Factors for the Early Revision of Misdiagnosed Tuberculosis to Lung Cancer: A Multicenter Study in A Tuberculosis-Prevalent Area
Abstract
1. Introduction
2. Methods
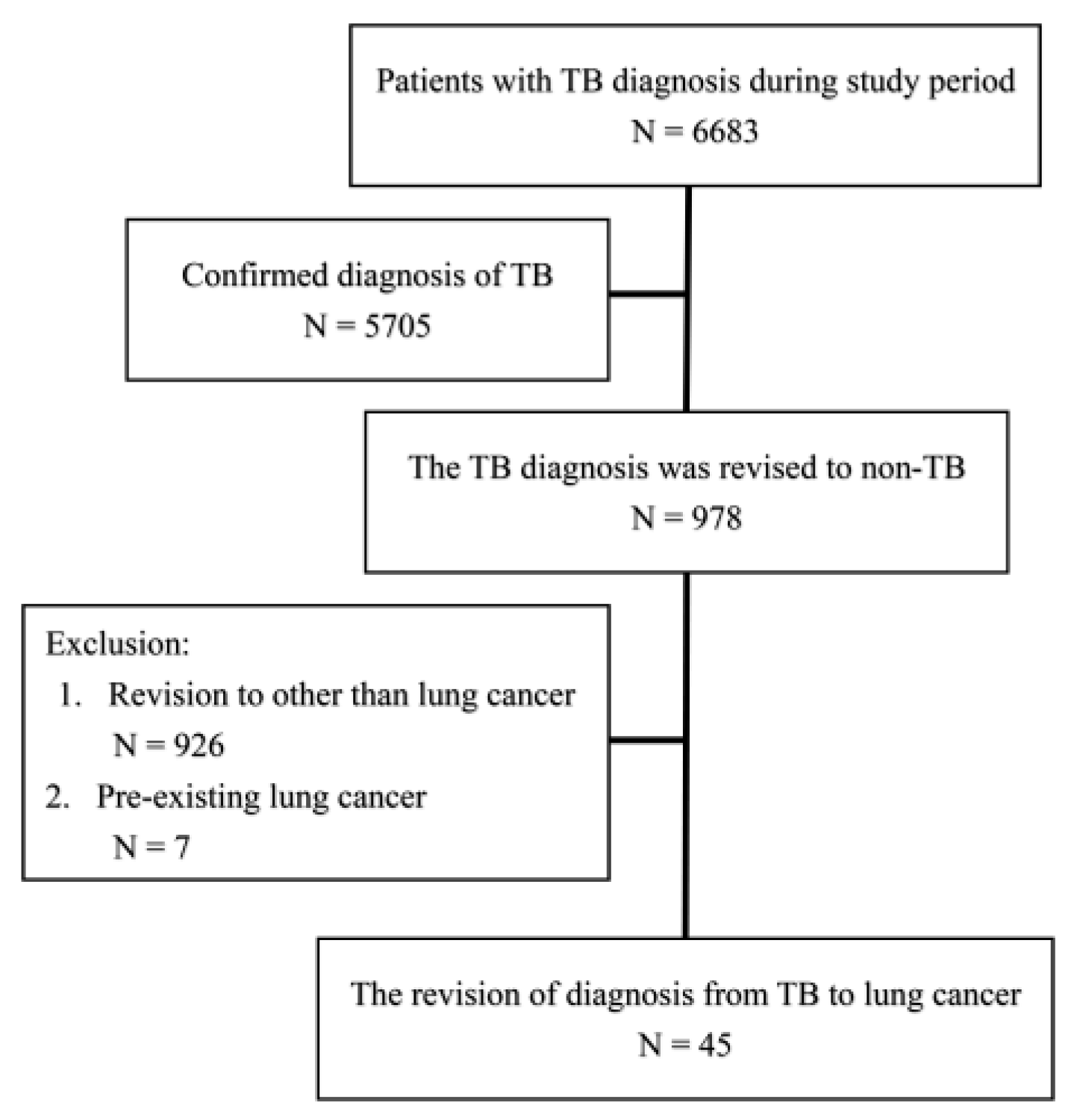
2.1. Participant Enrollment
2.2. Clinical Information
