Diverse Action of Selected Statins on Skeletal Muscle Cells—An Attempt to Explain the Protective Effect of Geranylgeraniol (GGOH) in Statin-Associated Myopathy (SAM)
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Antibodies
2.2. Cell Cultures and Treatments
2.3. Assessment of Cell Viability
2.4. Mitochondrial Bioenergetics Measurements
2.5. Western Blot Analysis
2.6. Statistical Analyses
3. Results
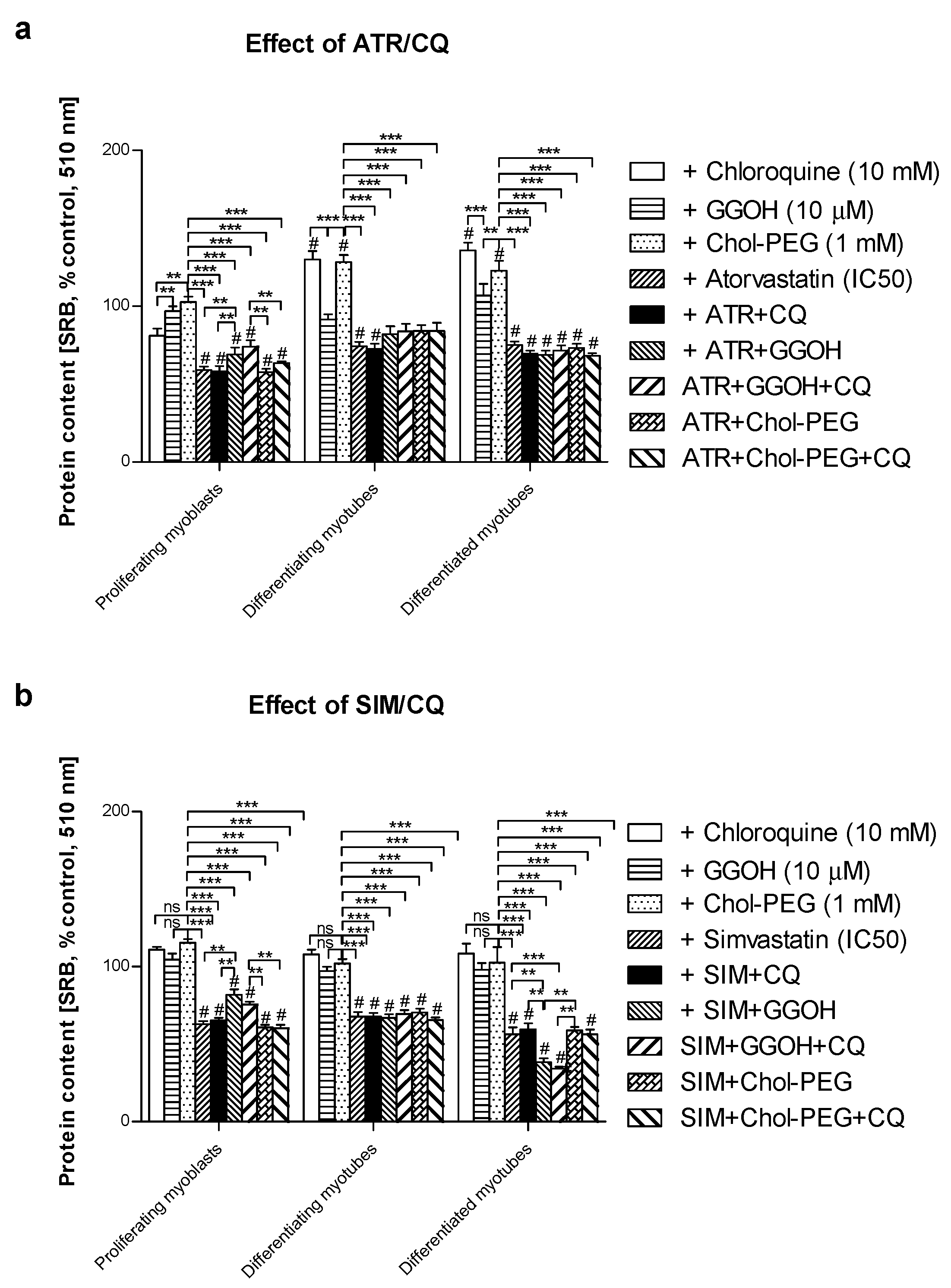
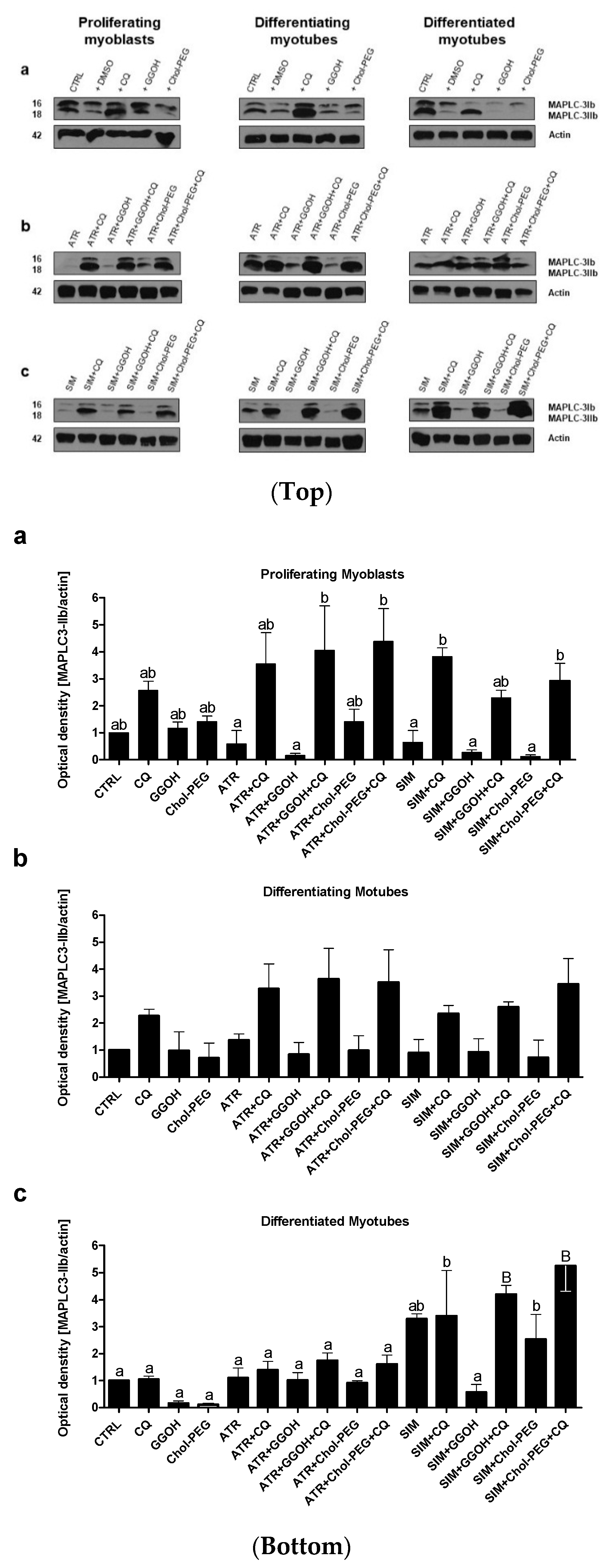
3.1. Magnitude of Autophagy Increases in Differentiating Skeletal Muscle Cells. Rescuing Skeletal Muscle Cells from Statin-Induced Myotoxicity by GGOH is Independent of Autophagy
