedics 2004, 27, 1289–1291. [Google Scholar] [PubMed]
- Chechik, O.; Khashan, M.; Lador, R.; Salai, M.; Amar, E. Surgical approach and prosthesis fixation in hip arthroplasty world wide. Arch. Orthop. Trauma Surg. 2013, 133, 1595–1600. [Google Scholar] [CrossRef]
- Pennington, M.; Grieve, R.; Sekhon, J.S.; Gregg, P.; Black, N.; van der Meulen, J.H. Cemented, cementless, and hybrid prostheses for total hip replacement: Cost effectiveness analysis. BMJ 2013, 346, f1026. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Jones, S.A.; Porter, M.L.; Blom, A.W. Revision for prosthetic joint infection following hip arthroplasty: Evidence from the National Joint Registry. Bone Joint Res. 2017, 6, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Dorey, F.; Grigoris, P.; Amstutz, H. Making do without randomised trials. J. Bone Joint Surg. Br. 1994, 76, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Board, T.; Kay, P.; Wroblewski, B.M.; Zeller, V.; Chen, S.Y.; Hsieh, P.H.; Masri, B.A.; et al. One- and two-stage surgical revision of peri-prosthetic joint infection of the hip: a pooled individual participant data analysis of 44 cohort studies. Eur. J. Epidemiol. 2018, 33, 933–946. [Google Scholar] [CrossRef]
| Author, Year of Publication | Year of Study | Country | Indication for THR | Average Age (Years) | Design, Source of Data | Fixation Types Compared | Average Follow-Up Duration, Years | No. of Participants/Hips | Infection Outcome | No. of PJIs | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wannske, 1979 | NR | Germany | NK | 63.7 | RCT | Cemented with antibiotics, cemented without antibiotics | NK | 476 | Infection | 15 | NA |
| Wykman, 1991 | 1982–1984 | Sweden | Osteoarthritis 77%, rheumatoid arthritis 10%, other 13% | 67.4/64.8 * | RCT | All cemented, uncemented | Over 5.0 | 150 | Infection | 2 | NA |
| Katz, 1992 | 1977–1987 | Canada | Avascular necrosis | 63.0/54.0 * | Observational cohort, Consecutive case series | All cemented, uncemented | 3.8 | 34 | Infection | 1 | 6 |
| Laupacis,2002 | 1987–1992 | Canada | Osteoarthritis | 64.0 | RCT | All cemented, uncemented | 6.3 | 250 | Infection | 2 | NA |
| Engesaeter, 2006 | 1987–2003 | Norway | Primary osteoarthritis | 71.0 | Observational cohort, Registry | Uncemented, cemented with or without antibiotics | 12.0 | 56,275 | Revision due to infection | 252 | 8 |
| Pospula, 2008 | 1994–2004 | Kuwait | Osteoarthritis 12.6%, other 87.4% | 53.7/46.7 * | Observational cohort, Consecutive case series | All cemented, uncemented | 5.0/3.0 | 182 | Infection | 5 | 6 |
| Hooper, 2009 | 1999–2006 | New Zealand | All indications | <55 to >75 | Observational cohort, Registry | All cemented, hybrid, uncemented | < and >90 days | 42,665 | Revision due to infection | 143 | 6 |
| Dale, 2009 | 1987–2007 | Norway | Osteoarthritis 72.2%, inflammatory 3.7%, other 24.1% | <40 to ≥80 | Observational cohort, Registry | Uncemented and cemented with or without antibiotics | 5.0 | 97,344 | Revision due to infection | 614 | 8 |
| Pedersen, 2010 | 1995–2008 | Denmark | Primary osteoarthritis 78.4%, others 21.6% | NR | Observational cohort, Registry | Hybrid, uncemented, cemented with or without antibiotics | 4.6 | 80,756 | Revision due to infection | 597 | 8 |
| Hailer, 2010 | 1992–2007 | Sweden | Primary osteoarthritis 76%, others 24% | <50 to >75 | Observational cohort, Registry | All cemented, uncemented | 5.8 | 170,413 | Revision due to infection | 852 | 8 |
| Dale, 2011 | 2005–2009 | Norway | NR | NR | Observational cohort, Registry | All cemented, uncemented, hybrid | 1.0 (median, 29 days) | 31,086 | Revision due to infection | 236 | 8 |
