Pleural Tap-Guided Antimicrobial Treatment for Pneumonia with Parapneumonic Effusion or Pleural Empyema in Children: A Single-Center Cohort Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethics Statement
2.2. Patients
2.3. Data Collection
2.4. Imaging
2.4.1. Quantification (Grade 1–3)
2.4.2. Classification (Stage 1–3)
2.5. Microbiological Analyses
2.5.1. Pleural Fluid
2.5.2. Blood Culture
2.6. Follow-up and Outcome
2.7. Statistical Analysis
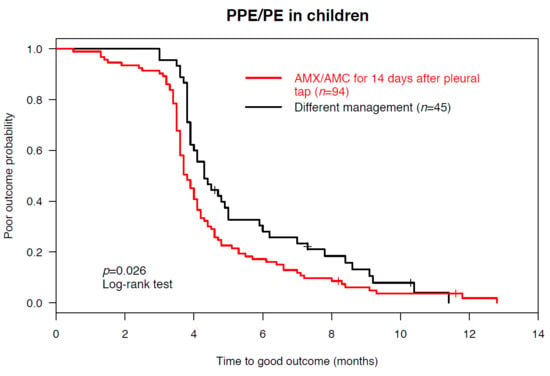
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harris, M.; Clark, J.; Coote, N.; Fletcher, P.; Harnden, A.; McKean, M.; Thomson, A.; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax 2011, 66, 1–23. [Google Scholar] [CrossRef]
- Li, S.T.; Tancredi, D.J. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics 2010, 125, 26–33. [Google Scholar] [CrossRef]
- Yu, D.; Buchvald, F.; Brandt, B.; Nielsen, K.G. Seventeen-year study shows rise in parapneumonic effusion and empyema with higher treatment failure after chest tube drainage. Acta Paediatr. 2014, 103, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Bou, S.; Garcia-Garcia, J.J.; Esteva, C.; Gene, A.; Luaces, C.; Munoz Almagro, C. Pediatric parapneumonic pleural effusion: Epidemiology, clinical characteristics, and microbiological diagnosis. Pediatr. Pulmonol. 2009, 44, 1192–1200. [Google Scholar] [CrossRef]
- Bradley, J.S.; Byington, C.L.; Shah, S.S.; Alverson, B.; Carter, E.R.; Harrison, C.; Kaplan, S.L.; Mace, S.E.; McCracken, G.H., Jr.; Moore, M.R.; et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, e25–e76. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.C.; King, M.D.; Miller, M.L. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr. Infect. Dis. J. 2003, 22, 499–504. [Google Scholar] [CrossRef]
- Ouldali, N.; Levy, C.; Minodier, P.; Morin, L.; Biscardi, S.; Aurel, M.; Dubos, F.; Dommergues, M.A.; Mezgueldi, E.; Levieux, K.; et al. Long-term association of 13-valent pneumococcal conjugate vaccine implementation with rates of community-acquired pneumonia in children. JAMA Pediatr. 2019. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.H.; Kim, W.S.; Kim, M.J.; Jung, J.Y.; Suh, J.H. US in the diagnosis of pediatric chest diseases. Radiographics 2000, 20, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.; Waldhausen, J.; Zhang, W.; Hoffman, L.; Redding, G. Management of children with empyema: Pleural drainage is not always necessary. Pediatr. Pulmonol. 2010, 45, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.A.; Winterhalter, K.M.; Connors, R.H.; Betz, B.W.; Winters, J.W. Therapy of parapneumonic effusions in children: Video-assisted thoracoscopic surgery versus conventional thoracostomy drainage. Pediatrics 2006, 118, e547–e553. [Google Scholar] [CrossRef] [PubMed]
- Balfour-Lynn, I.M.; Abrahamson, E.; Cohen, G.; Hartley, J.; King, S.; Parikh, D.; Spencer, D.; Thomson, A.H.; Urquhart, D. Paediatric Pleural Diseases Subcommittee of the BTS Standards of Care Committee. BTS guidelines for the management of pleural infection in children. Thorax 2005, 60, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Segerer, F.J.; Seeger, K.; Maier, A.; Hagemann, C.; Schoen, C.; van der Linden, M.; Streng, A.; Rose, M.A.; Liese, J.G. Therapy of 645 children with parapneumonic effusion and empyema-A German nationwide surveillance study. Pediatr. Pulmonol. 2017, 52, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Le Mee, A.; Mordacq, C.; Lagree, M.; Deschildre, A.; Martinot, A.; Dubos, F. Survey of hospital procedures for parapneumonic effusion in children highlights need for standardised management. Acta Paediatr. 2014, 103, e393–e398. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing, EUCAST. Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. 2017. Available online: http://www.eucast.org/resistance_mechanisms/ (accessed on 15 April 2019).