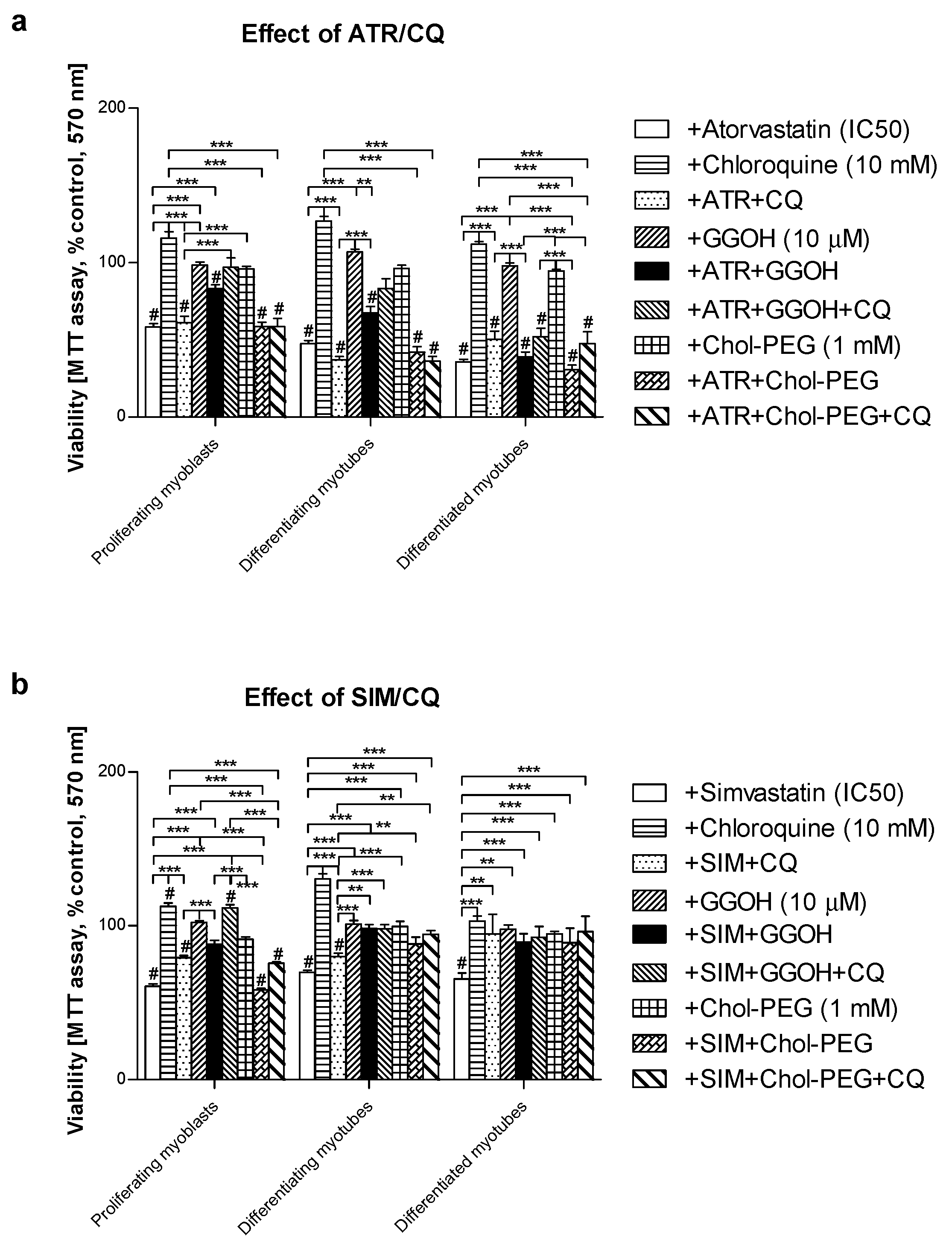
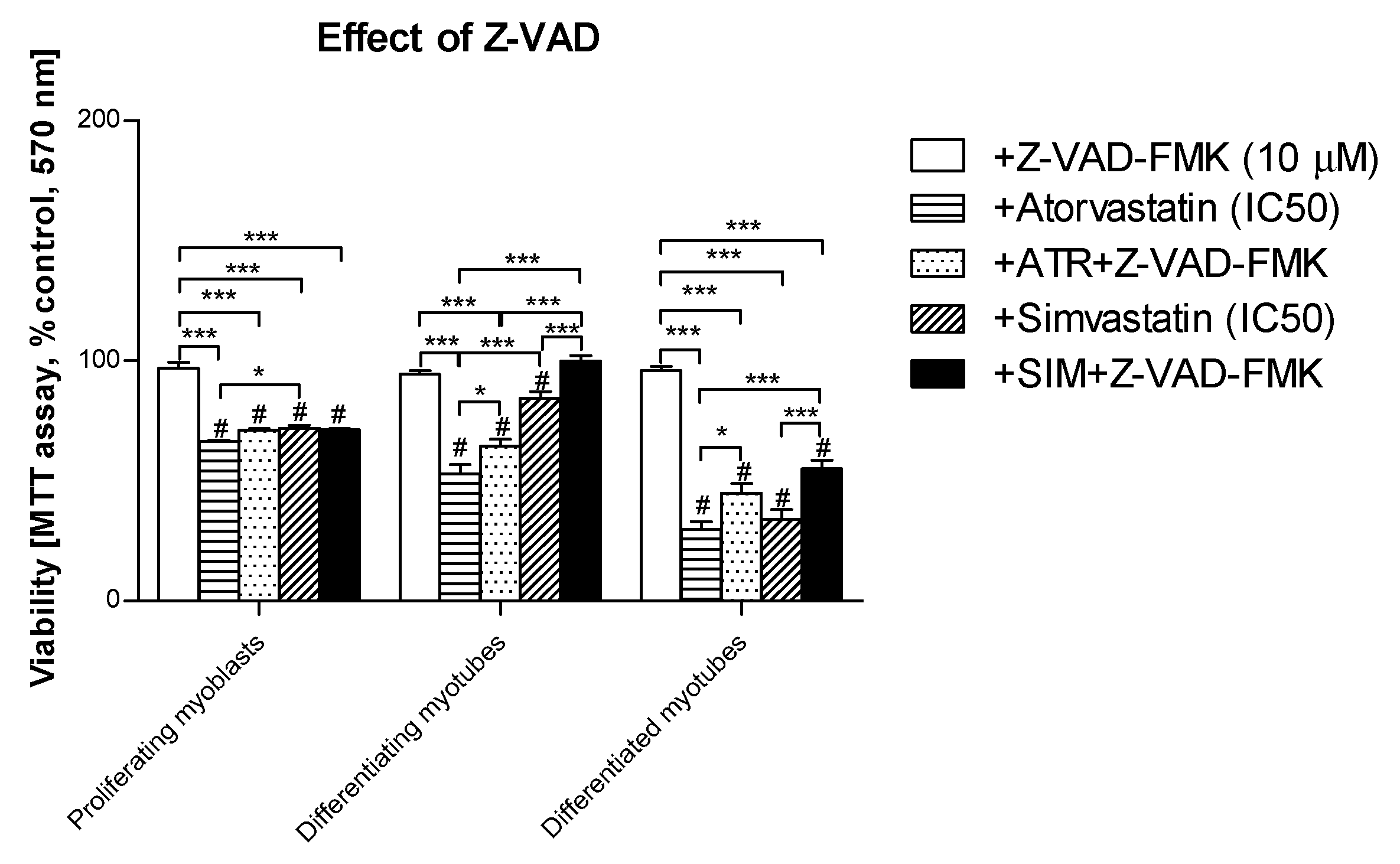
3.2. Pan-Caspase (Z-VAD-FMK) Inhibitor Could Partially Retain Muscle Cell Viability Reduced by Statins in Differentiating and differentiated myotubes but Not in Proliferating Myoblasts
3.3. Increased Magnitude of Autophagy in Differentiating Skeletal Muscle Cells Is Inversely Proportional to Procaspase-3 Cleavage
3.4. Inhibition of Calpains (μ- And m-) But Not Z-VAD-FMK Mimics the Effect of GGOH on Statin-Induced Myotoxicity
