Prospective Study of Sex-Specific Adiponectin Changes and Incident Metabolic Syndrome: The ARIRANG Study
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Data Collection
2.3. Definition of Incident Metabolic Syndrome
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.1.1. The Risk of Incident MetS According to the Baseline Adiponectin Level and the Change in Adiponectin Level
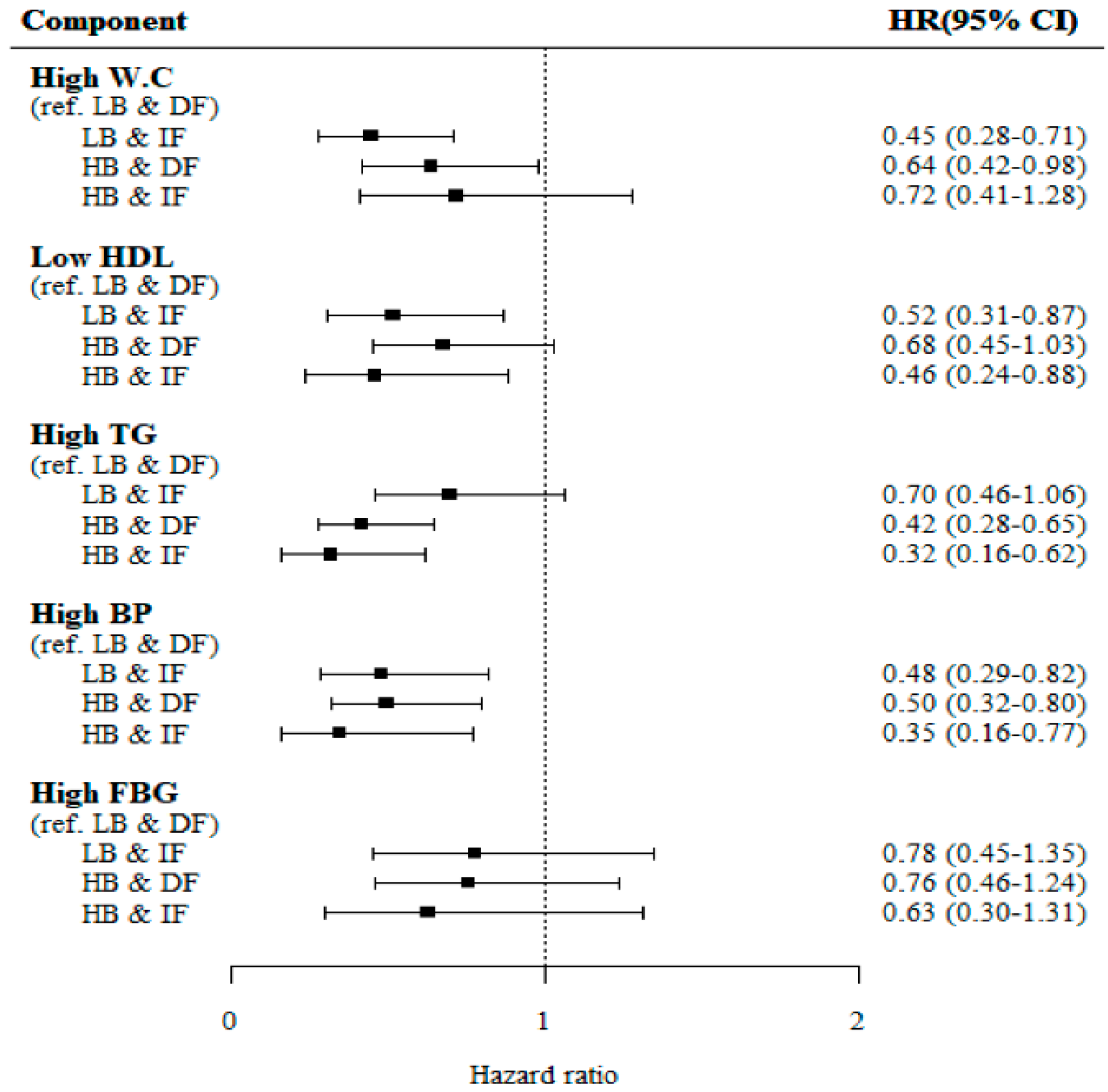
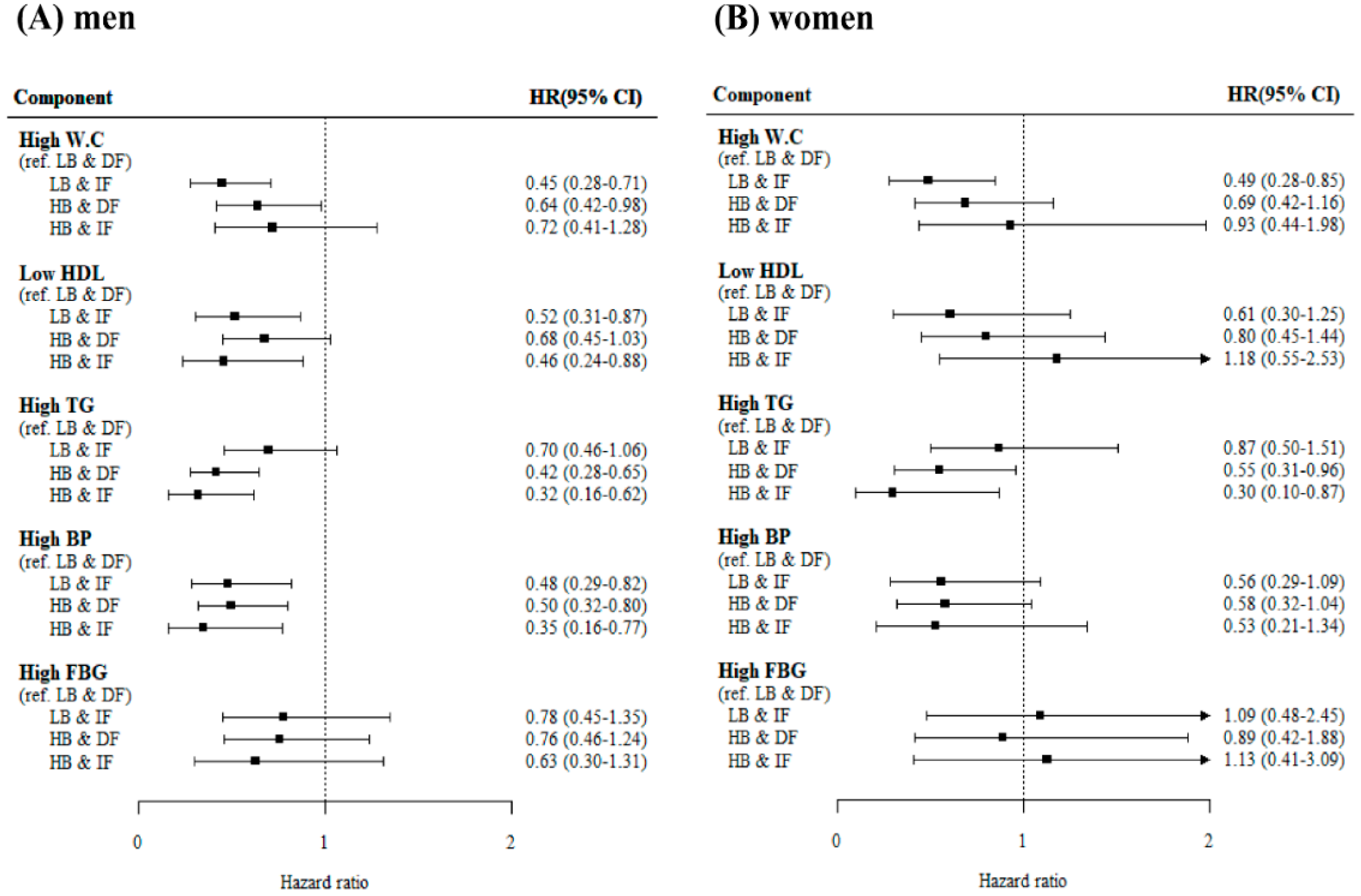
3.1.2. The Incident Risk of Individual MetS Components According to the Baseline Adiponectin Level and the Change in Adiponectin Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An american heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Huh, J.H.; Kang, D.R.; Jang, J.Y.; Shin, J.H.; Kim, J.Y.; Choi, S.; Cho, E.J.; Park, J.S.; Sohn, I.S.; Jo, S.H.; et al. Metabolic syndrome epidemic among korean adults: Korean survey of cardiometabolic syndrome (2018). Atherosclerosis 2018, 277, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef]
- Huh, J.H.; Yadav, D.; Kim, J.S.; Son, J.W.; Choi, E.; Kim, S.H.; Shin, C.; Sung, K.C.; Kim, J.Y. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism 2017, 67, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, E.A.; Celoria, B.M.; Duarte, S.F.; da Silva, E.G.; Santos, I.J.; Cabello, P.H.; Genelhu, V.A. Hypoadiponectinemia is associated with blood pressure increase in obese insulin-resistant individuals. Metabolism 2007, 56, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Drolet, R.; Belanger, C.; Fortier, M.; Huot, C.; Mailloux, J.; Legare, D.; Tchernof, A. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity 2009, 17, 424–430. [Google Scholar] [CrossRef]
- De Rosa, A.; Monaco, M.L.; Capasso, M.; Forestieri, P.; Pilone, V.; Nardelli, C.; Buono, P.; Daniele, A. Adiponectin oligomers as potential indicators of adipose tissue improvement in obese subjects. Eur. J. Endocrinol. 2013, 169, 37–43. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, S.V.; Yoon, J.H.; Koh, S.B.; Yoon, J.; Yoo, B.S.; Lee, S.H.; Park, J.K.; Choe, K.H.; Guallar, E. Prospective study of serum adiponectin and incident metabolic syndrome the arirang study. Diabetes Care 2013, 36, 1547–1553. [Google Scholar] [CrossRef]