2.3. Outcome and Statistical Analysis
3. Results
3.1. Participant Demographics and Reasons for TB Suspicions
3.2. Final Diagnosis of Lung Cancer Status
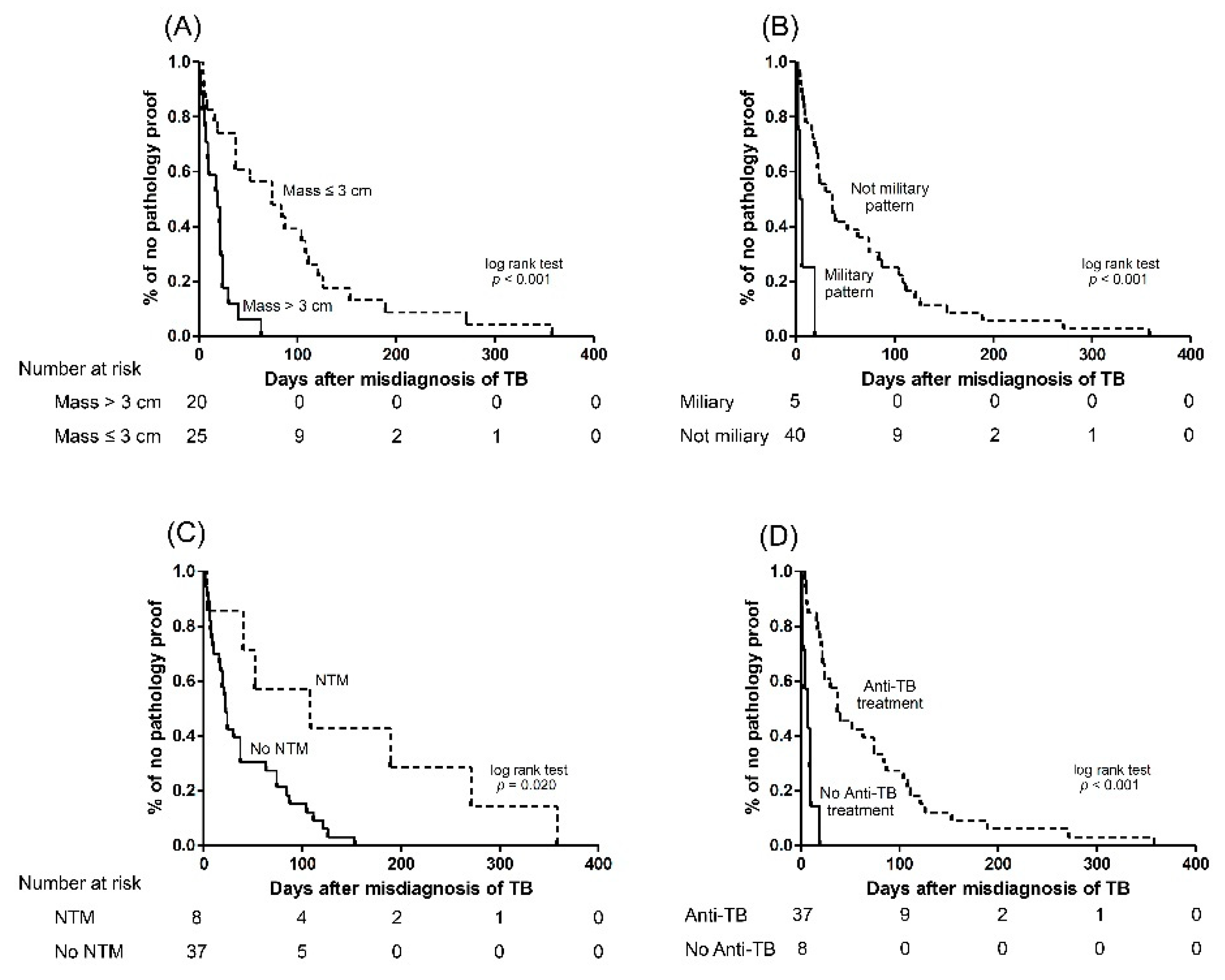
3.3. The Factors Favored Early Revising Diagnosis