3.5. The Effect of GGOH or Chol-PEG co-Treatment Upon Mitochondrial Cytochrome C Oxidase Protein Expression Levels in Statin-Treated C2C12 Muscle Cells
3.6. Mitochondrial Respiration in Statin-Treated C2C12 Muscle Cells. The Effect of GGOH and Chol-PEG Co-Treatment
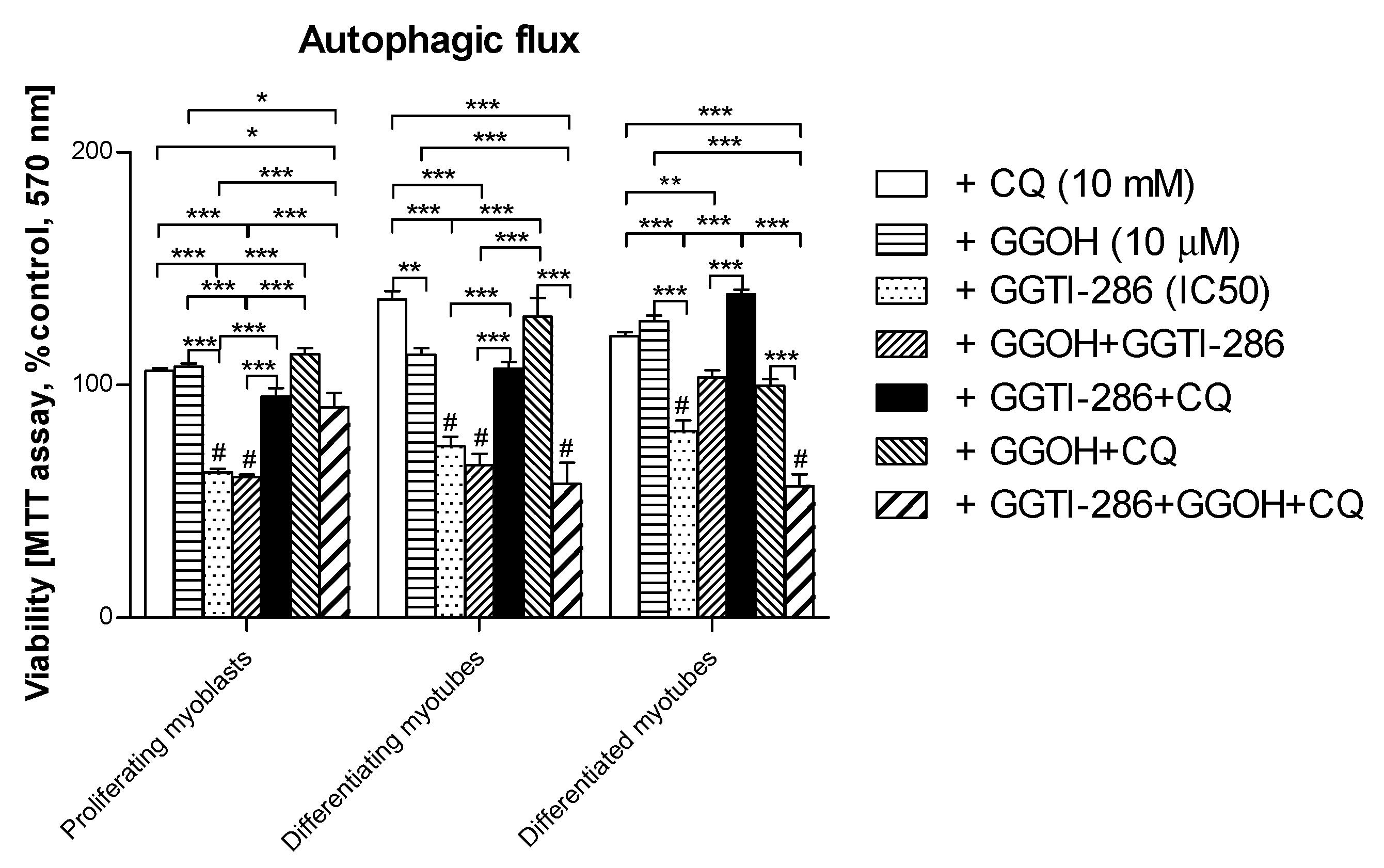
3.7. Viability of C2C12 Muscle Cells during Autophagic Flux. The Cytoprotective Effect of GGOH Is Fully Dependent on Geranylgeraniol Transferase I (GGT-I) and Could Be Reversed by Inhibition of Autophagy with Chloroquine
4. Discussion
5. Conclusions
- (i)
- Autophagy is essential for myogenesis.