| Dale, 2011 | 2005–2009 | Norway | NR | NR | Observational cohort, Registry | All cemented, uncemented, hybrid | 1.0 (median, 16 days) | 5540 | Infection | 167 | 8 |
| Kim, 2011 | 1991–1993 | Korea | Osteonecrosis 66%, others 34% | 43.4/46.8 * | Observational cohort, Consecutive case series | Hybrid, uncemented | 18.4 | 219 | Infection | 4 | 7 |
| Dale, 2012 | 1995–2009 | NARA | Osteoarthritis 80%, others 20% | <40 to ≥90 | Observational cohort, Registry | Hybrid, all cemented, reverse hybrid, uncemented | 5.0 | 432,168 | Revision due to infection | 2778 | 8 |
| Takenaga, 2012 | 1994–1999/1970–1976 | USA | Osteoarthritis 11% in cemented cohort, 39% in uncemented cohort | 42.0/40.1 * | Observational cohort | All cemented, uncemented | 18.0/12.0 * | 208 | Infection | 4 | 6 |
| Bolland, 2012 | 2003–2008 | UK | NR | 64.7 | Observational cohort, Registry | All cemented, hybrid, uncemented | 3.0 | 220,399 | Revision due to infection | 406 | 7 |
| Makela, 2014 | 1995–2011 | NARA | Primary osteoarthritis 90.6%, other 9.4% | 55 and older | Observational cohort, Registry | All cemented, hybrid, reverse hybrid, uncemented | 10.0 | 347,899 | Revision due to infection | 877 | 6 |
| Wyatt, 2014 | NR | New Zealand | NR | NR | Observational cohort, Registry | All cemented, hybrid, reverse hybrid, uncemented | 13.0 | 3319 | Revision due to infection | 390 | 7 |
| Schrama, 2015 | 1995–2010 | NARA | Osteoarthritis 96.6%, rheumatoid arthritis 3.4% | 68.8 | Observational cohort, Registry | Uncemented, cemented with or without antibiotics | 16.0 | 390,671 | Revision due to infection | 2315 | 8 |
| Wangen, 2017 | 2000–2013 | NARA | Osteoarthritis 81%, others 20% | 64.0/73.0 * | Observational cohort, Registry | Reverse hybrid, all cemented | 3.3/6.2 * | 496,567 | Revision due to infection | 2309 | 8 |
| Chammout, 2017 | 2009–2014 | Sweden | Displaced femoral neck fracture | 73.0 | RCT | Reverse hybrid, all cemented | 2.0 | 69 | Infection | 1 | NA |
| Trela-Larsen, 2018 | 2003–2013 | UK | Osteoarthritis | 63.7 | Observational cohort, Registry | Cemented with antibiotics, cemented without antibiotics | 4.1 | 199,205 | Revision due to infection | 595 | 8 |
| Lenguerrand, 2018 | 2003–2013 | UK | Osteoarthritis 93%, others 7% | 68.0 | Observational cohort, Registry | All cemented, uncemented | 4.6 | 623,253 | Revision due to infection | 2705 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunutsor, S.K.; Beswick, A.D.; Whitehouse, M.R.; Blom, A.W.; Lenguerrand, E. Implant Fixation and Risk of Prosthetic Joint Infection Following Primary Total Hip Replacement: Meta-Analysis of Observational Cohort and Randomised Intervention Studies. J. Clin. Med. 2019, 8, 722. https://doi.org/10.3390/jcm8050722
Kunutsor SK, Beswick AD, Whitehouse MR, Blom AW, Lenguerrand E. Implant Fixation and Risk of Prosthetic Joint Infection Following Primary Total Hip Replacement: Meta-Analysis of Observational Cohort and Randomised Intervention Studies. Journal of Clinical Medicine. 2019; 8(5):722. https://doi.org/10.3390/jcm8050722
Chicago/Turabian StyleKunutsor, Setor K., Andrew D. Beswick, Michael R. Whitehouse, Ashley W. Blom, and Erik Lenguerrand. 2019. "Implant Fixation and Risk of Prosthetic Joint Infection Following Primary Total Hip Replacement: Meta-Analysis of Observational Cohort and Randomised Intervention Studies" Journal of Clinical Medicine 8, no. 5: 722. https://doi.org/10.3390/jcm8050722
APA StyleKunutsor, S. K., Beswick, A. D., Whitehouse, M. R., Blom, A. W., & Lenguerrand, E. (2019). Implant Fixation and Risk of Prosthetic Joint Infection Following Primary Total Hip Replacement: Meta-Analysis of Observational Cohort and Randomised Intervention Studies. Journal of Clinical Medicine, 8(5), 722. https://doi.org/10.3390/jcm8050722