- Koh, S.B.; Park, J.K.; Yoon, J.H.; Chang, S.J.; Oh, S.S.; Kim, J.Y.; Ryu, S.Y.; Kim, K.S.; Lee, T.Y.; You, J.H. Preliminary report: A serious link between adiponectin levels and metabolic syndrome in a korean nondiabetic population. Metabolism 2010, 59, 333–337. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, J.Y.; Kim, J.K.; Koh, S.B.; Park, J.K.; Ahn, S.V. Relative contribution of obesity and serum adiponectin to the development of hypertension. Diabetes Res. Clin. Pract. 2014, 103, 51–56. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, H.S.; Kim, D.J.; Han, J.H.; Kim, S.M.; Cho, G.J.; Kim, D.Y.; Kwon, H.S.; Kim, S.R.; Lee, C.B.; et al. Appropriate waist circumference cutoff points for central obesity in korean adults. Diabetes Res. Clin. Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef]
- Menzaghi, C.; Trischitta, V. The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes 2018, 67, 12–22. [Google Scholar] [CrossRef]
- Choi, S.H.; Ku, E.J.; Hong, E.S.; Lim, S.; Kim, K.W.; Moon, J.H.; Kim, K.M.; Park, Y.J.; Park, K.S.; Jang, H.C. High serum adiponectin concentration and low body mass index are significantly associated with increased all-cause and cardiovascular mortality in an elderly cohort, “adiponectin paradox”: The korean longitudinal study on health and aging (klosha). Int. J. Cardiol. 2015, 183, 91–97. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; May, S.; Langenberg, C. Association of adiponectin with coronary heart disease and mortality: The rancho bernardo study. Am. J. Epidemiol. 2007, 165, 164–174. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Tabak, A.G.; Carstensen, M.; Witte, D.R.; Brunner, E.J.; Shipley, M.J.; Jokela, M.; Roden, M.; Kivimaki, M.; Herder, C. Adiponectin trajectories before type 2 diabetes diagnosis: Whitehall ii study. Diabetes Care 2012, 35, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Kollerits, B.; Fliser, D.; Heid, I.M.; Ritz, E.; Kronenberg, F. Gender-specific association of adiponectin as a predictor of progression of chronic kidney disease: The mild to moderate kidney disease study. Kidney Int. 2007, 71, 1279–1286. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Du, X.L.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-function studies of the adipocyte-secreted hormone acrp30/adiponectin—Implications for metabolic regulation and bioactivity. J. Biol. Chem. 2003, 278, 9073–9085. [Google Scholar] [CrossRef]
- Rasouli, N.; Kern, P.A. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocr. Metab. 2008, 93, S64–S73. [Google Scholar] [CrossRef]
- Nakashima, R.; Yamane, K.; Kamei, N.; Nakanishi, S.; Kohno, N. Low serum levels of total and high-molecular-weight adiponectin predict the development of metabolic syndrome in japanese-americans. J. Endocrinol. Investig. 2011, 34, 615–619. [Google Scholar] [CrossRef]
- Daniele, A.; De Rosa, A.; De Cristofaro, M.; Monaco, M.L.; Masullo, M.; Porcile, C.; Capasso, M.; Tedeschi, G.; Oriani, G.; Di Costanzo, A. Decreased concentration of adiponectin together with a selective reduction of its high molecular weight oligomers is involved in metabolic complications of myotonic dystrophy type 1. Eur. J. Endocrinol. 2011, 165, 969–975. [Google Scholar] [CrossRef] [PubMed]
| Low Adiponectin at Baseline | High Adiponectin at Baseline | ||||
|---|---|---|---|---|---|
| Variable | Decreased Adiponectin during Follow-Up (n = 334) | Increased Adiponectin during Follow-Up (n = 221) | Decreased Adiponectin during Follow-Up (n = 401) | Increased Adiponectin during Follow-Up (n = 154) | p-Value |
| Age (years) | 53.01 ± 7.86 ‡§ | 54.70 ± 7.95 | 55.71 ± 8.47 * | 56.71 ± 8.13 * | <0.0001 |
| Sex (male, %) | 136 (40.72) | 93 (42.08) | 169 (42.14) | 60 (38.96) | 0.904 |
| BMI (kg/m2) | 24.00 ± 2.74 § | 23.80 ± 2.65 | 23.63 ± 2.81 | 23.09 ± 3.04 * | 0.009 |
| BMI change | −0.32 ± 1.19 † | 0.00 ± 1.18 *‡ | −0.29 ± 1.12 † | −0.25 ± 1.22 | 0.008 |
| Waist circumference | 81.38 ± 7.46 § | 80.11 ± 7.53 | 80.96 ± 7.89 | 79.28 ± 8.36 * | 0.026 |
| Systolic BP (mmHg) | 121.28 ± 16.26 | 123.48 ± 16.26 | 123.74 ± 16.35 | 124.96 ± 16.29 | 0.075 |
| Diastolic BP (mmHg) | 75.56 ± 10.64 | 80.45 ± 11.19 | 80.67 ± 11.06 | 81.97 ± 11.08 | 0.151 |