- (ii)
- The myoprotective effect of GGOH is not dependent on autophagy activation.
- (iii)
- Atorvastatin does, whereas simvastatin does not impair mitochondrial bioenergetics.
- (iv)
- GGOH prevents muscle cell viability in SAM through the inhibition of calpains rather than caspases.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gotto, A.M.G. Statins, cardiovascular disease, and drug safety. Am. J. Cardiol. 2006, 97, 3C–5C. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 1992, 33, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Rosenon, R.S. Current overview of statin-induced myopathy. Am. J. Med. 2002, 116, 408–416. [Google Scholar] [CrossRef]
- Harper, C.R.; Jacobson, T.A. The broad spectrum of statin myopathy: From myalgia to rhabmomylysis. Curr. Opin. Lipidol. 2007, 18, 401–408. [Google Scholar] [CrossRef]
- Law, M.; Rudnicka, A.R. Statin safety: A systemic review. Am. J. Cardiol. 2006, 97, 52C–60C. [Google Scholar] [CrossRef]
- Bruckert, E.; Hayem, G.; Dejager, S.; Yau, C.; Begaud, B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—The PRIMO study. Cardiovasc. Drugs Ther. 2005, 19, 403–414. [Google Scholar] [CrossRef]
- Urso, M.L.; Clarkson, P.M.; Hittel, D.; Hoffman, E.P.; Thompson, P.D. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2560–2566. [Google Scholar] [CrossRef]
- Newman, C.; Tsai, J.; Szarek, M.; Luo, D.; Gibson, E. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,326 patients. Am. J. Cardiol. 2006, 97, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Cannon, C.P.; Morrow, D.A.; Ray, K.K.; Pfeffer, M.A.; Braunwald, E. Can low-density lipoprotein be too low? The safety and afficacy of achieving very low low-density lipoprotein with intensive statin therapy: A PROVE IT-TIMI 22 substugy. J. Am. Coll. Cardiol. 2005, 46, 1411–1416. [Google Scholar] [CrossRef]
- Baker, S. Molecular clues into the pathogenesis of statin-mediated muscle toxicity. Muscle Nerve 2005, 31, 572–580. [Google Scholar] [CrossRef]
- Hodel, C. Myopathy and rhabdomyolysis with lipid-lowering drugs. Toxicol. Lett. 2002, 128, 159–168. [Google Scholar] [CrossRef]
- Marcoff, L.; Thompson, P.D. The role of coenzyme Q10 in statin associated myopathy: A systemic review. J. Am. Coll. Cardiol. 2007, 49, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Dirks, A.J.; Jones, K.M. Statin-induced aoptosis and skeletal muscle myopathy. Am. J. Physiol. Cell Physiol. 2006, 291, C1208–C1212. [Google Scholar] [CrossRef] [PubMed]
- Ameziane-Le Hir, A.; Raguenes-Nicol, C.; Paboeuf, G.; Nicolas, A.; Le Rumeur, E.; Vie, V. Cholesterol favors the anchorage of human dystrophin repeats 16 to 21 in membrane at physiological surface pressure. Biochim. Biophys. Acta 2014, 1838, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Flint, O.P.; Masters, B.A.; Gregg, R.E.; Durham, S.K. Inhibition of cholesterol synthesis by squalene synthase inhibitors does not induce myotoxicity in vitro. Toxicol. Appl. Pharmacol. 1997, 145, 91–98. [Google Scholar] [CrossRef]