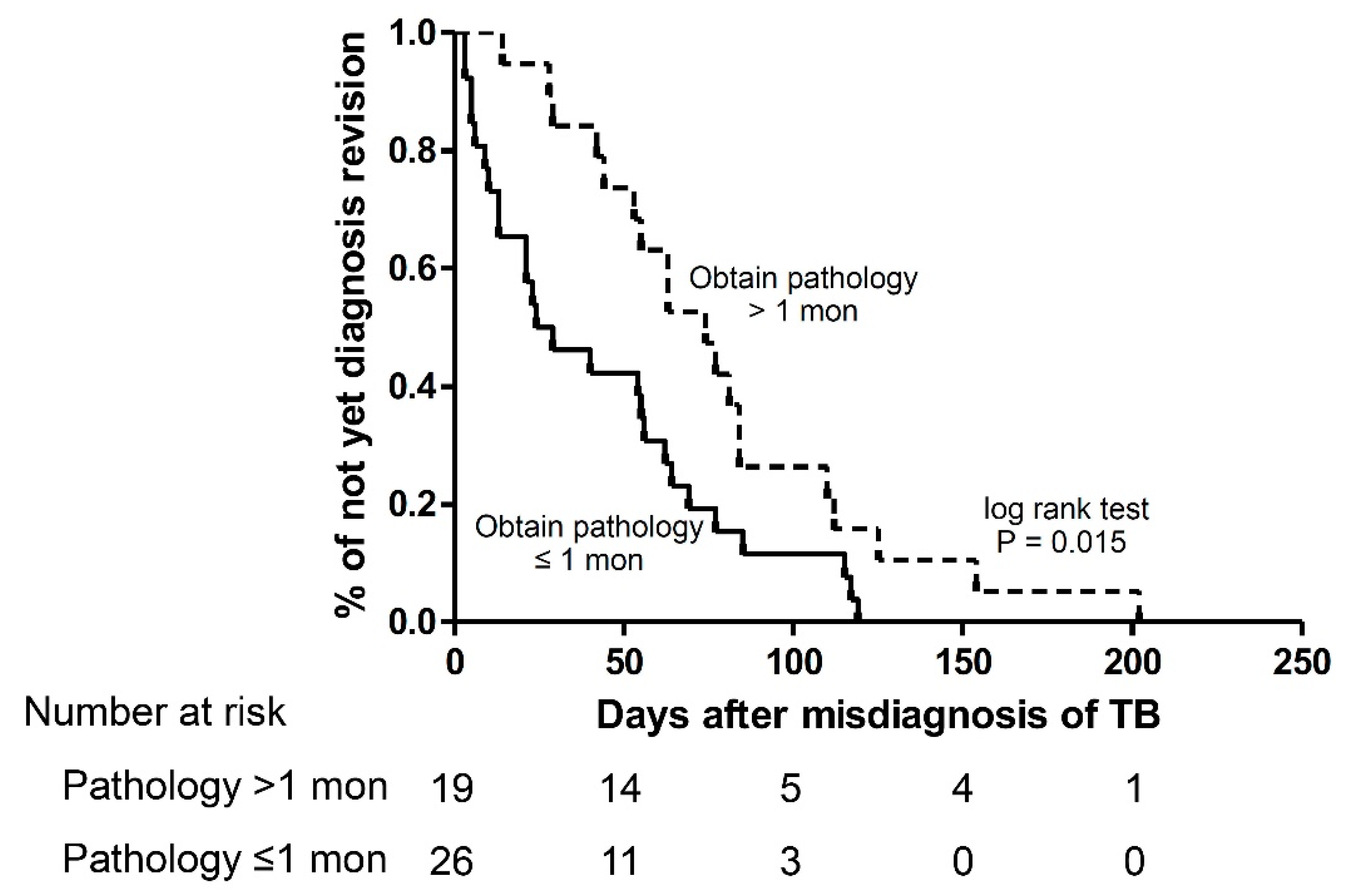
3.4. The Factors Associated with Early Obtained Pathology
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Group at Risk: WHO Report on the Tuberculosis Epidemic; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018.