| hsCRP (mg/L) | 1.91 ± 4.69 | 2.01 ± 6.63 | 1.96 ± 4.34 | 2.50 ± 10.85 | 0.788 |
| BUN (mg/dL) | 15.32 ± 4.05 | 14.77 ± 4.00 ‡§ | 15.74 ± 4.15 † | 15.97 ± 4.73 † | 0.016 |
| Cr (mg/dL) | 0.95 ± 0.15 | 0.94 ± 0.14 | 0.94 ± 0.16 | 0.96 ± 0.20 | 0.623 |
| Fasting glucose (mg/dL) | 91.78 ± 18.31 | 90.47 ± 10.83 | 90.12 ± 12.44 | 89.66 ± 15.57 | 0.356 |
| Total cholesterol (mg/dL) | 200.51 ± 35.55 | 196.06 ± 35.66 | 202.75 ± 36.23 | 198.43 ± 37.18 | 0.151 |
| HDL-C (mg/dL) | 47.89 ± 9.92 ‡ | 48.52 ± 10.58 ‡ | 51.90 ± 12.49 *† | 50.55 ± 10.36 | <0.0001 |
| LDL-C (mg/dL) | 117.77 ± 30.12 | 117.35 ± 29.91 | 117.89 ± 30.77 | 117.73 ± 32.33 | 0.997 |
| Triglyceride (mg/dL) | 107.5 (81.0–143.0) § | 99.0 (75.0–129.0) | 102.0 (77.0–133.0) | 94.0 (71.0–122.0) * | 0.001 |
| Albumin (g/dL) | 4.66 ± 0.26 †§ | 4.58 ± 0.25 * | 4.62 ± 0.27 § | 4.54 ± 0.25 *‡ | <0.0001 |
| Adiponectin (baseline) | 7.20 (5.45–8.66) ‡§ | 6.25 (4.87–7.90) ‡§ | 13.65 (11.68–16.61) *† | 13.01 (11.35–15.31) *† | <0.0001 |
| Adiponectin (follow-up) | 5.23 (3.60–6.94) †‡§ | 8.89 (6.66–11.89) *§ | 9.75 (7.30–12.20) *§ | 15.54 (13.43–18.76) *†‡ | <0.0001 |
| Leptin | 4.61 (2.30–8.54) | 4.87 (1.81–8.18) | 4.00 (1.92–7.68) | 3.96 (1.94–6.89) | 0.106 |
| HOMA-IR | 1.54 (1.24–1.98) § | 1.48 (1.21–1.89) | 1.49 (1.20–1.89) | 1.34 (1.08–1.75) * | 0.006 |
| Alcohol intake (%) | 155 (46.41) | 85 (38.46) | 185 (46.13) | 62 (40.26) | 0.161 |
| Regular exercise (%) | 90 (27.27) | 60 (27.15) | 103 (25.88) | 38 (24.68) | 0.922 |
| Current smoker (%) | 72 (21.56) | 37 (16.74) | 65 (16.21) | 17 (11.04) | 0.031 |
| Low Baseline Decreased FU | Low Baseline Increased FU | High Baseline Decreased FU | High Baseline Increased FU | p for Trend | |
|---|---|---|---|---|---|
| Total (n = 1110) | 334 | 221 | 401 | 154 | |
| Number of incident case (%) | 61 (18.26) | 51 (23.08) | 56 (13.97) | 12 (7.79) | 0.001 |
| Person-years | 880.83 | 759.08 | 1188.83 | 422.25 | |
| Incidence rate (1000 person-year) | 69.25 | 67.19 | 47.11 | 28.42 | |
| Crude HR | reference | 0.79 (0.54–1.15) | 0.59 (0.41–0.85) | 0.41 (0.22–0.77) | <0.0001 |
| Model 1 | reference | 0.76 (0.52–1.11) | 0.55 (0.38–0.80) | 0.38 (0.20–0.71) | <0.0001 |