- Matzno, S.; Yamauchi, T.; Gohda, M.; Ishida, N.; Katsuura, K.; Hanasaki, Y.; Tokunaga, T.; Itoh, H.; Nakamura, N. Inhibition of cholesterol biosynthesis by squalene epoxidase inhibitor avoids apoptotic cell death in L6 myoblasts. J. Lipid Res. 1997, 38, 1639–1648. [Google Scholar]
- Nishimoto, T.; Tozawa, R.; Amano, Y.; Wada, T.; Imura, Y.; Sugiyama, Y. Comparing myotoxic effects of squalene synthase inhibitor, T-91485, and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors in human myocytes. Biochem. Pharmacol. 2003, 66, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Langsjoen, P.; Willis, R.; Richardson, P.; Xia, L.-J.; Ye, C.-Q.; Tamagawa, H. Lovastatin decreases coenzyme Q levels in humans. Proc. Natl. Acad. Sci. USA 1990, 87, 8931–8934. [Google Scholar] [CrossRef]
- Johnson, T.E.; Zhang, X.; Bleicher, K.B.; Dysart, G.; Luoghlin, A.F.; Schaefer, W.H.; Umbenhauer, D.R. Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol. Appl. Pharmacol. 2004, 200, 237–250. [Google Scholar] [CrossRef]
- Laaksonen, R.; Jokelainen, K.; Sahi, T.; Tikkanen, M.J.; Himberg, J.J. Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin. Pharmacol. Ther. 1995, 57, 62–66. [Google Scholar] [CrossRef]
- Kaufmann, P.; Torok, M.; Zahno, A.; Waldhauser, K.M.; Brecht, K.; Krahenbuhl, S. Toxicity of statins on rat skeletal muscle mitochondria. Cell. Mol. Life Sci. 2006, 63, 2415–2425. [Google Scholar] [CrossRef]
- Bouitbir, J.; Charles, A.-L.; Rasseneur, L.; Dufour, S.; Piquard, F.; Geny, B.; Zoll, J. Atorvastatin treatment reduces exercise capacities in rats: Involvement of mitochondrial impairments and oxidative stress. J. Appl. Physiol. 2011, 111, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Hubal, M.J.; Reich, K.A.; De Biase, A.; Bilbie, C.; Clarkson, P.M.; Hoffman, E.P.; Thompson, P.D. Transcriptional deficits in oxidative phosphorylation with statin myopathy. Muscle Nerve 2011, 44, 393–401. [Google Scholar] [CrossRef]
- Sirvent, P.; Bordenave, S.; Vermaelen, M.; Roels, B.; Vassort, G.; Mercier, J.; Raynaud, E.; Lacampagne, A. Simvastatin induces impairment in skeletal muscle while heart is protected. Biochem. Biophys. Res. Commun. 2005, 338, 1426–1434. [Google Scholar] [CrossRef]
- Sirvent, P.; Fabre, O.; Bordenave, S.; Hillaire-Buys, D.; Raynaud De Mauverger, E.; Lacampagne, A.; Mercier, J. Muscle mitochondrial metabolism and calcium signaling impairment in patients treated with statins. Toxicol. Appl. Pharmacol. 2012, 259, 263–268. [Google Scholar] [CrossRef]
- Kwak, H.-B.; Thalacker-Mercer, A.; Anderson, E.J.; Lin, C.-T.; Kane, D.A.; Lee, N.-S.; Cortright, R.N.; Bamman, M.M.; Neufer, P.D. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal muotubes. Free Radic. Bio Med. 2012, 52, 198–207. [Google Scholar] [CrossRef]