- Tarrago, D.; Fenoll, A.; Sanchez-Tatay, D.; Arroyo, L.A.; Munoz-Almagro, C.; Esteva, C.; Hausdorff, W.P.; Casal, J.; Obando, I. Identification of pneumococcal serotypes from culture-negative clinical specimens by novel real-time PCR. Clin. Microbiol. Infect. 2008, 14, 828–834. [Google Scholar] [CrossRef]
- Rampini, S.K.; Bloemberg, G.V.; Keller, P.M.; Buchler, A.C.; Dollenmaier, G.; Speck, R.F.; Bottger, E.C. Broad-range 16S rRNA gene polymerase chain reaction for diagnosis of culture-negative bacterial infections. Clin. Infect. Dis. 2011, 53, 1245–1251. [Google Scholar] [CrossRef]
- Asner, S.A.; Agyeman, P.K.A.; Gradoux, E.; Posfay-Barbe, K.M.; Heininger, U.; Giannoni, E.; Crisinel, P.A.; Stocker, M.; Bernhard-Stirnemann, S.; Niederer-Loher, A.; et al. Burden of Streptococcus pneumoniae sepsis in children after introduction of pneumococcal conjugate vaccines - a prospective population-based cohort study. Clin. Infect. Dis. 2019. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 April 2019).
- Chan, K.P.; Fitzgerald, D.B.; Lee, Y.C.G. Emerging concepts in pleural infection. Curr. Opin. Pulm. Med. 2018, 24, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Redden, M.D.; Chin, T.Y.; van Driel, M.L. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst. Rev. 2017, 3, CD010651. [Google Scholar] [CrossRef]
- Hafen, G.M.; Grenzbach, A.C.; Moeller, A.; Rochat, M.K. Lack of concordance in parapneumonic effusion management in children in central Europe. Pediatr. Pulmonol. 2016, 51, 411–417. [Google Scholar] [CrossRef]
- Dorman, R.M.; Vali, K.; Rothstein, D.H. Trends in treatment of infectious parapneumonic effusions in U.S. children’s hospitals, 2004–2014. J. Pediatr. Surg. 2016, 51, 885–890. [Google Scholar] [CrossRef]
- Islam, S.; Calkins, C.M.; Goldin, A.B.; Chen, C.; Downard, C.D.; Huang, E.Y.; Cassidy, L.; Saito, J.; Blakely, M.L.; Rangel, S.J.; et al. The diagnosis and management of empyema in children: A comprehensive review from the APSA Outcomes and Clinical Trials Committee. J. Pediatr. Surg. 2012, 47, 2101–2110. [Google Scholar] [CrossRef]
- Le Monnier, A.; Carbonnelle, E.; Zahar, J.R.; Le Bourgeois, M.; Abachin, E.; Quesne, G.; Varon, E.; Descamps, P.; De Blic, J.; Scheinmann, P.; et al. Microbiological diagnosis of empyema in children: Comparative evaluations by culture, polymerase chain reaction, and pneumococcal antigen detection in pleural fluids. Clin. Infect. Dis. 2006, 42, 1135–1140. [Google Scholar] [CrossRef]
- Casado Flores, J.; Nieto Moro, M.; Berron, S.; Jimenez, R.; Casal, J. Usefulness of pneumococcal antigen detection in pleural effusion for the rapid diagnosis of infection by Streptococcus pneumoniae. Eur. J. Pediatr. 2010, 169, 581–584. [Google Scholar] [CrossRef]
- Langley, J.M.; Kellner, J.D.; Solomon, N.; Robinson, J.L.; Le Saux, N.; McDonald, J.; Ulloa-Gutierrez, R.; Tan, B.; Allen, U.; Dobson, S.; et al. Empyema associated with community-acquired pneumonia: A Pediatric Investigator’s Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect. Dis. 2008, 8, 129. [Google Scholar] [CrossRef]