- MacNeil, A.; Glaziou, P.; Sismanidis, C.; Maloney, S.; Floyd, K. Global epidemiology of tuberculosis and progress toward achieving global targets—2017. MMWR Morb. Mortal. Wkly Rep. 2019, 68, 263–266. [Google Scholar] [CrossRef] [PubMed]
- WHO. The End TB Strategy. 2015. Available online: https://www.who.int/tb/post2015_strategy/en/.
- Lönnroth, K.; Migliori, G.B.; Abubakar, I.; D’Ambrosio, L.; de Vries, G.; Diel, R.; Douglas, P.; Falzon, D.; Gaudreau, M.A.; Goletti, D.; et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur. Respir. J. 2015, 45, 928–952. [Google Scholar] [CrossRef]
- Goletti, D.; Lee, M.R.; Wang, J.Y.; Walter, N.; Ottenhoff, T.H.M. Update on tuberculosis biomarkers: From correlates of risk, to correlates of active disease and of cure from disease. Respirology 2018, 23, 455–466. [Google Scholar] [CrossRef]
- Chiang, C.Y.W.J.; Yu, M.C.; Lee, J.J.; Lee, P.I.; Lee, P.H.; Chou, J.H.; Lin, H.H.; Chiang, I.H.; Hung, C.C.; So, R.; et al. Taiwan Guidelines for TB Diangosis and Treatment, 5th ed.; Center for Disease Control, Executive Yuan: Taipei, Taiwan, 2017.
- Rozenshtein, A.; Hao, F.; Starc, M.T.; Pearson, G.D. Radiographic appearance of pulmonary tuberculosis: Dogma disproved. AJR Am. J. Roentgenol. 2015, 204, 974–978. [Google Scholar] [CrossRef]
- Curley, C.A. Rule out pulmonary tuberculosis: Clinical and radiographic clues for the internist. Cleve. Clin. J. Med. 2015, 82, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Masamba, L.P.L.; Jere, Y.; Brown, E.R.S.; Gorman, D.R. Tuberculosis diagnosis delaying treatment of cancer: Experience from a New Oncology Unit in Blantyre, Malawi. J. Glob. Oncol. 2016, 2, 26–29. [Google Scholar] [CrossRef]
- Lu, D.; Heeren, B.; Dunne, W.M. Comparison of the Automated Mycobacteria Growth Indicator Tube System (BACTEC 960/MGIT) with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Am. J. Clin. Pathol. 2002, 118, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; Lo, W.C.; Yang, Y.W.; You, S.L.; Chen, C.J.; Lai, M.S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. 2016, 115, 1076–1088. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Oh, C.M.; Kong, H.J.; Lee, D.H.; Lee, K.H. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2014. Cancer Res. Treat. 2017, 49, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Li, Q.; McGettigan, M.J.; Balagurunathan, Y.; Garcia, A.L.; Thompson, Z.J.; Heine, J.J.; Ye, Z.; Gillies, R.J.; et al. Radiologic features of small pulmonary nodules and lung cancer risk in the National Lung Screening Trial: A nested case-control study. Radiology 2018, 286, 298–306. [Google Scholar] [CrossRef]