- Schirris, T.J.J.; Renkema, G.H.; Ritschel, T.; Voermans, N.C.; Bilos, A.; van Engelen, B.G.M.; Brandt, U.; Koopman, W.J.H.; Beyrath, J.D.; Rodenburg, R.J.; et al. Statin-induced myopathy is associated with mitochondrial complex III inhibition. Cell Metab. 2015, 22, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.J.; Luscher, B.; Scharnagl, H.; Krahenbuhl, S.; Brecht, K. Effect of simvastatin on cholesterol metabolism in C2C12 myotubes and HepG2 cells, and consequences for statin-induced myopathy. Biochem. Pharmacol. 2010, 79, 1200–1209. [Google Scholar] [CrossRef]
- Mookerjea, S.; Coolbear, T.; Sarkar, M.L. Key role of dolichol phosphate in glycprotein biosynthesis. Can. J. Biochem. 1983, 61, 1032–1040. [Google Scholar]
- Dricu, A.; Wang, M.; Hjertman, M.; Malec, M.; Blegen, H.; Wejde, J.; Carlberg, M.; Larsson, O. Mevalonate-regulated mechanisms in cell growth control; role of dolichyl phosphate in expression of the insulin-like growth factor-1 receptor (IGF-1R) in comparison to Ras prenylation and expression of c-myc. Glycobiology 1997, 7, 625–633. [Google Scholar] [CrossRef]
- Esapa, C.T.; Bentham, G.R.; Schroder, J.E.; Kroger, S.; Blake, D.J. The effects of posttranslational processing on dystroglycan synthesis and trafficking. FEBS Lett. 2003, 555, 2019–2216. [Google Scholar] [CrossRef]
- Liao, J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Investig. 2002, 110, 285–288. [Google Scholar] [CrossRef]
- Jaśkiewicz, A.; Pająk, B.; Litwiniuk, A.; Urbańska, K.; Orzechowski, A. Geranylgeraniol prevents statin-dependent myotoxicity in C2C12 muscle cells through RAP1 GTPase prenylation and cytoprotective autophagy. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef]
- Crick, D.C.; Andres, D.A.; Waechter, C.J. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem. Biophys. Res. Commun. 1997, 237, 483–487. [Google Scholar] [CrossRef]
- Mohaupt, M.G.; Karas, R.H.; Babiyuchuk, E.B.; Sanchez-Freire, V.; Monasturskaya, K.; Lakshmanan, L.; Hoppeler, H.; Breil, F.; Draeger, A. Association betwenn statin-associated myopathy and skeletal damage. CMAJ 2009, 181, E11–E18. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Sorimachi, H. Calpains—An elaborate proteolytic system. Biochim. Biophys. Acta 2012, 1824, 224–236. [Google Scholar] [CrossRef]
- Jacobson, M.D.; Burne, J.F.; Raff, M.C. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994, 13, 1899–1910. [Google Scholar] [CrossRef]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio Protoc. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives in mitochondrial physiology. Int. J. Biochem. Cell Biol. 2009, 41, 1837–1845. [Google Scholar] [CrossRef]