- Gaudelus, J.; Dubos, F.; Dommergues, M.A.; Vu Thien, H.; Bingen, E.; Cohen, R.; Groupe de Pathologie Infectieuse Pediatrique de la Societe Francaise de Pediatrie. Antibiotic treatment of child empyema: Lessons from published studies and therapeutic options. Arch. Pediatr. 2008, S15, S84–S92. [Google Scholar] [CrossRef]
- Liese, J.G.; Schoen, C.; van der Linden, M.; Lehmann, L.; Goettler, D.; Keller, S.; Maier, A.; Segerer, F.; Rose, M.A.; Streng, A. Changes in the incidence and bacterial aetiology of paediatric parapneumonic pleural effusions/empyema in Germany, 2010-2017: A nationwide surveillance study. Clin. Microbiol. Infect. 2018. Epub ahead of print. [Google Scholar] [CrossRef]
- Krenke, K.; Sadowy, E.; Podsiadly, E.; Hryniewicz, W.; Demkow, U.; Kulus, M. Etiology of parapneumonic effusion and pleural empyema in children. The role of conventional and molecular microbiological tests. Respir. Med. 2016, 116, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.R.; Morgenthaler, T.I.; Ryu, J.H.; Daniels, C.E. Reducing iatrogenic risk in thoracentesis: Establishing best practice via experiential training in a zero-risk environment. Chest 2009, 135, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Niemi, E.; Korppi, M. Parapneumonic empyema in children before the era of pneumococcal vaccination. Acta Paediatr. 2011, 100, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Byington, C.L.; Spencer, L.Y.; Johnson, T.A.; Pavia, A.T.; Allen, D.; Mason, E.O.; Kaplan, S.; Carroll, K.C.; Daly, J.A.; Christenson, J.C.; et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin. Infect. Dis. 2002, 34, 434–440. [Google Scholar] [CrossRef]
- Tan, T.Q.; Mason, E.O., Jr.; Wald, E.R.; Barson, W.J.; Schutze, G.E.; Bradley, J.S.; Givner, L.B.; Yogev, R.; Kim, K.S.; Kaplan, S.L. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics 2002, 110, 1–6. [Google Scholar] [CrossRef]
- Lahti, E.; Peltola, V.; Virkki, R.; Alanen, M.; Ruuskanen, O. Development of parapneumonic empyema in children. Acta Paediatr. 2007, 96, 1686–1692. [Google Scholar] [CrossRef]
- Madhi, F.; Levy, C.; Morin, L.; Minodier, P.; Dubos, F.; Zenkhri, F.; Dommergues, M.A.; Mezgueldi, E.; Levieux, K.; Pneumonia Study Group. Change in bacterial causes of community-acquired parapneumonic effusion and pleural empyema in children 6 years after 13-valent pneumococcal conjugate vaccine implementation. J. Pediatric Infect. Dis. Soc. 2018. Epub ahead of print. [Google Scholar] [CrossRef]
- Krenke, K.; Urbankowska, E.; Urbankowski, T.; Lange, J.; Kulus, M. Clinical characteristics of 323 children with parapneumonic pleural effusion and pleural empyema due to community acquired pneumonia. J. Infect. Chemother. 2016, 22, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.; Wheeler, R.; Legg, J. Update on the causes, investigation and management of empyema in childhood. Arch. Dis. Child. 2011, 96, 482–488. [Google Scholar] [CrossRef]
- Hauser, C.; Kronenberg, A.; Allemann, A.; Muhlemann, K.; Hilty, M. Serotype/serogroup-specific antibiotic non-susceptibility of invasive and non-invasive Streptococcus pneumoniae, Switzerland, 2004 to 2014. Euro Surveill. 2016, 21. [Google Scholar] [CrossRef]