- Nachiappan, A.C.; Rahbar, K.; Shi, X.; Guy, E.S.; Barbosa, E.J.M.; Shroff, G.S.; Ocazionez, D.; Schlesinger, A.E.; Katz, S.I.; Hammer, M.M. Pulmonary tuberculosis: Role of radiology in diagnosis and management. Radiographics 2017, 37, 52–72. [Google Scholar] [CrossRef]
- Light, R.W. Update on tuberculous pleural effusion. Respirology 2010, 15, 451–458. [Google Scholar] [CrossRef]
- Light, R.W. Clinical practice. Pleural effusion. N. Engl. J. Med. 2002, 346, 1971–1977. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, J.Y.; Chien, Y.C.; Chen, K.Y.; Yu, C.J.; Yang, P.C. Lung cancer mimicking pulmonary tuberculosis in a TB-endemic country: The role of early invasive diagnostic procedures. Lung Cancer Manag. 2015, 4, 9–16. [Google Scholar] [CrossRef]
- Lobrano, M.B. Partnerships in oncology and radiology: The role of radiology in the detection, staging, and follow-up of lung cancer. Oncologist 2006, 11, 774–779. [Google Scholar] [CrossRef]
- Burki, T.K. Late detection of lung cancer. Lancet Oncol. 2014, 15, e590. [Google Scholar] [CrossRef]
- Vinas, F.; Ben Hassen, I.; Jabot, L.; Monnet, I.; Chouaid, C. Delays for diagnosis and treatment of lung cancers: A systematic review. Clin. Respir. J. 2016, 10, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Kestin, L.L.; Grills, I.S.; Battu, M.; Fitch, D.W.; Wong, C.O.; Margolis, J.H.; Chmielewski, G.W.; Welsh, R.J. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Lee, C.H.; Lee, M.C.; Wang, J.Y.; Yu, C.J.; Lee, L.N. Hepatotoxicity due to first-line anti-tuberculosis drugs: A five-year experience in a Taiwan medical centre. Int. J. Tuberc. Lung Dis. 2013, 17, 934–939. [Google Scholar] [CrossRef]
- Centers of Disease Control DoH, R.O.C. (Taiwan). CDC Annual Report 2018; Centers of Disease Control, Department of Health: Taipei, Taiwan, 2018.
- Morales-Garcia, C.; Parra-Ruiz, J.; Sanchez-Martinez, J.A.; Delgado-Martin, A.E.; Amzouz-Amzouz, A.; Hernandez-Quero, J. Concomitant tuberculosis and lung cancer diagnosed by bronchoscopy. Int. J. Tuberc. Lung Dis. 2015, 19, 1027–1032. [Google Scholar] [CrossRef]
