Macular Hole Surgery Using Gas Tamponade—An Outcome from the Oslo Retrospective Cross-Sectional Study
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Duker, J.S.; Kaiser, P.K.; Binder, S.; De Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The International Vitreomacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef]
- Heegaard, S. Morphology of the vitreoretinal border region. Acta Ophthalmol. Scand. Suppl. 1997, 1–31. [Google Scholar]
- Ezra, E.; Gregor, Z.J.; Morfields, N. Macular Hole Study Ggroup Report, Surgery for idiopathic full-thickness macular hole: two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Morfields Macular Hole Study Group RAeport no. 1. Arch. Ophthalmol. 2004, 122, 224–236. [Google Scholar]
- Freeman, W.R.; Azen, S.P.; Kim, J.W.; el-Haig, W.; Mishell, D.R., III; Bailey, I. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch. Ophthalmol. 1997, 115, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Christensen, U.C.; Kroyer, K.; Sander, B.; Jorgensen, T.M.; Larsen, M.; la Cour, M. Macular morphology and visual acuity after macular hole surgery with or without internal limiting membrane peeling. Br. J. Ophthalmol. 2010, 94, 41–47. [Google Scholar] [CrossRef]
- Jaycock, P.D.; Bunce, C.; Xing, W.; Thomas, D.; Poon, W.; Gazzard, G.; Williamson, T.H.; Laidlaw, D.A. Outcomes of macular hole surgery: Implications for surgical management and clinical governance. Eye 2005, 19, 879–884. [Google Scholar] [CrossRef]
- Kannan, N.B.; Adenuga, O.O.; Kumar, K.; Ramasamy, K. Outcome of 2 cc pure sulfur hexafluoride gas tamponade for macular hole surgery. BMC Ophthalmol. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Smiddy, W.E.; Feuer, W.J.; Shi, W. OUTCOMES OF SULFUR HEXAFLUORIDE (SF6) VERSUS PERFLUOROPROPANE (C3F8) GAS TAMPONADE FOR MACULAR HOLE SURGERY. Retina 2008, 28, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Negi, A. Predicting Visual Outcome following Surgery for Idiopathic Macular Holes. Ophthalmologica 2014, 231, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mancino, R.; Ciuffoletti, E.; Martucci, A.; Aiello, F.; Cedrone, C.; Cerulli, L.; Nucci, C. Anatomical and Functional Results of Macular Hole Retinal Detachment Surgery in Patients with High Myopia and Posterior Staphyloma Treated with Perfluoropropane Gas or Silicone Oil. Retina 2013, 33, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.R.P.; Wong, J.; Belliveau, M.; Rayat, J.; Gale, J. Anatomical and Visual Outcomes of Macular Hole Surgery with Short-Duration 3-Day Face-Down Positioning. Retina 2012, 32, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Mittra, R.A.; E Kim, J.; Han, D.P.; Pollack, J.S. Sustained postoperative face-down positioning is unnecessary for successful macular hole surgery. Br. J. Ophthalmol. 2009, 93, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Tadayoni, R.; Vicaut, E.; Devin, F.; Creuzot-Garcher, C.; Berrod, J.-P.; Le Mer, Y.; Korobelnik, J.-F.; Aout, M.; Massin, P.; Gaudric, A. A Randomized Controlled Trial of Alleviated Positioning after Small Macular Hole Surgery. Ophthalmology 2011, 118, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Tsipursky, M.S.; Heller, M.A.; De Souza, S.A.; Gordon, A.J.; Bryan, J.S.; Ziemianski, M.C.; Sell, C.H. Comparative evaluation of no dye assistance, indocyanine green and triamcinolone acetonide for internal limiting membrane peeling during macular hole surgery. Retina 2013, 33, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, J.; Moroz, I.; Katz, G. Effect of ocriplasmin on the management of macular holes: Assessment of the clinical relevance of ocriplasmin. JAMA Ophthalmol. 2014, 132, 709–713. [Google Scholar] [CrossRef]