- Heininger, U.; Datta, F.; Gervaix, A.; Schaad, U.B.; Berger, C.; Vaudaux, B.; Aebi, C.; Hitzler, M.; Kind, C.; Gnehm, H.E.; et al. Prevalence of nasal colonization with methicillin-resistant Staphylococcus aureus (MRSA) in children a multicenter cross-sectional study. Pediatr. Infect. Dis. J. 2007, 26, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Proesmans, M.; De Boeck, K. Clinical practice: Treatment of childhood empyema. Eur. J. Pediatr. 2009, 168, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.; Cohen, E. Hold those scalpels. Arch. Pediatr. Adolesc. Med. 2008, 162, 698–699. [Google Scholar] [CrossRef]
- Picazo, J.; Ruiz-Contreras, J.; Casado-Flores, J.; Negreira, S.; Del Castillo, F.; Hernandez-Sampelayo, T.; Bueno, M.; Calvo, C.; Rios, E.; Mendez, C.; et al. Laboratory-based, 2-year surveillance of pediatric parapneumonic pneumococcal empyema following heptavalent pneumococcal conjugate vaccine universal vaccination in Madrid. Pediatr. Infect. Dis. J. 2011, 30, 471–474. [Google Scholar] [CrossRef]
- Grisaru-Soen, G.; Eisenstadt, M.; Paret, G.; Schwartz, D.; Keller, N.; Nagar, H.; Reif, S. Pediatric parapneumonic empyema: Risk factors, clinical characteristics, microbiology, and management. Pediatr. Emerg. Care. 2013, 29, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Avansino, J.R.; Goldman, B.; Sawin, R.S.; Flum, D.R. Primary operative versus nonoperative therapy for pediatric empyema: A meta-analysis. Pediatrics 2005, 115, 1652–1659. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | Total Number of PPE/PE Patients | PPE/PE Patients Without Surgical Intervention | PPE/PE Patients with Surgical Intervention | OR (95% CI) | p Value |
|---|---|---|---|---|---|
| (n = 147) | (n = 89, 61%) | (n = 58, 39%) | |||
| Demographic characteristics | |||||
| Sex (male), n (%) | 73 (50) | 48 (54) | 25 (43) | 1.5 (0.8–3.2) | 0.238 |
| Age (years), median (IQR) | 4 (3–6) | 4 (3–7) | 4 (2–5) | 0.098 | |
| Clinical characteristics | |||||
| Duration of prodromal fever and/or respiratory symptoms (days), median (IQR) | 7 (4–10) | 7 (5–11) | 6 (4–9) | 0.060 | |
| Diagnosis of pneumonia prior to diagnosis of PPE/PE, n (%) | 67 (46) | 49 (55) | 18 (31) | 2.7 (1.3–5.8) | 0.007 |
| Antibiotics prior to diagnosis of PPE/PE, n (%) | 110 (75) | 72 (81) | 38 (66) | 2.2 (1.0–5.1) | 0.051 |
| Duration from start antibiotics to diagnosis of PPE/PE (days), median (IQR) | 3 (1–5) | 4 (2–6) | 2 (0–4) | <0.001 | |
| Symptoms at diagnosis of PPE/PE: | |||||
| • Chest pain, n (%) | 29 (20) | 21 (24) | 8 (14) | 1.9 (0.7–5.4) | 0.212 |
| • Sepsis, n (%) | 32 (22) | 10 (11) | 22 (38) | 0.2 (0.1–0.5) | <0.001 |
| Inflammatory parameters: | |||||
| • WBC count (G/L), median (IQR) | 15.1 (8.6–22.4) | 15.8 (10.0–21.2) | 13.6 (8.1–22.9) | 0.636 | |
| • CRP (mg/L), median (IQR) | 200 (157–317) | 195 (152–314) | 202 (160–318) | 0.370 | |
| Pleural effusion characteristics | |||||
| Pleural fluid: | |||||