- Varol, Y.; Varol, U.; Unlu, M.; Kayaalp, I.; Ayranci, A.; Dereli, M.S.; Guclu, S.Z. Primary lung cancer coexisting with active pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2014, 18, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Seon, H.J.; Kim, Y.I.; Lim, S.C.; Kim, Y.H.; Kwon, Y.S. Clinical significance of residual lesions in chest computed tomography after anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2014, 18, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Su, W.J.; Feng, J.Y.; Chiu, Y.C.; Huang, S.F.; Lee, Y.C. Role of 2-month sputum smears in predicting culture conversion in pulmonary tuberculosis. Eur. Respir. J. 2011, 37, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.; Karanth, S.; Ocazionez, D.; Estrada, Y.M.R.M.; Cherian, S.V. Clinical characteristics and etiologies of miliary nodules in the US: A single-center study. Am. J. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Furqan, M.; Butler, J. Miliary pattern on chest radiography: TB or not TB? Mayo Clin. Proc. 2010, 85, 108. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; Bendien, S.A.; de Lange, W.C.M.; Hoefsloot, W.; Dekhuijzen, P.N.R.; Boeree, M.J.; van Soolingen, D. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax 2009, 64, 502–506. [Google Scholar] [CrossRef] [PubMed]
| All (n = 45) | Early Revision (n = 17) | Late Revision (n = 28) | p Value | |
|---|---|---|---|---|
| Age (years) | 66.1 ± 13.3 | 69.3 ± 11.8 | 64.1 ± 13.9 | 0.242 |
| Male sex | 32 (71%) | 11 (75%) | 21 (75%) | 0.460 |
| Microbiology | ||||
| AFS * | 0.368 | |||
| Positive | 9 (20%) | 4 (24%) | 5 (18%) | |
| Negative | 35 (78%) | 12 (71%) | 23 (82%) | |
| Culture * | 0.147 | |||
| NTM | 8 (18%) | 4 (24%) | 4 (14%) | |
| Negative | 16 (36%) | 3 (18%) | 13 (46%) | |
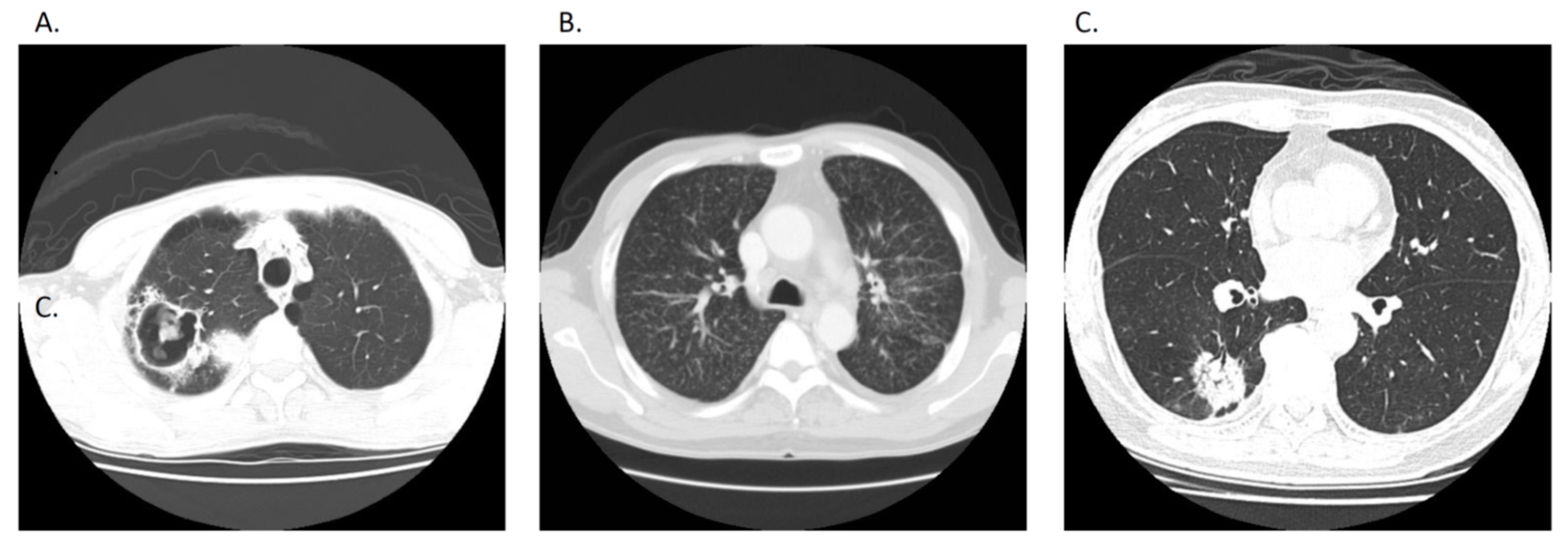
| Chest CT radiographic pattern | 0.529 | |||
| Nodular | 28 (62%) | 11 (65%) | 17 (61%) | |
| Consolidation | 15 (33%) | 6 (35%) | 9 (32%) | |
| Pleural effusion | 2 (4%) | 0 | 2 (7%) | |
| Lesion size (cm) | 4.7 ± 2.8 | 5.2 ± 3.0 | 4.4 ± 2.7 | 0.235 |
| Bilateral | 6 (13%) | 3 (18%) | 3 (11%) | 0.710 |
| Cavitation | 10 (22%) | 1 (6%) | 9 (32%) | 0.040 |
| Miliary pattern | 5 (11%) | 3 (18%) | 2 (7%) | 0.277 |
| Pleural effusion amount | 0.388 | |||
| <1/3 hemithorax | 3 (7%) | 2 (12%) | 1 (4%) | |
| 1/3–2/3 hemithorax | 5 (11%) | 2 (12%) | 3 (11%) | |
| >2/3 hemithorax | 1 (2%) | 1 (6%) | 0 | |
