Effects of β-Adrenergic Antagonists on Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Survival Analysis
2.3. Western Blot Analysis
2.4. Lung Cancer Patient Cohort
2.5. Study Variables and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Propranolol Enhances Cisplatin and Radiation Sensitivity of PC9 and A549 Tumor Cells In Vitro
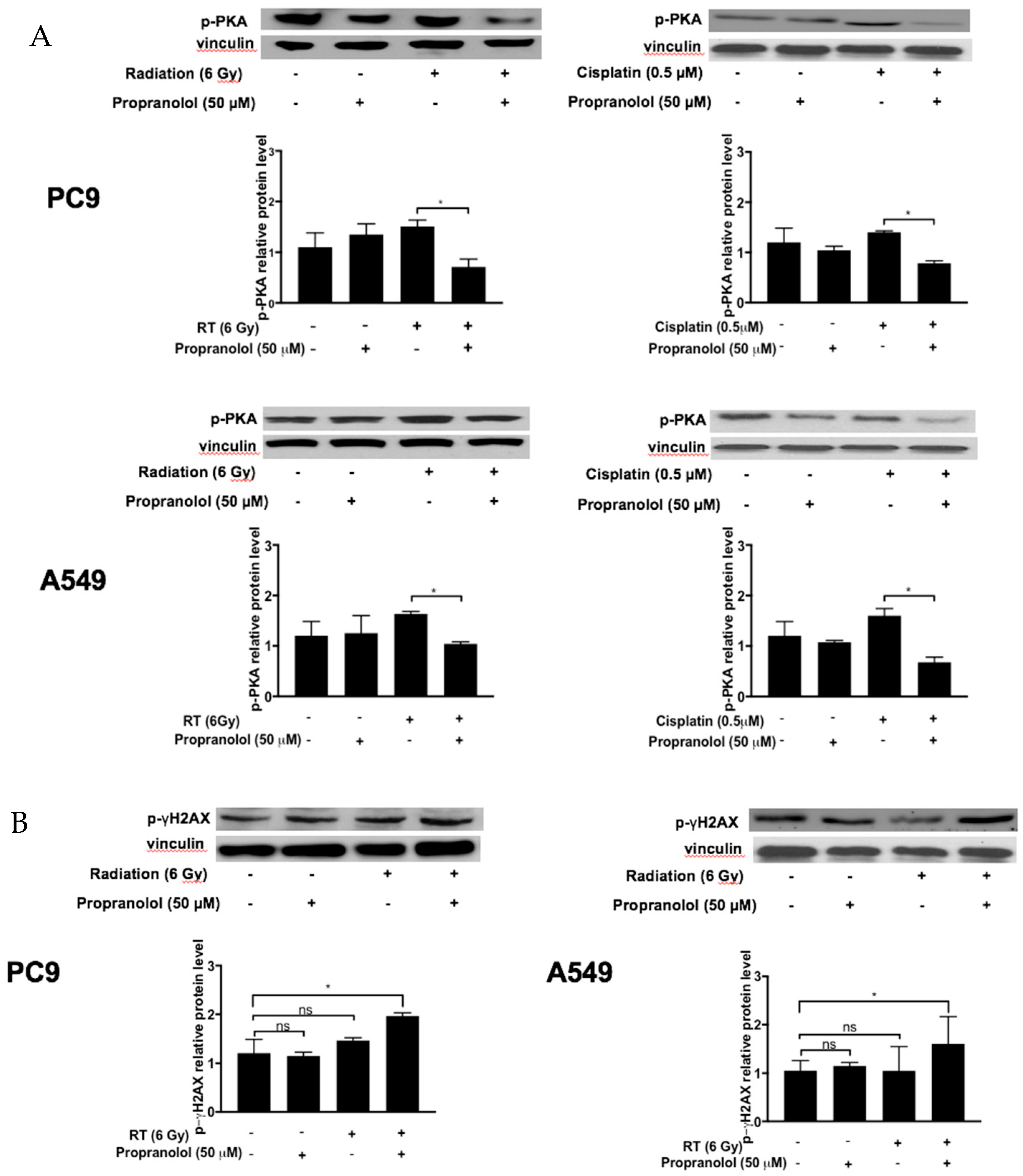
3.2. Propranolol Downregulates the β-AR Mediator p-PKA in PC9 and A549 Tumor Cells In Vitro
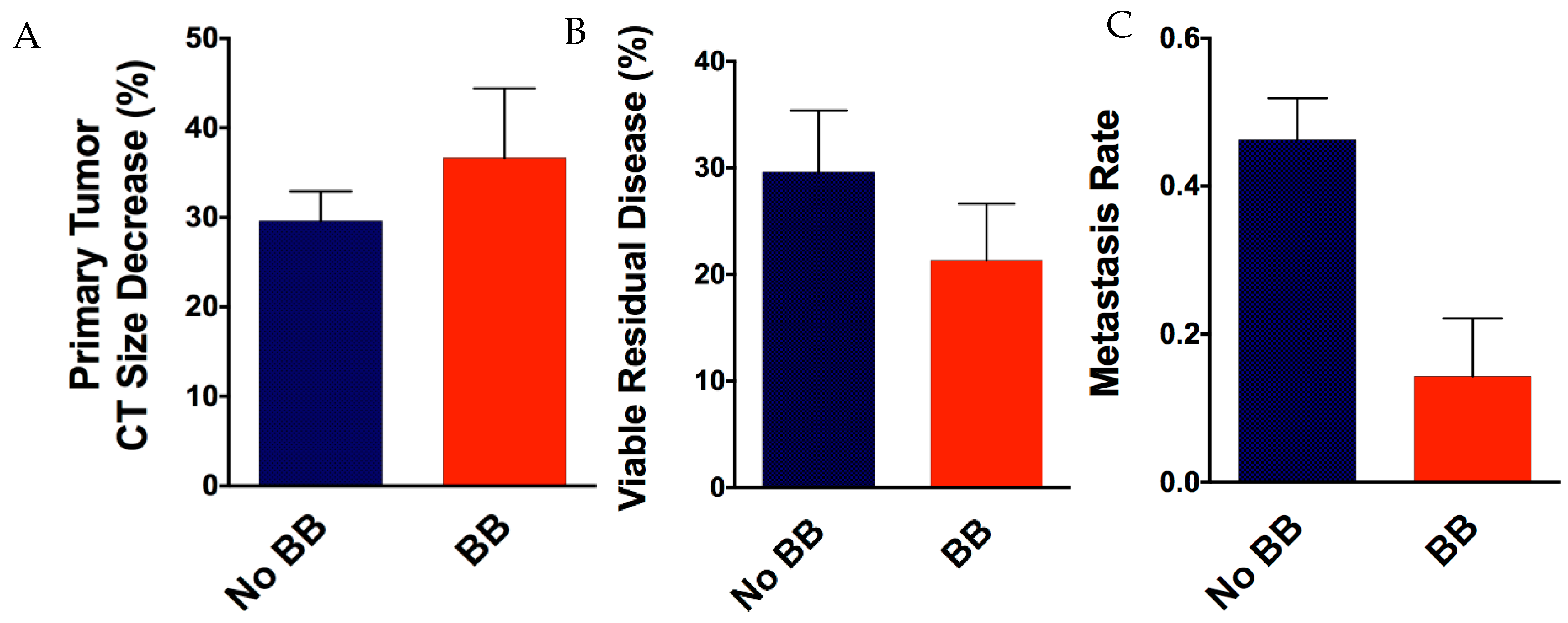
3.3. Patient Beta-Blocker Use and Association with Therapy Responses and Patient Outcomes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Howlander, N.; Noone, A.M.; Krapcho, M. SEER Cancer Statistics Review. 1975–2011. Available online: http://seer.cancer.gov/statfacts/html/lungb.html (accessed on 9 April 2019).
- Carlisle, D.L.; Liu, X.; Hopkins, T.M.; Swick, M.C.; Dhir, R.; Siegfried, J.M. Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm. Pharmacol. Ther. 2007, 20, 629–641. [Google Scholar] [CrossRef]
- Schuller, H.M.; Cole, B. Regulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis 1989, 10, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Orloff, M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem. Pharmacol. 1998, 55, 1377–1384. [Google Scholar] [CrossRef]
- Schaal, C.; Chellappan, S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Laag, E.; Majidi, M.; Cekanova, M.; Masi, T.; Takahashi, T.; Schuller, H.M. NNK activates ERK1/2 and CREB/ATF-1 via beta-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int. J. Cancer 2006, 119, 1547–1552. [Google Scholar]
- Schuller, H.M.; Tithof, P.K.; Williams, M.; Plummer, H., 3rd. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999, 59, 4510–4515. [Google Scholar] [PubMed]
- Majidi, M.; Al-Wadei, H.A.; Takahashi, T.; Schuller, H.M. Nongenomic beta estrogen receptors enhance beta1 adrenergic signaling induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human small airway epithelial cells. Cancer Res. 2007, 67, 6863–6871. [Google Scholar] [CrossRef]
- Weddle, D.L.; Tithoff, P.; Williams, M.; Schuller, H.M. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis 2001, 22, 473–479. [Google Scholar] [CrossRef]
- Chen, R.J.; Ho, Y.S.; Guo, H.R.; Wang, Y.J. Long-term nicotine exposure-induced chemoresistance is mediated by activation of Stat3 and downregulation of ERK1/2 via nAChR and beta-adrenoceptors in human bladder cancer cells. Toxicol Sci. 2010, 115, 118–130. [Google Scholar] [CrossRef]
- Yao, H.; Duan, Z.; Wang, M.; Awonuga, A.O.; Rappolee, D.; Xie, Y. Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet. Cytogenet. 2009, 190, 81–87. [Google Scholar] [CrossRef]