- Lemieux, H.; Semsroth, S.; Antretter, A.; Hofer, D.; Gnaiger, E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int. J. Biochem. Cell Biol. 2011, 43, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski, A.; Jank, M.; Gajkowska, B.; Sadkowski, T.; Godlewski, M.M. A novel antioxidant-inhibited dexamethasone-mediated and caspase-3 independent muscle cell death. Ann. N. Y. Acad. Sci. 2003, 1010, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Campia, I.; Lussiana, C.; Pescarmona, G.; Ghigo, D.; Bosia, A.; Riganti, C. Geranylgeraniol prevents the cytotoxic effects of mevastatin in THP-1 cells, without decreasing the beneficial effects on cholesterol synthesis. Br. J. Pharmacol. 2009, 158, 1777–1786. [Google Scholar] [CrossRef]
- Cao, P.; Hanai, J.-I.; Tanksale, P.; Imamura, S.; Sukhatme, V.P.; Lecker, S.H. Statin-induced muscle damage and atrogin-1 induction is the result of geranylgeranylation defect. FASEB J. 2009, 23, 2844–2854. [Google Scholar] [CrossRef]
- Anderson, H.C.; Kratz, L.; Kelley, R. Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay. Am. J. Med. Genet. 2002, 113, 315–319. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Corso, G.; Rossi, M.; Ferrari, P.; Balli, F.; Rivasi, F.; Annunziata, I.; Ballabio, A.; Russo, A.D.; Andria, G.; et al. Lathosterolosis, a novel multiple-malformation/mental retardation syndrome due to deficiency of 3beta-hydroxysteroid-delta5-desaturase. Am. J. Hum. Genet. 2002, 71, 952–958. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Aloj, S.M.; Garbi, C. Mevalonate controls cytoskeleton organiztion and cell morphology in thyroid epithelial cells. J. Cell. Physiol. 1993, 155, 340–348. [Google Scholar] [CrossRef]
- Jaśkiewicz, A.; Pająk, B.; Orzechowski, A. The Many Faces of Rap1 GTPase. Int. J. Mol. Sci. 2018, 19, 2848. [Google Scholar] [CrossRef]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive involvement of autophagy in Alzhemer’s disease: An immunoelectron microscopy study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Pająk, B.; Kania, E.; Orzechowski, A. Nucleofection of rat pheochromocytoma PC-12 cells with human mutated beta-amyloid precursor protein gene (APP-sw) leads to reduced viability, autophagy-like process, and increased expression and secretion of beta amyloid. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Eskelinen, E.L.; Deretic, V. Autophagosomes, phagosomes, autolysosomes, phagolysosomes, autophagolysosomes... wait, I’m confused. Autophagy 2014, 10, 549–551. [Google Scholar] [CrossRef]
- Bonifacio, A.; Sanvee, G.M.; Boutibir, J.; Krahenbuhl, S. The AKT/mTOR signaling pathway plays a key role in statin-induced myotoxicity. Biochim. Biophys. Acta 2015, 1853, 1841–1849. [Google Scholar] [CrossRef]