| The cause for TB suspicion | 0.732 | |||
| AFS (+) | 12 (27%) | 5 (29%) | 7 (25%) | |
| Pathology suspicion | 2 (4%) | 0 | 2 (7%) | |
| Radiological suspicion | 23 (51%) | 9 (53%) | 14 (50%) | |
| Clinical suspicion | 8 (18%) | 3 (18%) | 5 (18%) | |
| Anti-TB treatment | 37 (82%) | 11 (65%) | 26 (93%) | 0.017 |
| Days to obtain pathology | 52.9 ± 74.4 | 46.9 ± 104.0 | 56.5 ± 50.7 | 0.009 |
| Days to diagnosis revision | 57.6 ± 44.7 | 15.1 ± 9.4 | 83.4 ± 37.0 | <0.001 |
| Characteristics | Multivariate | |
|---|---|---|
| HR (95% C.I.) | p Value | |
| Early obtaining pathology vs late group | 2.079 (1.041–4.154) | 0.038 |
| Empirical anti-TB treatment vs none | 0.363 (0.120–1.097) | 0.073 |
| AFS positive vs negative/not done | 0.409 (0.146–1.148) | 0.089 |
| Characteristics | Multivariate | |
|---|---|---|
| HR (95% C.I.) | p Value | |
| Miliary radiographic pattern vs. others | 14.739 (4.096–53.038) | <0.001 |
| Lesion size per 1 cm increment | 4.258 (1.659–10.924) | 0.003 |
| Empirical anti-TB treatment vs. no treatment | 0.305 (0.094–0.994) | 0.049 |
| Culture (+) for NTM vs. negative and not done | 0.310 (0.114–0.841) | 0.021 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, C.-C.; Chang, S.-C.; Lai, Y.-C.; Chang, C.-Y.; Wei, Y.-F.; Chen, C.-Y. Factors for the Early Revision of Misdiagnosed Tuberculosis to Lung Cancer: A Multicenter Study in A Tuberculosis-Prevalent Area. J. Clin. Med. 2019, 8, 700. https://doi.org/10.3390/jcm8050700
Shu C-C, Chang S-C, Lai Y-C, Chang C-Y, Wei Y-F, Chen C-Y. Factors for the Early Revision of Misdiagnosed Tuberculosis to Lung Cancer: A Multicenter Study in A Tuberculosis-Prevalent Area. Journal of Clinical Medicine. 2019; 8(5):700. https://doi.org/10.3390/jcm8050700
Chicago/Turabian StyleShu, Chin-Chung, Shih-Chieh Chang, Yi-Chun Lai, Cheng-Yu Chang, Yu-Feng Wei, and Chung-Yu Chen. 2019. "Factors for the Early Revision of Misdiagnosed Tuberculosis to Lung Cancer: A Multicenter Study in A Tuberculosis-Prevalent Area" Journal of Clinical Medicine 8, no. 5: 700. https://doi.org/10.3390/jcm8050700
APA StyleShu, C.-C., Chang, S.-C., Lai, Y.-C., Chang, C.-Y., Wei, Y.-F., & Chen, C.-Y. (2019). Factors for the Early Revision of Misdiagnosed Tuberculosis to Lung Cancer: A Multicenter Study in A Tuberculosis-Prevalent Area. Journal of Clinical Medicine, 8(5), 700. https://doi.org/10.3390/jcm8050700