- Flint, M.S.; Baum, A.; Episcopo, B.; Knickelbein, K.Z.; Liegey Dougall, A.J.; Chambers, W.H.; Jenkins, F.J. Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress 2013, 16, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Al-Wadei, M.H.; Al-Wadei, H.A.; Schuller, H.M. Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors alpha3, alpha5, and alpha7. Mol. Cancer Res. 2012, 10, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Ma, Q.; Zhang, D.; Guo, K.; Liu, H.; Wang, F.; Wu, E. β2-adrenoceptor blocker synergizes with gemcitabine to inhibit the proliferation of pancreatic cancer cells via apoptosis induction. Eur. J. Pharmacol. 2011, 665, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.S.; Karpova, Y.; Prokopovich, S.; Smith, A.J.; Essau, B.; Gersappe, A.; Carson, J.P.; Weber, M.J.; Register, T.C.; Chen, Y.Q.; et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J. Biol. Chem. 2007, 282, 14094–14100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, Q.; Wang, Z.; Zhang, M.; Guo, K.; Wang, F.; Wu, E. β2-adrenoceptor blockage induces G 1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NF κB pathway. Mol. Cancer 2011, 10, 146. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Capitano, M.L.; Lee, C.T.; Eng, J.W.; Waight, J.D.; Hylander, B.L.; Sexton, S.; Hong, C.C.; Gordon, C.J.; Abrams, S.I.; et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 20176–20181. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.W.; Reed, C.B.; Kokolus, K.M.; Pitoniak, R.; Utley, A.; Bucsek, M.J.; Ma, W.W.; Repasky, E.A.; Hylander, B.L. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat. Commun. 2015, 6, 6426. [Google Scholar] [CrossRef]
- Drell, T.L.; Joseph, J.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res. Treat 2003, 80, 63–70. [Google Scholar] [CrossRef]
- Lang, K.; Drell, T.L., 4th; Lindecke, A.; Niggemann, B.; Kaltschmidt, C.; Zaenker, K.S.; Entschladen, F. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer 2004, 112, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Badino, G.R.; Novelli, A.; Girardi, C.; Di Carlo, F. Evidence for functional beta-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacol. Res. 1996, 33, 255–260. [Google Scholar] [PubMed]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.; Al-Wadei, M.H.; Schuller, H.M. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: A novel target for intervention. PLoS ONE 2012, 7, e29915. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Che, X.; Zhao, W.; Zhang, D.; Long, H.; Chaudhary, P.; Li, H. Effects of propranolol in combination with radiation on apoptosis and survival of gastric cancer cells in vitro. Radiat. Oncol. 2010, 5, 98. [Google Scholar] [CrossRef]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011, 29, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Grytli, H.H.; Fagerland, M.W.; Fosså, S.D.; Tasken, K.A. Association between use of beta-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 2014, 65, 635–641. [Google Scholar] [CrossRef]