- Stalmans, P.; Benz, M.S.; Gandorfer, A.; Girach, A.; Pakola, S.; Kampik, A.; Haller, J.A. Enzymatic Vitreolysis with Ocriplasmin for Vitreomacular Traction and Macular Holes. N. Engl. J. Med. 2012, 367, 606–615. [Google Scholar] [CrossRef]
- Chan, C.K.; Wessels, I.F.; Friedrichsen, E.J. Treatment of Idiopathic Macular Holes by Induced Posterior Vitreous Detachment. Ophthalmology 1995, 102, 757–767. [Google Scholar]
- Holekamp, N.M.; Shui, Y.-B.; Beebe, D.C. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am. J. Ophthalmol. 2005, 139, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yagi, F.; Takagi, S.; Tomita, G. Combined Idiopathic Macular Hole Vitrectomy with Phacoemulsification without Face-Down Positioning. J. Ophthalmol. 2012, 2012, 1–4. [Google Scholar]
- Seider, M.I.; Lahey, J.M.; Fellenbaum, P.S. Cost of phacovitrectomy versus vitrectomy and sequential phacoemulsification. Retina 2014, 34, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000, 107, 1939–1948. [Google Scholar] [CrossRef]
- Kelly, N.E.; Wendel, R.T. Vitreous Surgery for Idiopathic Macular Holes. Arch. Ophthalmol. 1991, 109, 654. [Google Scholar] [CrossRef]
- Sebag, J.; Wang, M.Y.; Nguyen, D.; Sadun, A.A. Vitreopapillary Adhesion in Macular Diseases. Trans. Am. Ophthalmol. Soc. 2009, 107, 35–44. [Google Scholar]
- Wang, S.; Xu, L.; Jonas, J.B. Prevalence of Full-Thickness Macular Holes in Urban and Rural Adult Chinese: The Beijing Eye Study. Am. J. Ophthalmol. 2006, 141, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Kung, Y.H. Comparison of anatomical and visual outcomes of macular hole surgery in patients with high myopia vs. non-high myopia: A case-control study using optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tornambe, P.E.; Poliner, L.S.; Grote, K. Macular hole surgery without face-down positioning. Retina 1997, 17, 179–185. [Google Scholar] [CrossRef]