- Naba, H.; Kakinuma, C.; Ohnishi, S.; Ogihara, T. Improving effect of ethyl eicosapentanoate on statin-indiced rhabdomyolysis in Eisai hyperbilirubinemic rats. Biochem. Biophys. Res. Commun. 2006, 340, 215–220. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Mrcuzzi, A.; Piscianz, E.; Monasta, L.; Crovella, S.; Kleiner, G. Mevalonate kinase deficiency and neuroinflammation: Balance between apoptosis and pyroptosis. Int. J. Mol. Sci. 2013, 14, 23274–23288. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Crovella, S.; Celsi, F. Mevalonate pathway blockade, mitochondrial dysfunction and autophagy: A possible link. Int. J. Mol. Sci. 2015, 16, 16067–16084. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Kleiner, G.; Valencic, E.; Campisciano, G.; Girardelli, M.; Crovella, S.; Knowles, A.; Marcuzzi, A. Block of the mevalonate pathway triggers oxidative and inflammatory molecular mechanisms modulated by exogenous isoprenoid compounds. Int. J. Mol. Sci. 2014, 15, 6843–6856. [Google Scholar] [CrossRef]
- Ho, L.H.; Read, S.H.; Dorstyn, L.; Lambrusco, L.; Kumar, S. Caspase-2 is required for cell death induced by cytoskeletal disruption. Oncogene 2008, 27, 3393–3404. [Google Scholar] [CrossRef]
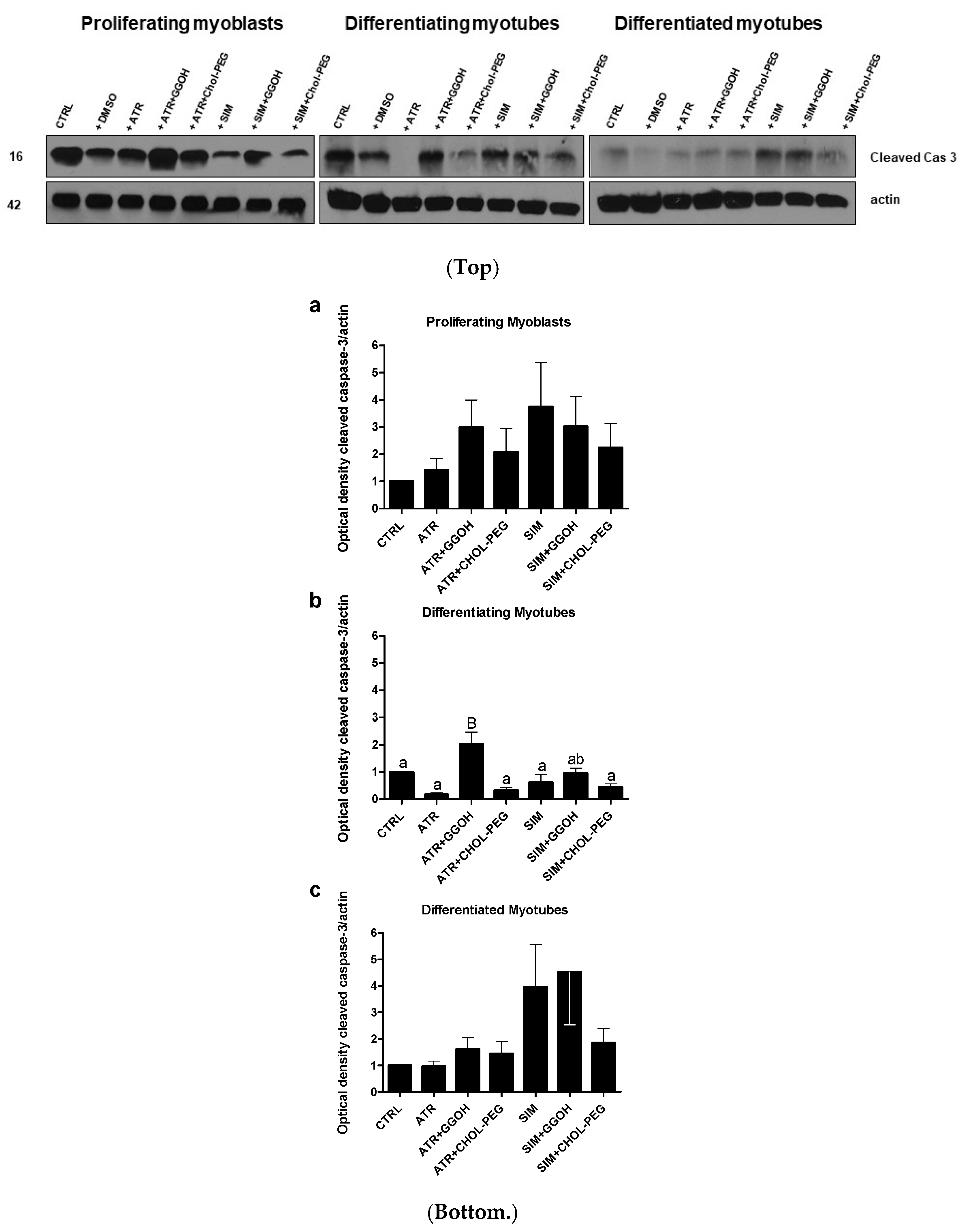
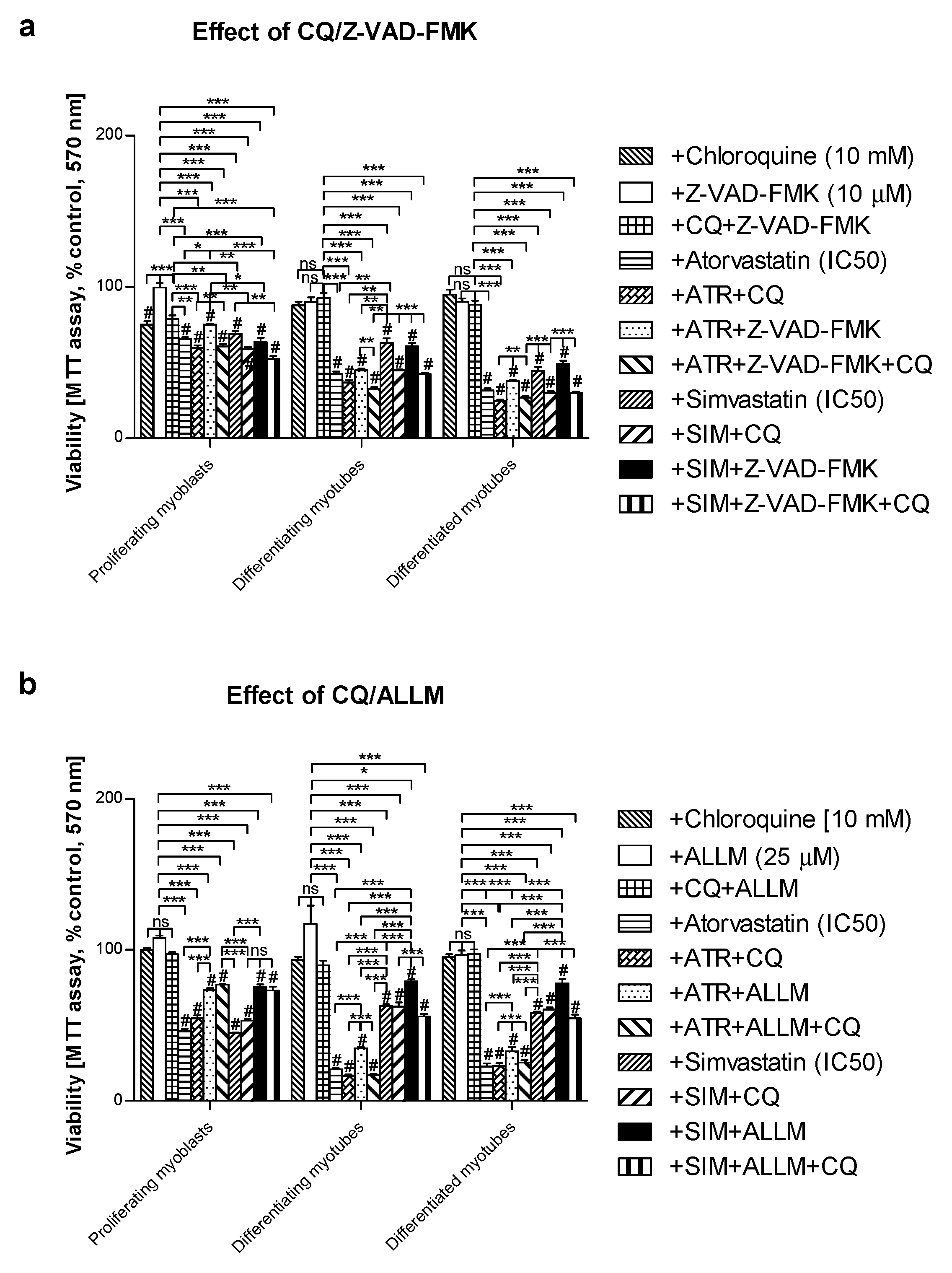
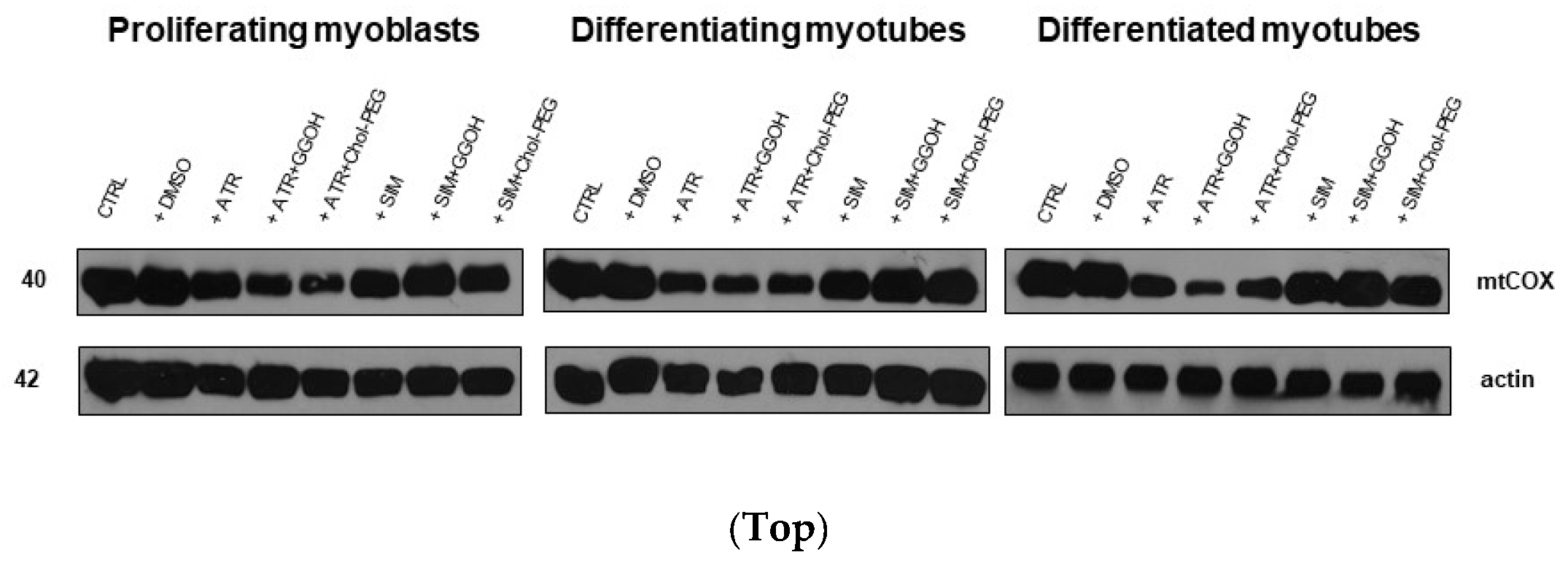
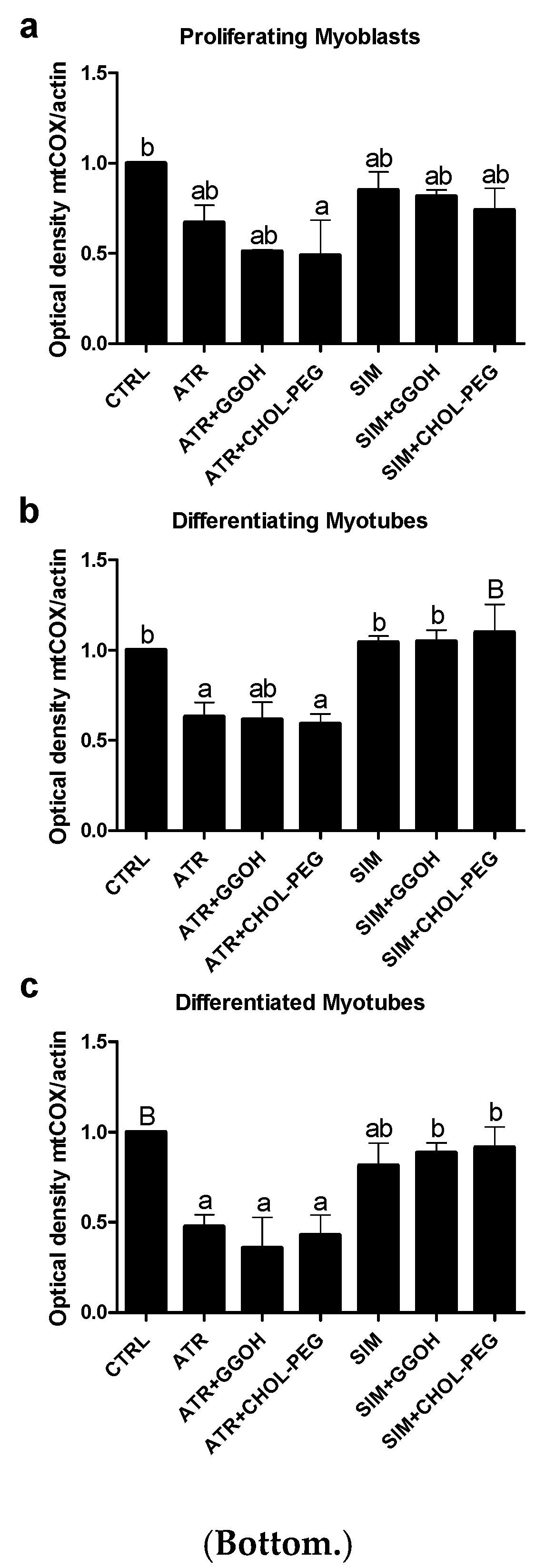
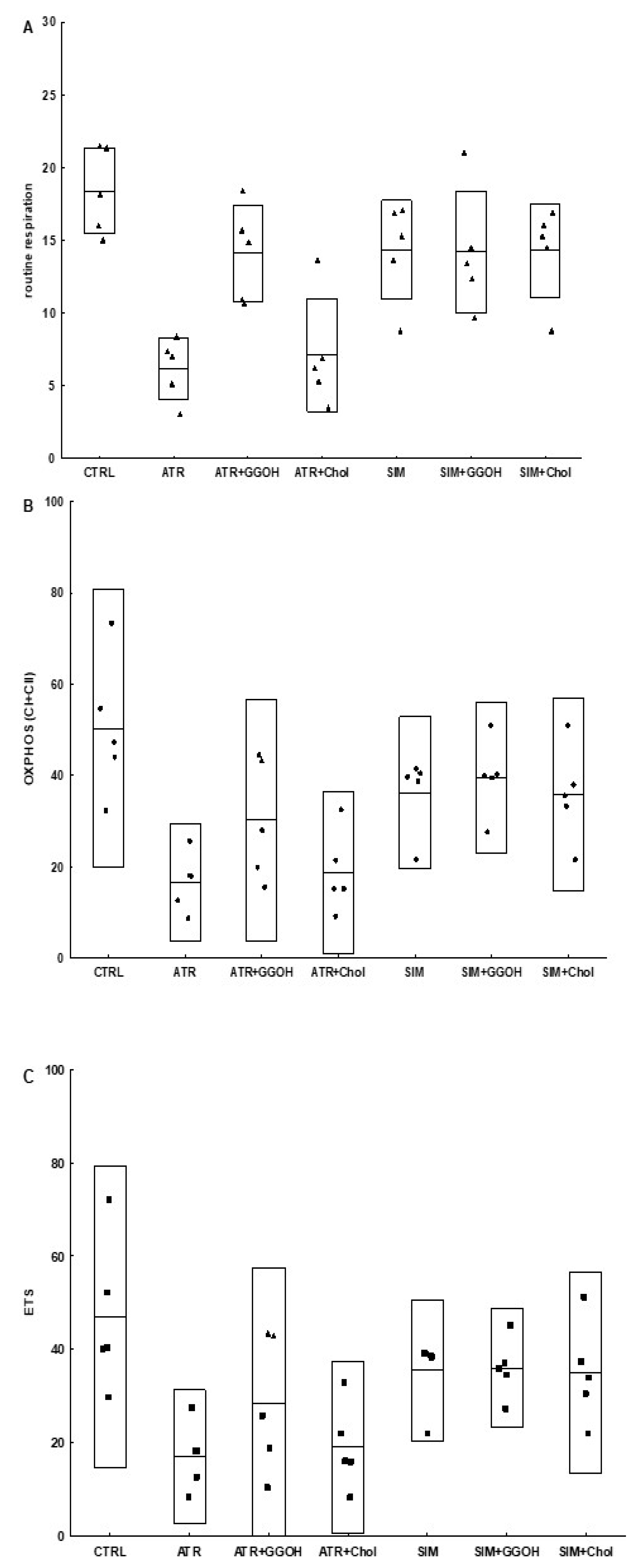
| Compound | OXPHOS I | CII |
|---|---|---|
| CTRL | 26.91 ± 8.01 | 34.08 ± 11.09 |
| ATR | 7.28 ± 3.36 | 9.24 ± 6.22 |
| ATR + GGOH | 20.35 ± 5.23 | 20.15 ± 11.57 |
| ATR + Chol-PEG | 9.20 ± 5.13 | 10.61 ± 4.69 |
| SIM | 19.65 ± 3.74 | 23.46 ± 7.89 |
| SIM + GGOH | 22.81 ± 5.00 | 18.23 ± 6.05 |
| SIM + Chol-PEG | 19.79 ± 6.58 | 22.32 ± 6.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśkiewicz, A.; Pająk, B.; Łabieniec-Watała, M.; Palma, C.D.; Orzechowski, A. Diverse Action of Selected Statins on Skeletal Muscle Cells—An Attempt to Explain the Protective Effect of Geranylgeraniol (GGOH) in Statin-Associated Myopathy (SAM). J. Clin. Med. 2019, 8, 694. https://doi.org/10.3390/jcm8050694
Jaśkiewicz A, Pająk B, Łabieniec-Watała M, Palma CD, Orzechowski A. Diverse Action of Selected Statins on Skeletal Muscle Cells—An Attempt to Explain the Protective Effect of Geranylgeraniol (GGOH) in Statin-Associated Myopathy (SAM). Journal of Clinical Medicine. 2019; 8(5):694. https://doi.org/10.3390/jcm8050694
Chicago/Turabian StyleJaśkiewicz, Anna, Beata Pająk, Magdalena Łabieniec-Watała, Clara De Palma, and Arkadiusz Orzechowski. 2019. "Diverse Action of Selected Statins on Skeletal Muscle Cells—An Attempt to Explain the Protective Effect of Geranylgeraniol (GGOH) in Statin-Associated Myopathy (SAM)" Journal of Clinical Medicine 8, no. 5: 694. https://doi.org/10.3390/jcm8050694
APA StyleJaśkiewicz, A., Pająk, B., Łabieniec-Watała, M., Palma, C. D., & Orzechowski, A. (2019). Diverse Action of Selected Statins on Skeletal Muscle Cells—An Attempt to Explain the Protective Effect of Geranylgeraniol (GGOH) in Statin-Associated Myopathy (SAM). Journal of Clinical Medicine, 8(5), 694. https://doi.org/10.3390/jcm8050694