- De Giorgi, V.; Gandini, S.; Grazzini, M.; Benemei, S.; Marchionni, N.; Geppetti, P. Effect of beta-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin. Proc. 2013, 88, 1196–1203. [Google Scholar] [CrossRef]
- Wang, H.M.; Liao, Z.X.; Komaki, R.; Welsh, J.W.; O’Reilly, M.S.; Chang, J.Y.; Zhuang, Y.; Levy, L.B.; Lu, C.; Gomez, D.R. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann. Oncol. 2013, 24, 1312–1319. [Google Scholar] [CrossRef]
- Wang, H.; Liao, Z.; Zhuang, Y.; Liu, Y.; Levy, L.B.; Xu, T.; Yusuf, S.W.; Gomez, D.R. Incidental receipt of cardiac medications and survival outcomes among patients with stage III non-small-cell lung cancer after definitive radiotherapy. Clin. Lung Cancer 2015, 16, 128–136. [Google Scholar] [CrossRef]
- Hall, E.J. Radiation dose-rate: A factor of importance in radiobiology and radiotherapy. Br. J. Radiol. 1972, 45, 81–97. [Google Scholar] [CrossRef]
- Wolter, N.E.; Wolter, J.K.; Enepekides, D.J.; Irwin, M.S. Propranolol as a novel adjunctive treatment for head and neck squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 2012, 41, 334–344. [Google Scholar]
- Kozanoglu, I.; Yandim, M.K.; Cincin, Z.B.; Ozdogu, H.; Cakmakoglu, B.; Baran, Y. New indication for therapeutic potential of an old well-known drug (propranolol) for multiple myeloma. J. Cancer Res. Clin. Oncol. 2013, 139, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Ho, Y.S.; Guo, H.R.; Wang, Y.J. Rapid activation of Stat3 and ERK1/2 by nicotine modulates cell proliferation in human bladder cancer cells. Toxicol. Sci. 2008, 104, 283–293. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Wu, Y.; Luan, B.; Wang, Y.; Qu, B.; Pei, G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell 2004, 14, 303–317. [Google Scholar] [CrossRef]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Hanusova, V.; Skalova, L.; Kralova, V.; Matouskova, P. Potential anti-cancer drugs commonly used for other indications. Curr. Cancer Drug Targets 2015, 15, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Masur, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001, 61, 2866–2869. [Google Scholar]
- Parsons, M.; Keppler, M.D.; Kline, A.; Messent, A.; Humphries, M.J.; Gilchrist, R.; Hart, I.R.; Quittau-Prevostel, C.; Hughes, W.E.; Parker, P.J.; et al. Site-directed perturbation of protein kinase C- integrin interaction blocks carcinoma cell chemotaxis. Mol. Cell Biol. 2002, 22, 5897–5911. [Google Scholar] [CrossRef]