- Guillaubey, A.; Malvitte, L.; Lafontaine, P.O.; Jay, N.; Hubert, I.; Bron, A.; Berrod, J.P.; Creuzot-Garcher, C. Comparison of Face-Down and Seated Position After Idiopathic Macular Hole Surgery: A Randomized Clinical Trial. Am. J. Ophthalmol. 2008, 146, 128–134.e1. [Google Scholar] [CrossRef]
- Hong, M.-C.; Wu, T.-T.; Sheu, S.-J. Primary gas tamponade in the management of macular hole with retinal detachment in highly myopic eyes. J. Chin. Med Assoc. 2011, 74, 121–124. [Google Scholar] [CrossRef]
- Krohn, J. Duration of face-down positioning after macular hole surgery: A comparison between 1 week and 3 days. Acta Ophthalmol. Scand. 2005, 83, 289–292. [Google Scholar] [CrossRef]
- Merkur, A.B.; Tuli, R. Macular hole repair with limited nonsupine positioning. Retina 2007, 27, 365–369. [Google Scholar] [CrossRef][Green Version]
- Park, D.W.; O Sipperley, J.; Sneed, S.R.; Dugel, P.U.; Jacobsen, J. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology 1999, 106, 1392–1398. [Google Scholar] [CrossRef]
- Tranos, P.G.; Peter, N.M.; Nath, R.; Singh, M.; Dimitrakos, S.; Charteris, D.; Kon, C. Macular hole surgery without prone positioning. Eye 2007, 21, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ho, L.Y.; Lai, M.; Capone, A. Surgical Outcomes of Idiopathic Macular Hole Repair With Limited Postoperative Positioning. Retina 2011, 31, 609–611. [Google Scholar] [CrossRef]
- Mohamed, S.; Lai, T.Y. Intraocular gas in vitreoretinal surgery. Hong Kong J. Ophthalmol. 2010, 14, 8–13. [Google Scholar]