| • Gram stain positive, n (%) | 32/125 (26) | 14/81 (17) | 18/44 (41) | 0.3 (0.1–0.7) | 0.002 |
| • WBC count (cells/μl), median (IQR) | 6604 (1899–69,112) | 4258 (1650–10,964) | 71,000 (5900–121,980) | <0.001 | |
| Size of effusion [5]: | |||||
| • Grade 1 (small, <¼ hemithorax), n (%) | 17/142 (12) | 13/86 (15) | 4/56 (7) | 2.3 (0.7–10.2) | 0.194 |
| • Grade 2 (moderate, ¼–½ hemithorax), n (%) | 35/142 (25) | 30/86 (35) | 5/56 (9) | 5.4 (1.9–19.2) | <0.001 |
| • Grade 3 (large, >½ hemithorax), n (%) | 90/142 (63) | 43/86 (50) | 47/56 (84) | 0.2 (0.1–0.5) | <0.001 |
| Sonographic staging [8]: | |||||
| • Stage 1 (exudative), n (%) | 48/115 (42) | 34/75 (45) | 14/40 (35) | 1.5 (0.7–3.7) | 0.325 |
| • Stage 2 (fibrino-purulent), n (%) | 54/115 (47) | 37/75 (49) | 17/40 (43) | 1.3 (0.6–3.1) | 0.558 |
| • Stage 3 (organized), n (%) | 13/115 (11) | 4/75 (5) | 9/40 (23) | 0.2 (0.1–0.8) | 0.011 |
| Pathogen Detection (n = 114, 78%) | Total | Pleural Fluid | PCR (n) | Antigen (n) | Blood |
|---|---|---|---|---|---|
| n (%) | Culture (n) | Culture (n) | |||
| Streptococcus pneumoniae | 90 (79) | 13 a | 39 | 84 b | 16 a |
| Vaccine serotypes c: | |||||
| 1 | 4 | 2 | 2 | 4 | 2 |
| 3 | 7 | 3 | 1 | 4 | 5 |
| 7F | 3 | 1 | 3 | 3 | |
| 9V | 1 | 1 | 1 | ||
| 19A | 5 | 3 | 1 | 4 | 3 |
| 14 | 3 | 3 | 3 | ||
| NA | 3 | 3 | 2 | ||
| Streptococcus pyogenes | 13 (11) | 12 d | 2 e | 1 | 4 d |
| Staphylococcus aureus | 7 (6) | 6 | 4 | ||
| MSSA | 6 | 5 f | 4 f | ||
| MRSA | 1 | 1 | 0 | ||
| Others g | 4 (4) | 3 h | 1 | 1 | |
| Total | 114 | 34 | 42 | 85 | 25 |
| Management | Total Number of PPE/PE Patients (n = 147) | PPE/PE Patients Without Surgical Intervention (n = 89, 61%) | PPE/PE Patients with Surgical Intervention (n = 58, 39%) | OR (95% CI) | p Value |
|---|---|---|---|---|---|
| Hospitalization | |||||
| LOS (days), median (IQR) | 11 (8–19) | 9 (6–13) | 21 (12–34) | <0.001 | |
| Antibiotic treatment | |||||
| Duration (d) after time point of diagnosis, median (range) | 14 (2–255) | 14 (4–20) | 14 (2–255) | 0.016 | |
| Total duration (d) including pretreatment, median (range) | 16 (13–255) | 16 (14–30) | 16 (13–255) | 0.750 | |
| Outcome | (n = 139) | (n = 82, 59%) | (n = 57, 41%) | ||
| Complete recovery, n (%) | 134 (96) | 80 (98) | 54 (95) | 2.2 (0.2–27.3) | 0.401 |
| Time to complete recovery (months), median (IQR) | 4.0 (3.6–5.1) | 3.8 (3.5–4.7) | 4.3 (3.7–6.0) | 0.023 | |
| Complete recovery achieved in: | |||||
| • ≤4 months FUP, n (%) | 71 (51) | 48 (59) | 23 (41) | 2.1 (1.0–4.4) | 0.040 |
| • 4–6 months FUP, n (%) | 39 (28) | 19 (23) | 20 (35) | 0.6 (0.3–1.3) | 0.131 |
| • >6 months FUP, n (%) | 24 (17) | 13 (16) | 11 (19) | 0.8 (0.3–2.1) | 0.652 |
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Diagnosis | PPE/PE | PPE/PE with ARDS and DIC | Necrotizing PPE/PE with MOF | PPE/PE | PPE/PE |
| Demographic characteristics | |||||
| Sex | Female | Female | Male | Male | Male |
| Age (y) | 1 | 12 | 3 | 2 | 8 |
| Previous medical history | Recurrent viral bronchitis, trisomy 21 | Recurrent viral bronchitis | |||
| Microbiology | |||||