- Wrobel, L.J.; Le Gal, F.A. Inhibition of human melanoma growth by a non-cardioselective beta-blocker. J. Investig. Dermatol. 2015, 135, 525–531. [Google Scholar] [CrossRef]
- Wolter, J.K.; Wolter, N.E.; Blanch, A.; Partridge, T.; Cheng, L.; Morgenstern, D.A.; Podkowa, M.; Kaplan, D.R.; Irwin, M.S. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 2014, 5, 161–172. [Google Scholar] [CrossRef]


| Characteristics | Number of Patients (%) Beta-Blockers (n = 16) | Number of Patients (%) No Beta-Blockers (n = 61) | p Value |
|---|---|---|---|
| Sex | 0.99 | ||
| Female | 6 (38) | 23 (38) | |
| Male | 10 (62) | 38 (62) | |
| Age, years | 0.29 | ||
| <65 | 5 (31) | 28 (46) | |
| ≥65 | 11 (69) | 33 (54) | |
| T Category | 0.24 | ||
| T1,2 | 10 (63) | 47 (77) | |
| T3,4 | 6 (38) | 14 (23) | |
| N Category | 0.32 | ||
| N0,1 | 3 (19) | 6 (10) | |
| N2 | 13 (81) | 55 (90) | |
| Tumor Histology | |||
| Squamous cell | 6 (38) | 13 (21) | 0.18 |
| Adenocarcinoma | 6 (38) | 31 (51) | |
| NSCLC, not otherwise specified (NOS) | 4 (25) | 17(28) | |
| Radiation Dose, Gy | 0.70 | ||
| <60 | 7 (54) | 23 (48) | |
| ≥60 | 6 (46) | 25 (52) | |
| Aspirin | 0.02 | ||
| No | 10 (62) | 53 (87) | |
| Yes | 6 (38) | 8 (13) | |
| ACE inhibitor | <0.01 | ||
| No | 11 (69) | 57 (93) | |
| Yes | 5 (31) | 4 (7) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, K.R.; Yan, S.X.; Heilbroner, S.P.; Sonett, J.R.; Stoopler, M.B.; Shu, C.; Halmos, B.; Wang, T.J.C.; Hei, T.K.; Cheng, S.K. Effects of β-Adrenergic Antagonists on Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. J. Clin. Med. 2019, 8, 575. https://doi.org/10.3390/jcm8050575
Chaudhary KR, Yan SX, Heilbroner SP, Sonett JR, Stoopler MB, Shu C, Halmos B, Wang TJC, Hei TK, Cheng SK. Effects of β-Adrenergic Antagonists on Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Journal of Clinical Medicine. 2019; 8(5):575. https://doi.org/10.3390/jcm8050575
Chicago/Turabian StyleChaudhary, Kunal R., Sherry X. Yan, Samuel P. Heilbroner, Joshua R. Sonett, Mark B. Stoopler, Catherine Shu, Balazs Halmos, Tony J.C. Wang, Tom K. Hei, and Simon K. Cheng. 2019. "Effects of β-Adrenergic Antagonists on Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer" Journal of Clinical Medicine 8, no. 5: 575. https://doi.org/10.3390/jcm8050575
APA StyleChaudhary, K. R., Yan, S. X., Heilbroner, S. P., Sonett, J. R., Stoopler, M. B., Shu, C., Halmos, B., Wang, T. J. C., Hei, T. K., & Cheng, S. K. (2019). Effects of β-Adrenergic Antagonists on Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Journal of Clinical Medicine, 8(5), 575. https://doi.org/10.3390/jcm8050575