- Kusuhara, S.; Ooto, S.; Kimura, D.; Itoi, K.; Mukuno, H.; Miyamoto, N.; Akimoto, M.; Takagi, H. Intraocular gas dynamics after 20-gauge and 23-gauge vitrectomy with sulfur hexafluoride gas tamponade. Retina 2011, 31, 250–256. [Google Scholar] [CrossRef]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted Internal Limiting Membrane Flap Technique for Large Macular Holes. Ophthalmology 2010, 117, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Hoerauf, H. Predictive values in macular hole repair. Br. J. Ophthalmol. 2007, 91, 1415–1416. [Google Scholar] [CrossRef] [PubMed]
- Tadayoni, R.; Gaudric, A.; Haouchine, B.; Massin, P. Relationship between macular hole size and the potential benefit of internal limiting membrane peeling. Br. J. Ophthalmol. 2006, 90, 1239–1241. [Google Scholar] [CrossRef]
- Wong, R.; Gupta, B.; Williamson, T.H.; Laidlaw, D.A. Day 1 postoperative intraocular pressure spike in vitreoretinal surgery (VDOP1). Acta Ophthalmol. 2011, 89, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Xirou, T.; Theodossiadis, P.G.; Apostolopoulos, M.; Kabanarou, S.A.; Feretis, E.; Ladas, I.D.; Koutsandrea, C. Macular hole surgery with short-acting gas and short-duration face-down positioning. Clin. Ophthalmol. 2012, 6, 1107–1112. [Google Scholar]
- Heath, G.; Rahman, R. Combined 23-gauge, sutureless transconjunctival vitrectomy with phacoemulsification without face down posturing for the repair of idiopathic macular holes. Eye 2010, 24, 214–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahn, S.J.; Woo, S.J.; Ahn, J.; Park, K.H. Comparison of postoperative intraocular pressure changes between 23-gauge transconjunctival sutureless vitrectomy and conventional 20-gauge vitrectomy. Eye 2012, 26, 796–802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gosse, E.; Newsom, R.; Hall, P.; Lochhead, J. Changes in Day 1 Post-Operative Intraocular Pressure Following Sutureless 23-Gauge and Conventional 20-Gauge Pars Plana Vitrectomy. Open Ophthalmol. J. 2013, 7, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Azen, S.P.; El-Bradey, M.H.; Scholz, B.M.; Chaidhawangul, S.; Toyoguchi, M.; Freeman, W.R. Duration of vitrectomy and postoperative cataract in the vitrectomy for macular hole study. Am. J. Ophthalmol. 2001, 132, 881–887. [Google Scholar] [CrossRef]