| Model 2 | reference | 0.62 (0.41–0.93) | 0.58 (0.40–0.84) | 0.33 (0.17–0.63) | <0.0001 |
| Men (n = 458) | 136 | 93 | 169 | 60 | |
| Number of incident cases (%) | 25 (18.38) | 22 (23.66) | 20 (11.83) | 4 (6.67) | 0.010 |
| Person-years | 357.08 | 318.17 | 475.67 | 183.50 | |
| Incidence rate (1000 person-year) | 70.01 | 69.15 | 42.05 | 21.80 | |
| Crude HR | reference | 0.70 (0.39–1.27) | 0.52 (0.29–0.93) | 0.25 (0.09–0.73) | 0.002 |
| Model 1 | reference | 0.65 (0.35–1.18) | 0.46 (0.25–0.86) | 0.21 (0.07–0.64) | 0.001 |
| Model 2 | reference | 0.39 (0.19–0.78) | 0.36 (0.18–0.69) | 0.08 (0.02–0.27) | <0.0001 |
| Women (n = 652) | 198 | 128 | 232 | 94 | |
| Number of incident case (%) | 36 (18.18) | 29 (22.66) | 36 (15.52) | 8 (8.51) | 0.036 |
| Person-years | 523.75 | 440.92 | 713.17 | 238.75 | |
| Incident rate (1000 person-year) | 68.74 | 65.77 | 50.48 | 33.51 | |
| Crude HR | reference | 0.84 (0.51–1.39) | 0.65 (0.41–1.03) | 0.55 (0.26–1.19) | 0.033 |
| Model 1 | reference | 0.81 (0.49–1.34) | 0.59 (0.37–0.94) | 0.51 (0.23–1.09) | 0.012 |
| Model 2 | reference | 0.73 (0.43–1.23) | 0.65 (0.40–1.05) | 0.69 (0.31–1.50) | 0.102 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, J.H.; Yoon, T.W.; Kang, D.R.; Kim, J.Y. Prospective Study of Sex-Specific Adiponectin Changes and Incident Metabolic Syndrome: The ARIRANG Study. J. Clin. Med. 2019, 8, 599. https://doi.org/10.3390/jcm8050599
Huh JH, Yoon TW, Kang DR, Kim JY. Prospective Study of Sex-Specific Adiponectin Changes and Incident Metabolic Syndrome: The ARIRANG Study. Journal of Clinical Medicine. 2019; 8(5):599. https://doi.org/10.3390/jcm8050599
Chicago/Turabian StyleHuh, Ji Hye, Tae Woong Yoon, Dae Ryong Kang, and Jang Young Kim. 2019. "Prospective Study of Sex-Specific Adiponectin Changes and Incident Metabolic Syndrome: The ARIRANG Study" Journal of Clinical Medicine 8, no. 5: 599. https://doi.org/10.3390/jcm8050599
APA StyleHuh, J. H., Yoon, T. W., Kang, D. R., & Kim, J. Y. (2019). Prospective Study of Sex-Specific Adiponectin Changes and Incident Metabolic Syndrome: The ARIRANG Study. Journal of Clinical Medicine, 8(5), 599. https://doi.org/10.3390/jcm8050599





