The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Ethics Statement
2.2. Sertoli Cell Isolation, Culture, Characterization and Function
2.3. Culture and Treatment
2.4. Western Blot Analysis
2.5. Reverse Transcription Polymerase Chain Reaction Analysis
2.6. Statistical Analysis
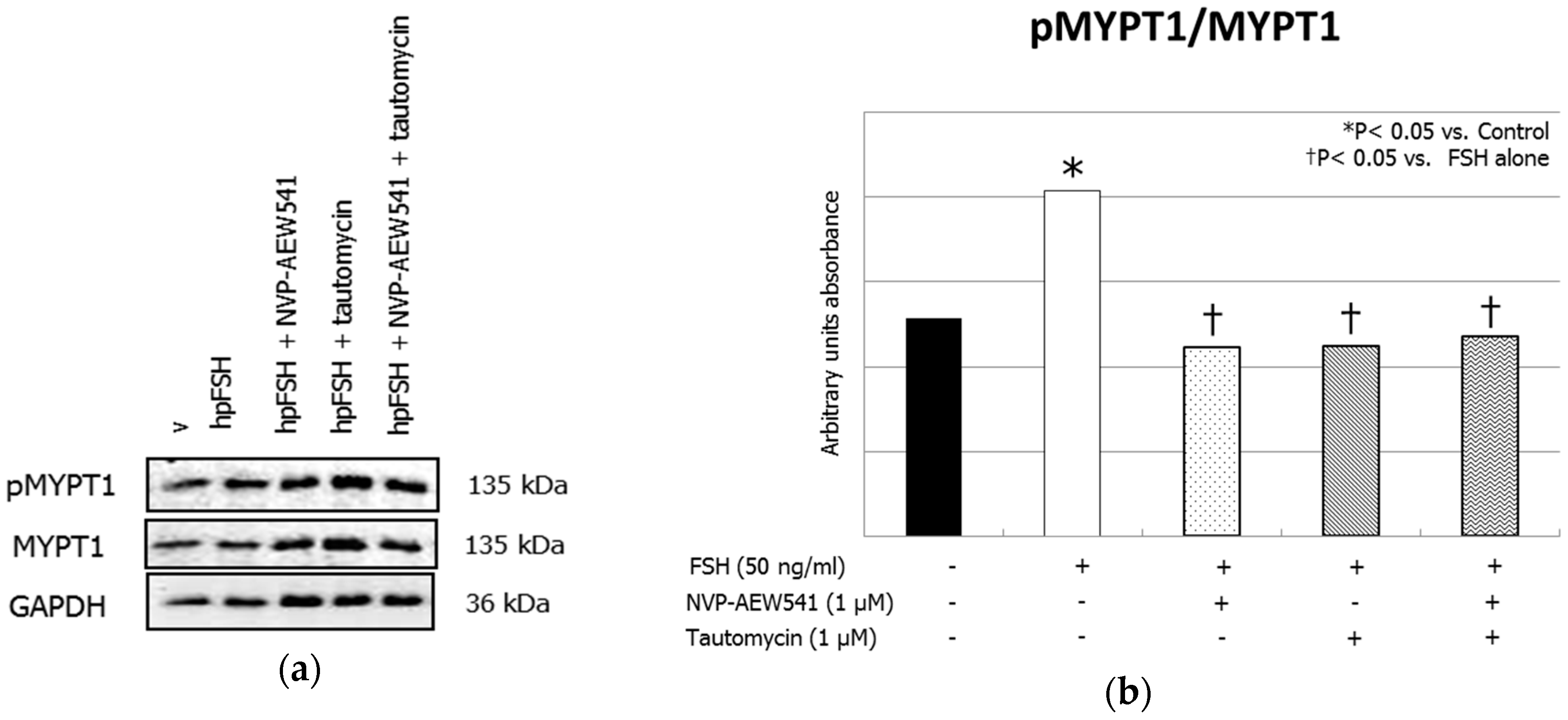
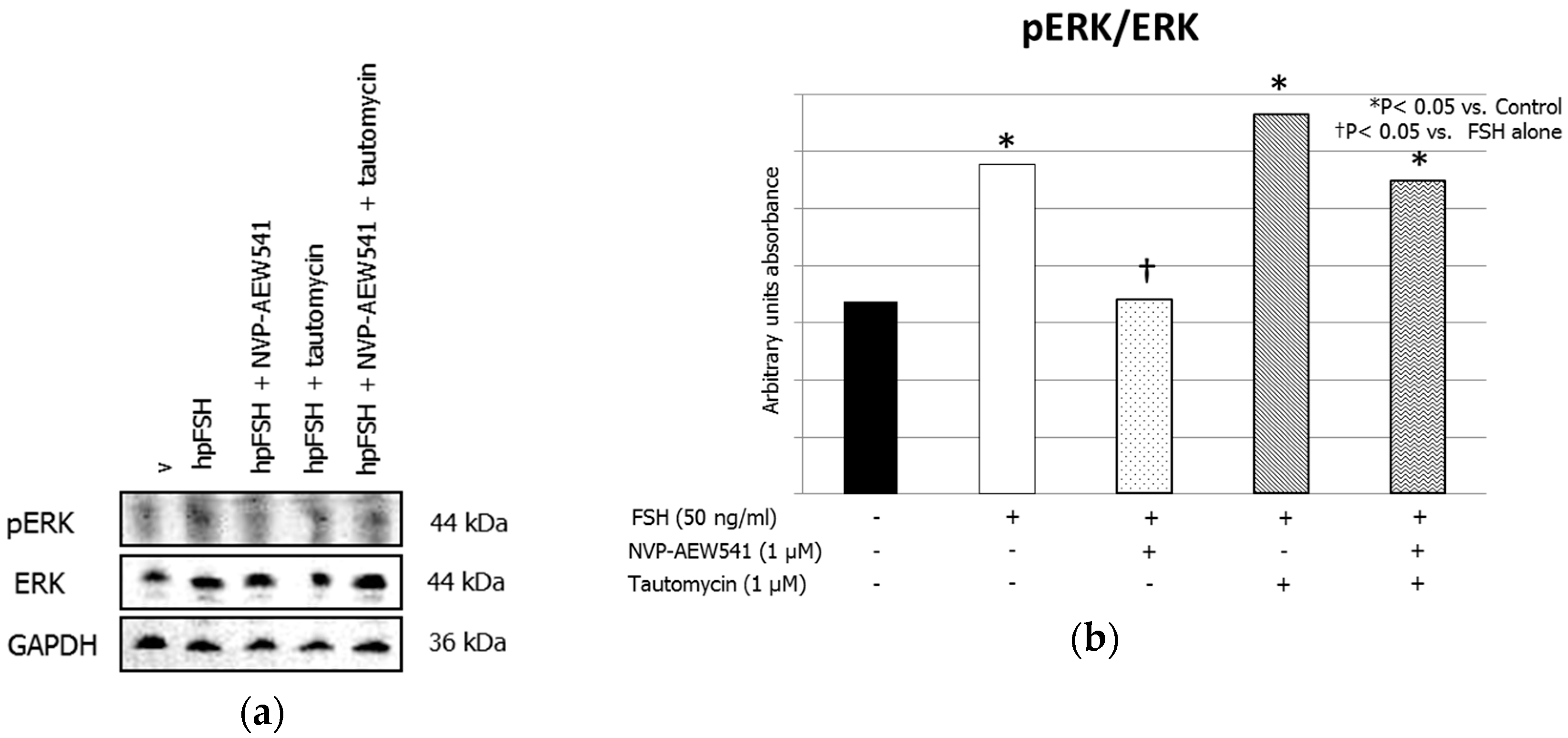
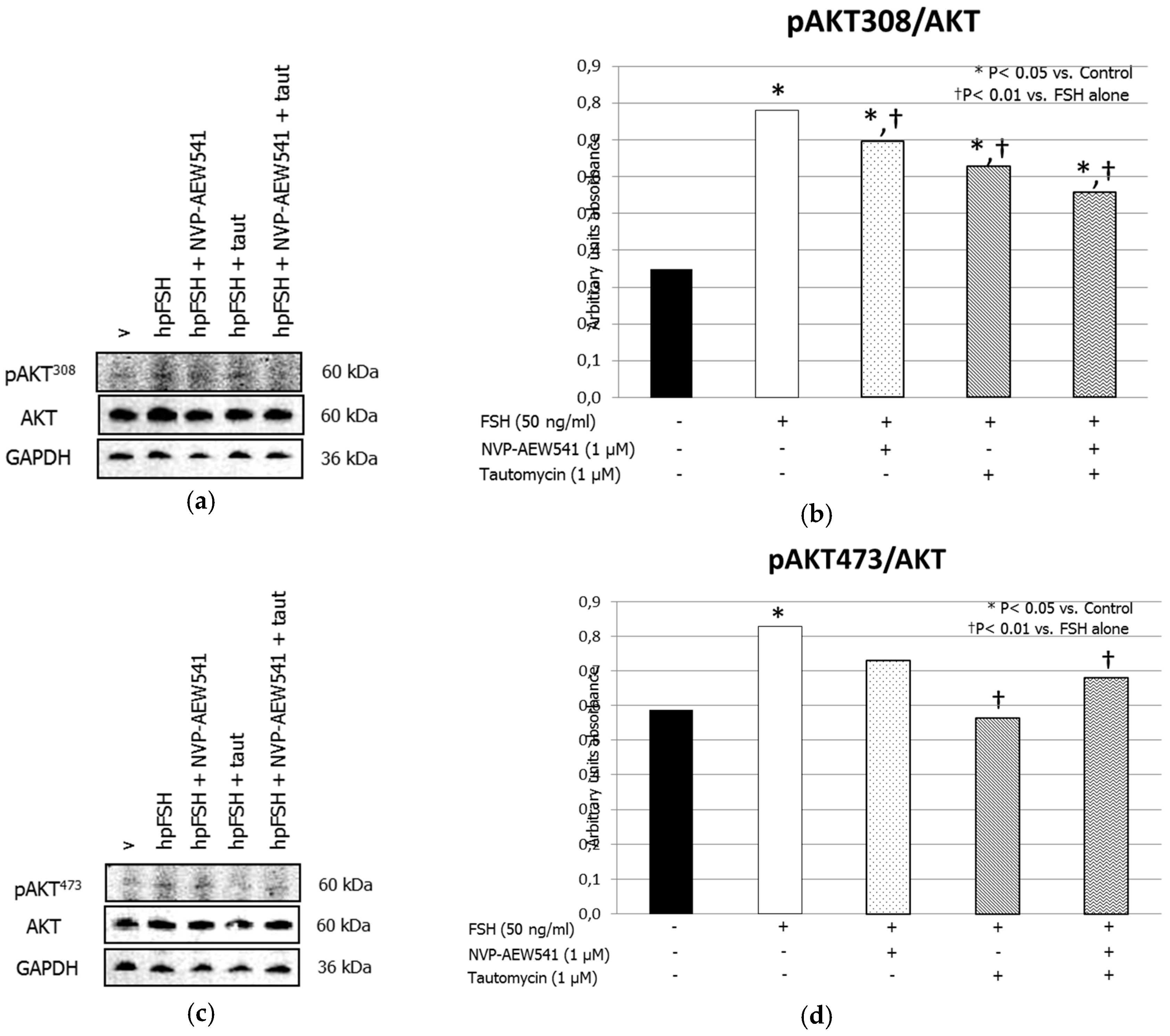
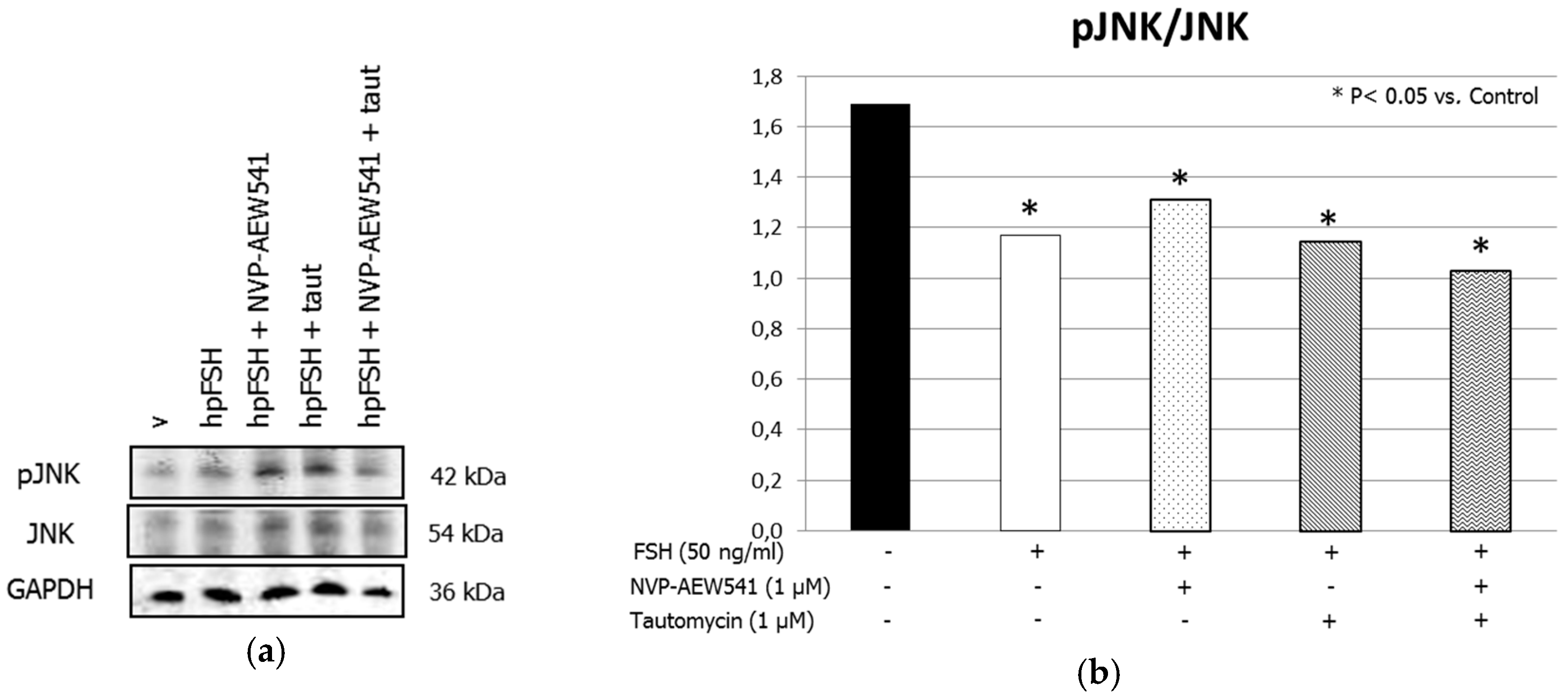
3. Results
3.1. Western Blot Analysis
3.2. mRNA Analysis
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Themmen, A.P.N.; Huhtaniemi, I.T. Mutations of gonadotropins and gonadotropin receptors: Elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000, 21, 551–583. [Google Scholar] [CrossRef]
- Gloaguen, P.; Crépieux, P.; Heitzler, D.; Poupon, A.; Reiter, E. Mapping the follicle-stimulating hormone-induced signaling networks. Front. Endocrinol. 2011, 2, 45. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Effects of the insulin-like growth factor system on testicular differentiation and function: A review of the literature. Andrology 2018, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nef, S.; Verma-Kurvari, S.; Merenmies, J.; Vassalli, J.D.; Efstratiadis, A.; Accili, D.; Parada, L.F. Testis determination requires insulin receptor family function in mice. Nature 2003, 426, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Pitetti, J.L.; Calvel, P.; Zimmermann, C.; Conne, B.; Papaioannou, M.D.; Aubry, F.; Cederroth, C.R.; Urner, F.; Fumel, B.; Crausaz, M.; et al. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol. Endocrinol. 2013, 27, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Law, N.C.; Hunzicker-Dunn, M.E. Insulin Receptor Substrate 1, the Hub Linking Follicle-stimulating Hormone to Phosphatidylinositol 3-Kinase Activation. J. Biol. Chem. 2016, 291, 4547–4560. [Google Scholar] [CrossRef] [PubMed]
- Hunzicker-Dunn, M.E.; Lopez-Biladeau, B.; Law, N.C.; Fiedler, S.E.; Carr, D.W.; Maizels, E.T. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 2012, 109, E2979–E2988. [Google Scholar] [CrossRef]
- Nobrega, R.H.; Morais, R.D.; Crespo, D.; de Waal, P.P.; de Franca, L.R.; Schulz, R.W.; Bogerd, J. Fsh stimulates spermatogonial proliferation and differentiation in Zebrafish via Igf3. Endocrinology 2015, 156, 3804–3817. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.T. Protein phosphatase 1—Targeted in many directions. J. Cell Sci. 2002, 115, 241–256. [Google Scholar]
- Terrak, M.; Kerff, F.; Langsetmo, K.; Tao, T.; Dominguez, R. Structural basis of protein phosphatase 1 regulation. Nature 2004, 429, 780–784. [Google Scholar] [CrossRef]
- Law, N.C.; Donaubauer, E.M.; Zeleznik, A.J.; Hunzicker-Dunn, M. How protein kinase A activates canonical tyrosine kinase signaling pathways to promote granulosa cell differentiation. Endocrinology 2017, 17, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Luca, G.; Mancuso, F.; Calvitti, M.; Arato, I.; Falabella, G.; Bufalari, A.; De Monte, V.; Tresoldi, E.; Nastruzzi, C.; Basta, G.; et al. Long-term stability, functional competence, and safety of microencapsulated specific pathogen-free neonatal porcine Sertoli cells: A potential product for cell transplant therapy. Xenotransplantation 2015, 22, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Luca, G.; Arato, I.; Mancuso, F.; Calvitti, M.; Falabella, G.; Murdolo, G.; Basta, G.; Cameron, D.F.; Hansen, B.C.; Fallarino, F.; et al. Xenograft of microencapsulated Sertoli cells restores glucose homeostasis in db/db mice with spontaneous diabetes mellitus. Xenotransplantation 2016, 23, 429–439. [Google Scholar] [CrossRef]
- Mather, J.P.; Philip, D.D. Primary culture of testicular somatic cells. In Methods for Serum Free Culture of Cells of the Endocrine System; Barnes, D.W., Sirbasku, D.A., Sato, G.H., Eds.; New York Liss: New York, NY, USA, 1999; pp. 24–45. [Google Scholar]
- Arato, I.; Luca, G.; Mancuso, F.; Bellucci, C.; Lilli, C.; Calvitti, M.; Hansen, B.C.; Milardi, D.; Grande, G.; Calafiore, R. An in vitro prototype of a porcine biomimetic testis-like cell culture system: A novel tool for the study of reassembled Sertoli and Leydig cells. Asian J. Androl. 2018, 20, 160–165. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Mital, P.; Kaur, G.; Dufour, J.M. Immunoprotective Sertoli cells: Making allogeneic and xenogeneic transplantation feasible. Reproduction 2010, 139, 495–504. [Google Scholar] [CrossRef]