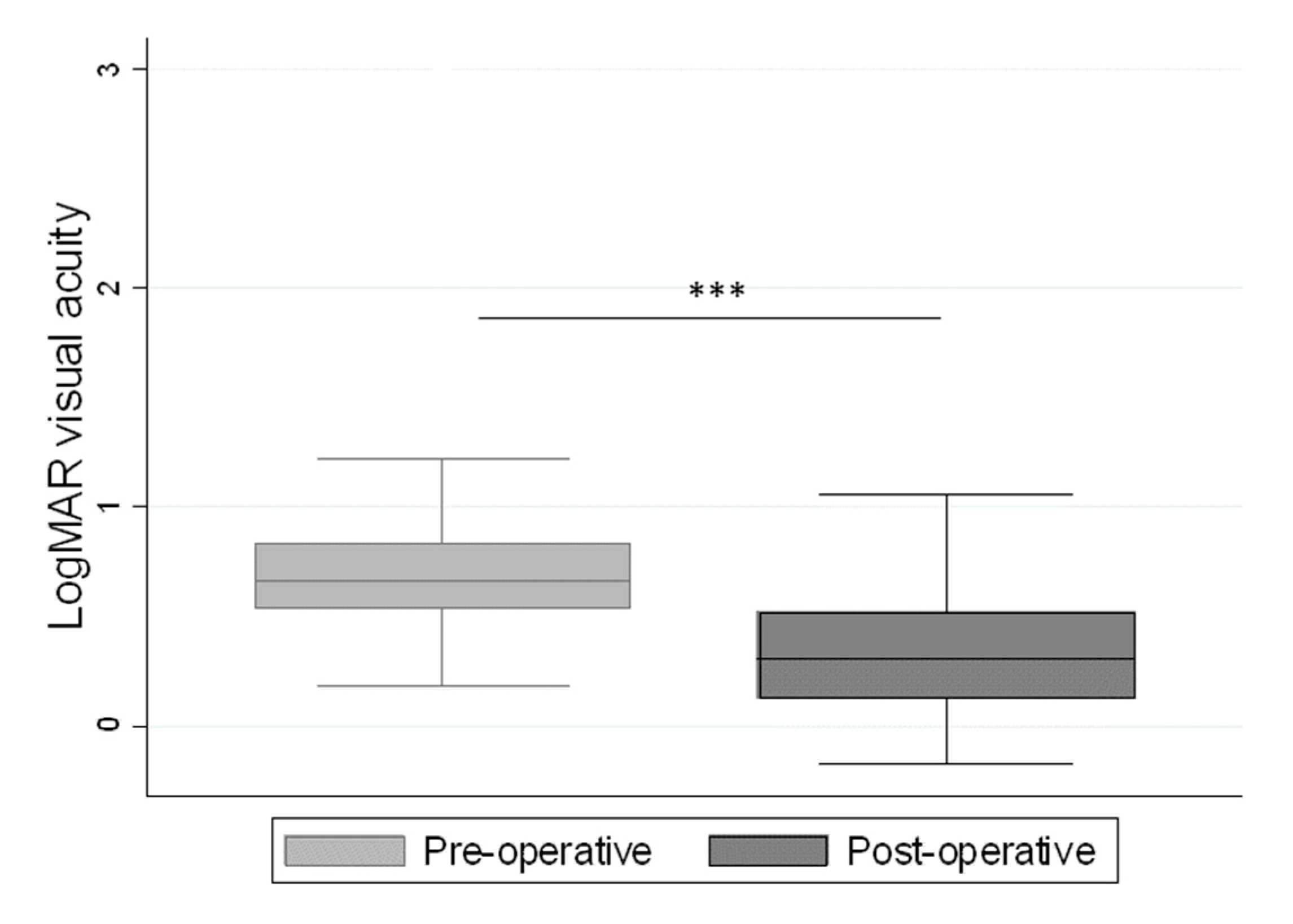
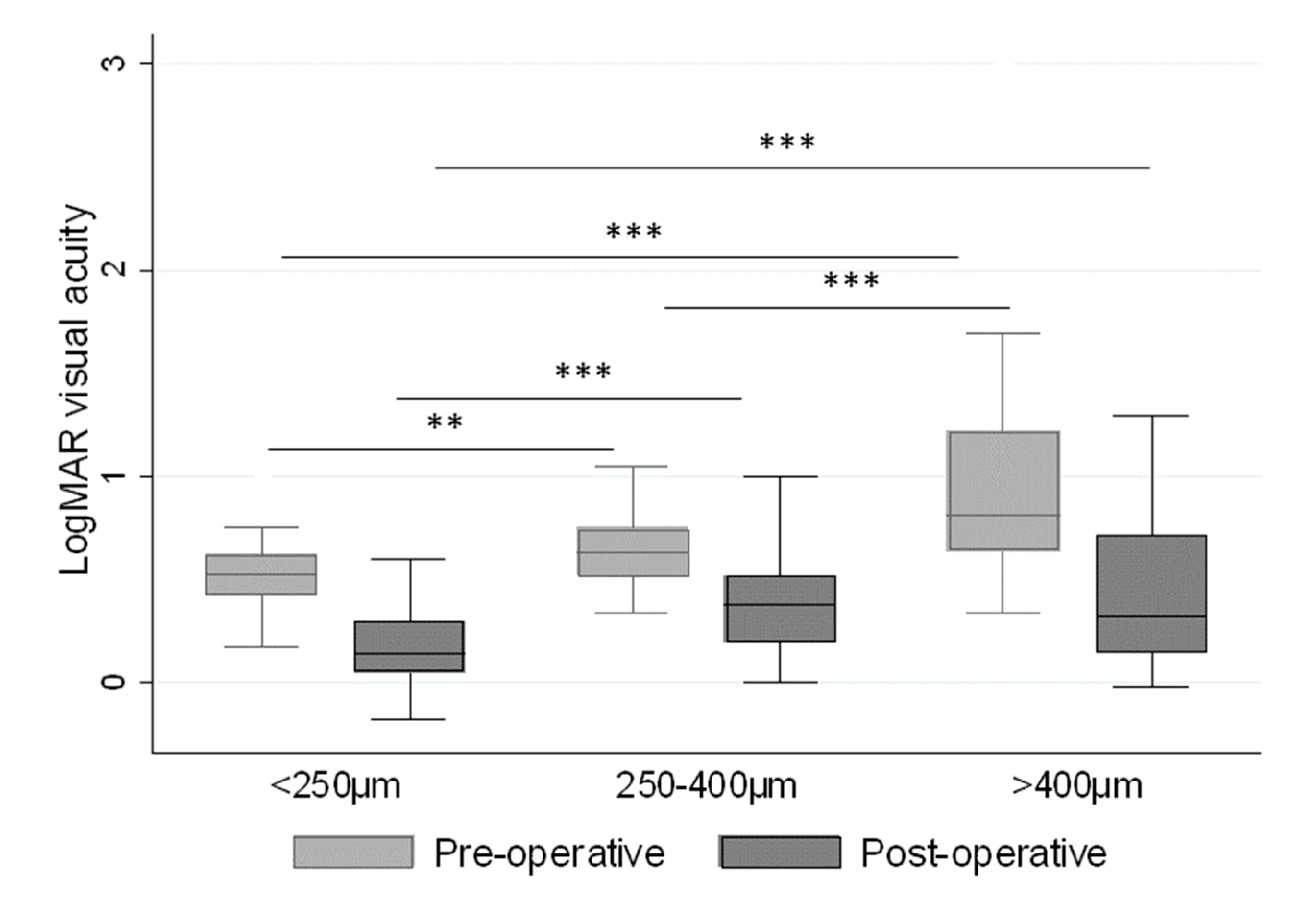
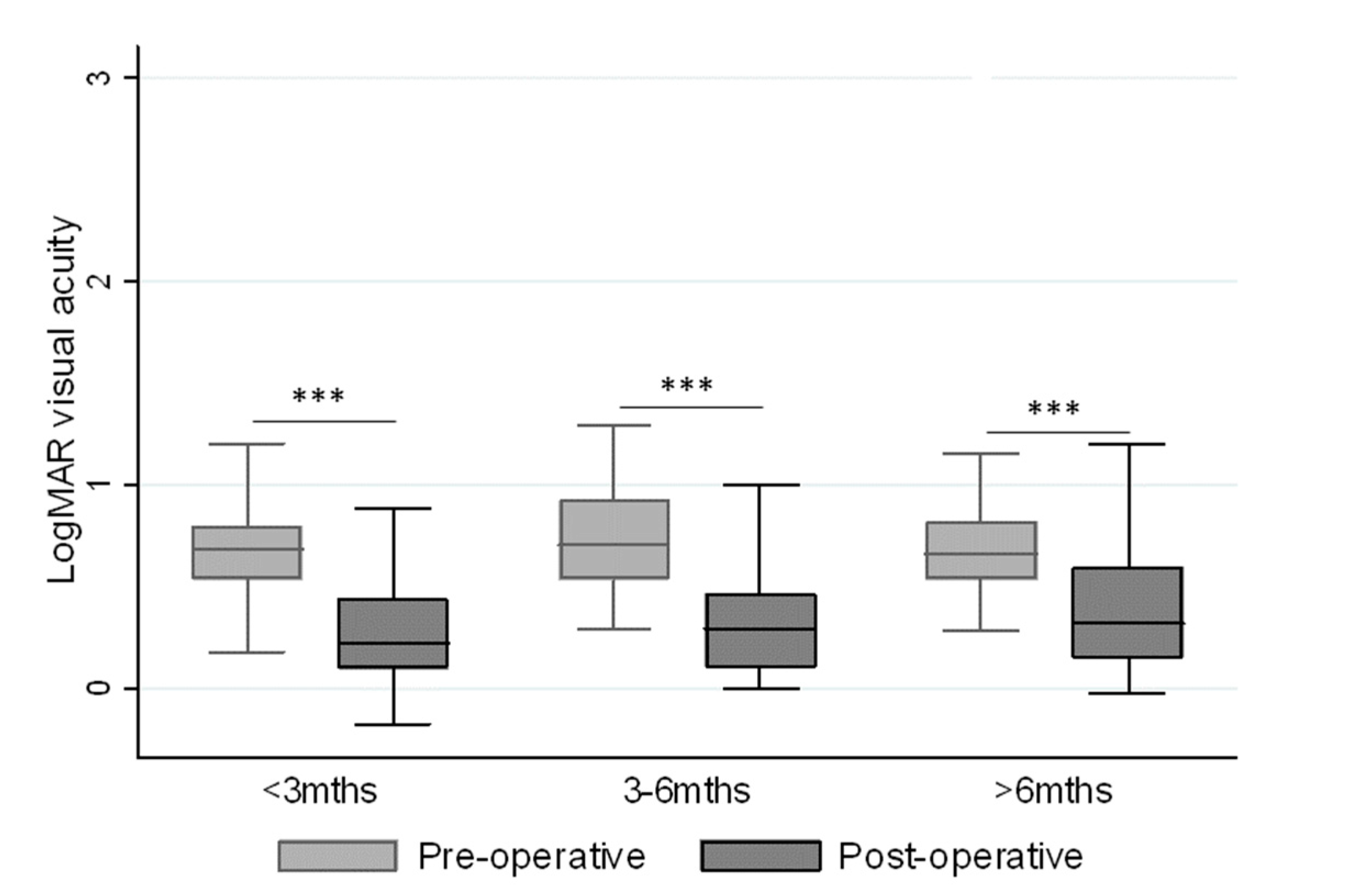
| Size of the Macular Hole | Percent of Subjects |
| Small (<250 µm) | 20.2% |
| Medium (between 250–400 µm) | 37.3% |
| Large (≥400 µm) | 42.5% |
| Duration of Symptoms | Percent of Subjects |
| ≤3 months | 15.7% |
| 3–6 months | 35.7% |
| >6 up to 48 months | 48.6% |
| LogMAR Difference Pre- and Post-Operatively | <0.2 | 0.2–0.4 | >0.4 |
|---|---|---|---|
| N (%) | 56 (28.3%) | 54 (27.3%) | 88 (44.4%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stene-Johansen, I.; Bragadóttir, R.; Petrovski, B.É.; Petrovski, G. Macular Hole Surgery Using Gas Tamponade—An Outcome from the Oslo Retrospective Cross-Sectional Study. J. Clin. Med. 2019, 8, 704. https://doi.org/10.3390/jcm8050704
Stene-Johansen I, Bragadóttir R, Petrovski BÉ, Petrovski G. Macular Hole Surgery Using Gas Tamponade—An Outcome from the Oslo Retrospective Cross-Sectional Study. Journal of Clinical Medicine. 2019; 8(5):704. https://doi.org/10.3390/jcm8050704
Chicago/Turabian StyleStene-Johansen, Ingar, Ragnheiður Bragadóttir, Beáta Éva Petrovski, and Goran Petrovski. 2019. "Macular Hole Surgery Using Gas Tamponade—An Outcome from the Oslo Retrospective Cross-Sectional Study" Journal of Clinical Medicine 8, no. 5: 704. https://doi.org/10.3390/jcm8050704
APA StyleStene-Johansen, I., Bragadóttir, R., Petrovski, B. É., & Petrovski, G. (2019). Macular Hole Surgery Using Gas Tamponade—An Outcome from the Oslo Retrospective Cross-Sectional Study. Journal of Clinical Medicine, 8(5), 704. https://doi.org/10.3390/jcm8050704