| Etiology | S. pneumoniae (serotype unknown) | MSSA | MRSA | S. pneumoniae (serotype 7F) | S. pneumoniae (serotype 1) |
| Diagnostic test | Antigen test (pleural fluid) | Culture (blood) | Culture (pleural fluid) | Culture (blood, pleural fluid) | Culture (blood), PCR and antigen test (pleural fluid) |
| Other detected pathogens | NA | Influenza B (NPS) | Candida (CRBI) | Influenza A (NPS) | NA |
| Pleural effusion | |||||
| Grading [5] | 3 (large) | 2 (moderate) | 3 (large) | 3 (large) | 3 (large) |
| Staging [8] | NA | NA | 1 (exudative) | 2 (fibrino-purulent) | 1 (exudative) |
| Hospitalization and management | |||||
| LOS (days) | 10 | 39 | 77 | 19 | 20 |
| ICU (days) | 15 | 55 | 5 | ||
| Surgical intervention | Pleural draining catheter | Pleural draining catheter | Thoracotomy and decortication following pleural draining catheter, VA-ECMO (9 days) | ||
| Complication | Pneumothorax, bronchopleural fistula | Pneumothorax, ARDS, DIC | Bronchopleural fistula, pneumothorax, necrotizing pneumonia, MOF, ischemic cerebral lesions, CRBI | ||
| Antibiotics (total duration in days) | AMC (14) | AMX, gentamicin, flucloxacillin, clindamycin (21) | AMC, teicoplanin, gentamicin, meropenem, vancomycin, linezolid (28) | AMC (14) | AMX (18) |
| FUP and outcome | |||||
| Last FUP (months) | 12 | 7 | 10 | 8 | 5 |
| Clinical recovery | + | – | + | – | + |
| Details | NA | Reduced lung function and exercise capacity | NA | Reduced lung function and exercise capacity | NA |
| Radiological recovery | – | – | – | – | – |
| Details | Persistent bulla, residual pleural thickening | Areas of air trapping | Residual pleural thickening | Emphysema, atelectasis | Residual pleural thickening |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer Sauteur, P.M.; Burkhard, A.; Moehrlen, U.; Relly, C.; Kellenberger, C.; Ruoss, K.; Berger, C. Pleural Tap-Guided Antimicrobial Treatment for Pneumonia with Parapneumonic Effusion or Pleural Empyema in Children: A Single-Center Cohort Study. J. Clin. Med. 2019, 8, 698. https://doi.org/10.3390/jcm8050698
Meyer Sauteur PM, Burkhard A, Moehrlen U, Relly C, Kellenberger C, Ruoss K, Berger C. Pleural Tap-Guided Antimicrobial Treatment for Pneumonia with Parapneumonic Effusion or Pleural Empyema in Children: A Single-Center Cohort Study. Journal of Clinical Medicine. 2019; 8(5):698. https://doi.org/10.3390/jcm8050698
Chicago/Turabian StyleMeyer Sauteur, Patrick M., Ariane Burkhard, Ueli Moehrlen, Christa Relly, Christian Kellenberger, Kerstin Ruoss, and Christoph Berger. 2019. "Pleural Tap-Guided Antimicrobial Treatment for Pneumonia with Parapneumonic Effusion or Pleural Empyema in Children: A Single-Center Cohort Study" Journal of Clinical Medicine 8, no. 5: 698. https://doi.org/10.3390/jcm8050698
APA StyleMeyer Sauteur, P. M., Burkhard, A., Moehrlen, U., Relly, C., Kellenberger, C., Ruoss, K., & Berger, C. (2019). Pleural Tap-Guided Antimicrobial Treatment for Pneumonia with Parapneumonic Effusion or Pleural Empyema in Children: A Single-Center Cohort Study. Journal of Clinical Medicine, 8(5), 698. https://doi.org/10.3390/jcm8050698