- Valdes-Gonzalez, R.A.; Dorantes, L.M.; Garibay, G.N.; Bracho-Blanchet, E.; Mendez, A.J.; Davila-Perez, R.; Elliott, R.B.; Teran, L.; White, D.J. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: A 4-year study. Eur. J. Endocrinol. 2005, 153, 419–427. [Google Scholar] [CrossRef]
- Valdes-Gonzalez, R.A.; White, D.J.; Dorantes, L.M.; Teran, L.; Garibay-Nieto, G.N.; Bracho-Blanchet, E.; Davila-Perez, R.; Evia-Viscarra, L.; Ormsby, C.E.; Ayala-Sumuano, J.T. Three-yr follow-up of a type 1 diabetes mellitus patient with an islet xenotransplant. Clin. Transplant. 2007, 21, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Juul, A.; Bang, P.; Hertel, N.T.; Main, K.; Dalgaard, P.; Jørgensen, K.; Müller, J.; Hall, K.; Skakkebaek, N.E. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: Relation to age, sex, stage of puberty, testicular size, and body mass index. J. Clin. Endocrinol. Metab. 1994, 78, 744–752. [Google Scholar] [PubMed]
- Koskenniemi, J.J.; Virtanen, H.E.; Wohlfahrt-Veje, C.; Löyttyniemi, E.; Skakkebaek, N.E.; Juul, A.; Andersson, A.M.; Main, K.M.; Toppari, J. Postnatal changes in testicular position are associated with IGF-I and function of Sertoli and Leydig cells. J. Clin. Endocrinol. Metab. 2018, 103, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, D.; Kolibianakis, E.M.; Venetis, C.A.; Papanikolaou, E.G.; Bontis, J.; Tarlatzis, B.C. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Fertil. Steril. 2009, 91, 749–766. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannarella, R.; Arato, I.; Condorelli, R.A.; Luca, G.; Barbagallo, F.; Alamo, A.; Bellucci, C.; Lilli, C.; La Vignera, S.; Calafiore, R.; et al. The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells. J. Clin. Med. 2019, 8, 577. https://doi.org/10.3390/jcm8050577
Cannarella R, Arato I, Condorelli RA, Luca G, Barbagallo F, Alamo A, Bellucci C, Lilli C, La Vignera S, Calafiore R, et al. The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells. Journal of Clinical Medicine. 2019; 8(5):577. https://doi.org/10.3390/jcm8050577
Chicago/Turabian StyleCannarella, Rossella, Iva Arato, Rosita A. Condorelli, Giovanni Luca, Federica Barbagallo, Angela Alamo, Catia Bellucci, Cinzia Lilli, Sandro La Vignera, Riccardo Calafiore, and et al. 2019. "The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells" Journal of Clinical Medicine 8, no. 5: 577. https://doi.org/10.3390/jcm8050577
APA StyleCannarella, R., Arato, I., Condorelli, R. A., Luca, G., Barbagallo, F., Alamo, A., Bellucci, C., Lilli, C., La Vignera, S., Calafiore, R., Mancuso, F., & Calogero, A. E. (2019). The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells. Journal of Clinical Medicine, 8(5), 577. https://doi.org/10.3390/jcm8050577







