Implication of Ventricular Assist Devices in Extracorporeal Membranous Oxygenation Patients Listed for Heart Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Statistical Analysis
3. Results
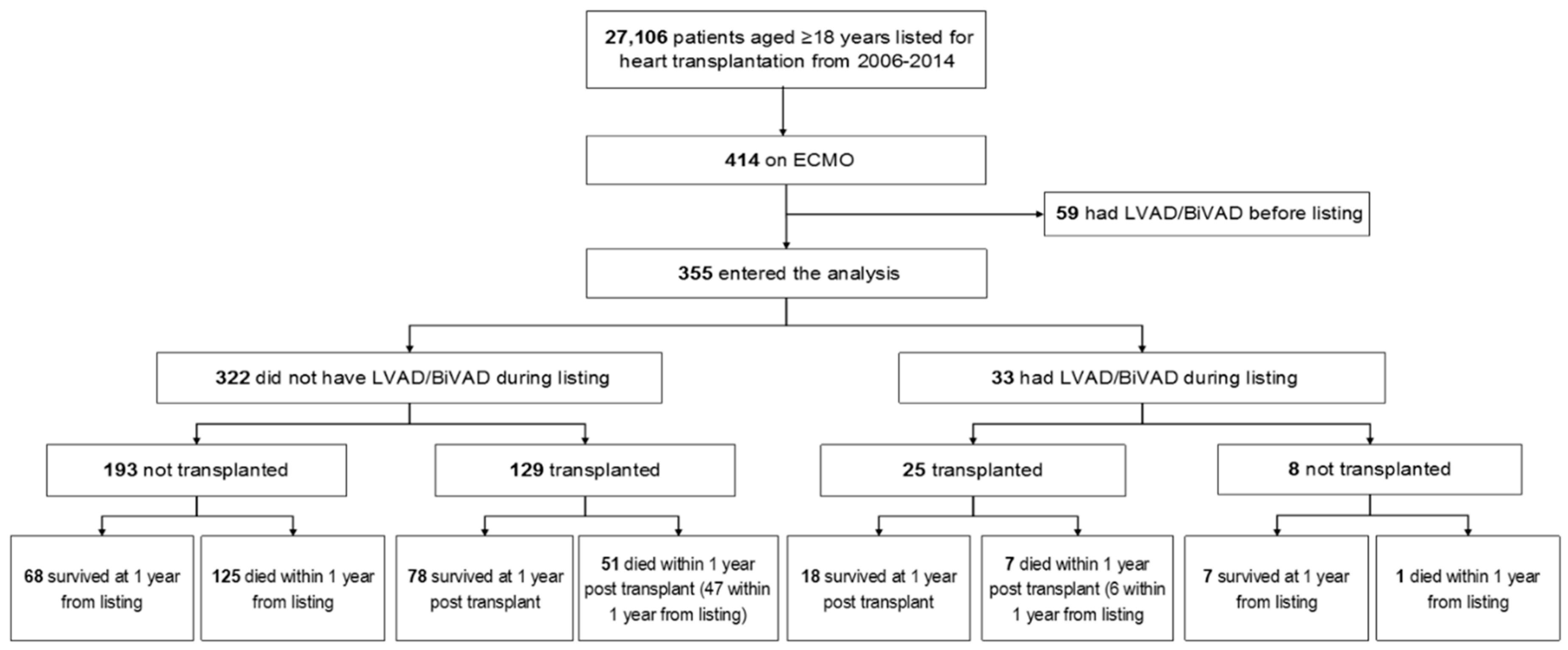
3.1. Baseline Characteristics and Outcomes
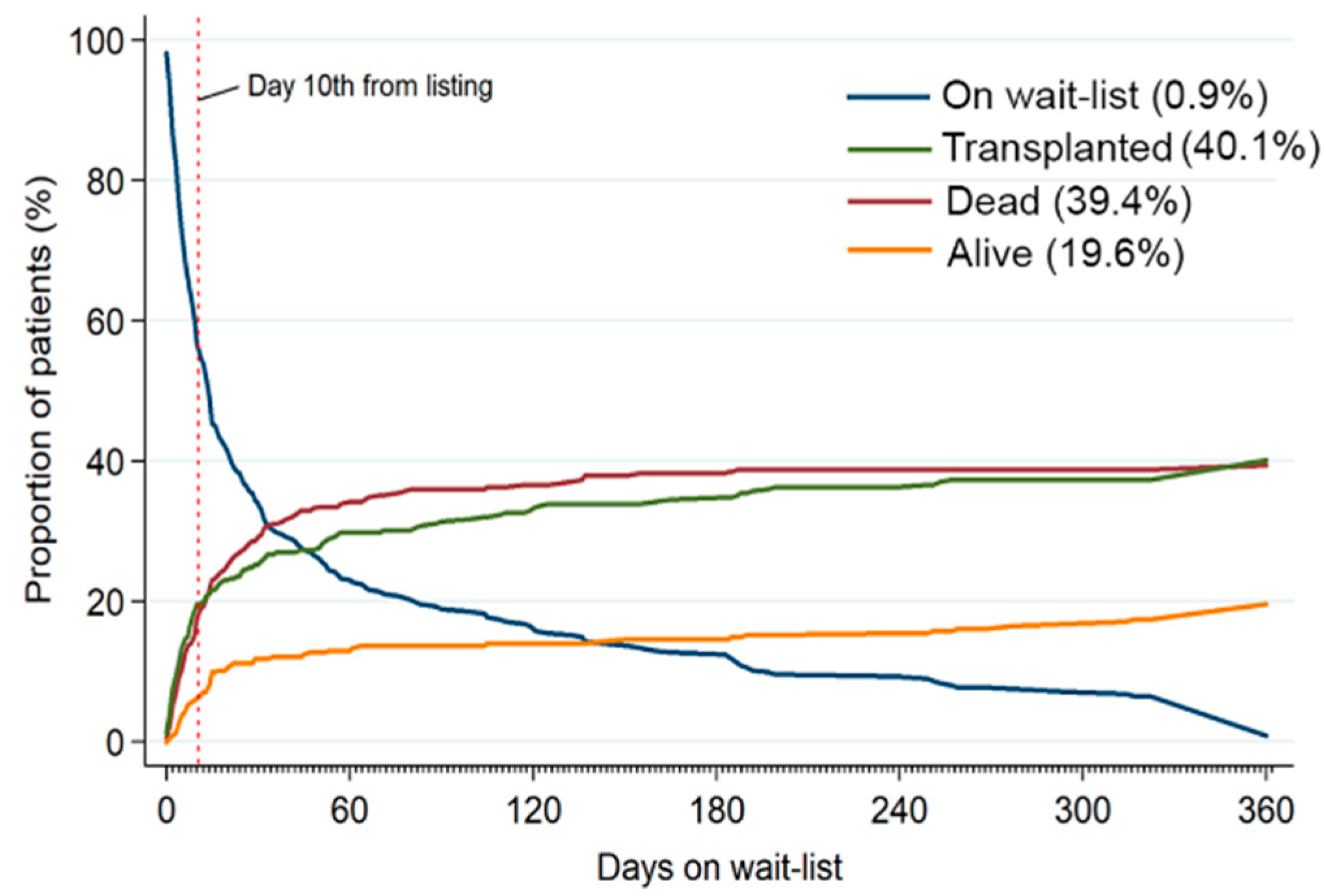
3.2. Waitlist Mortality
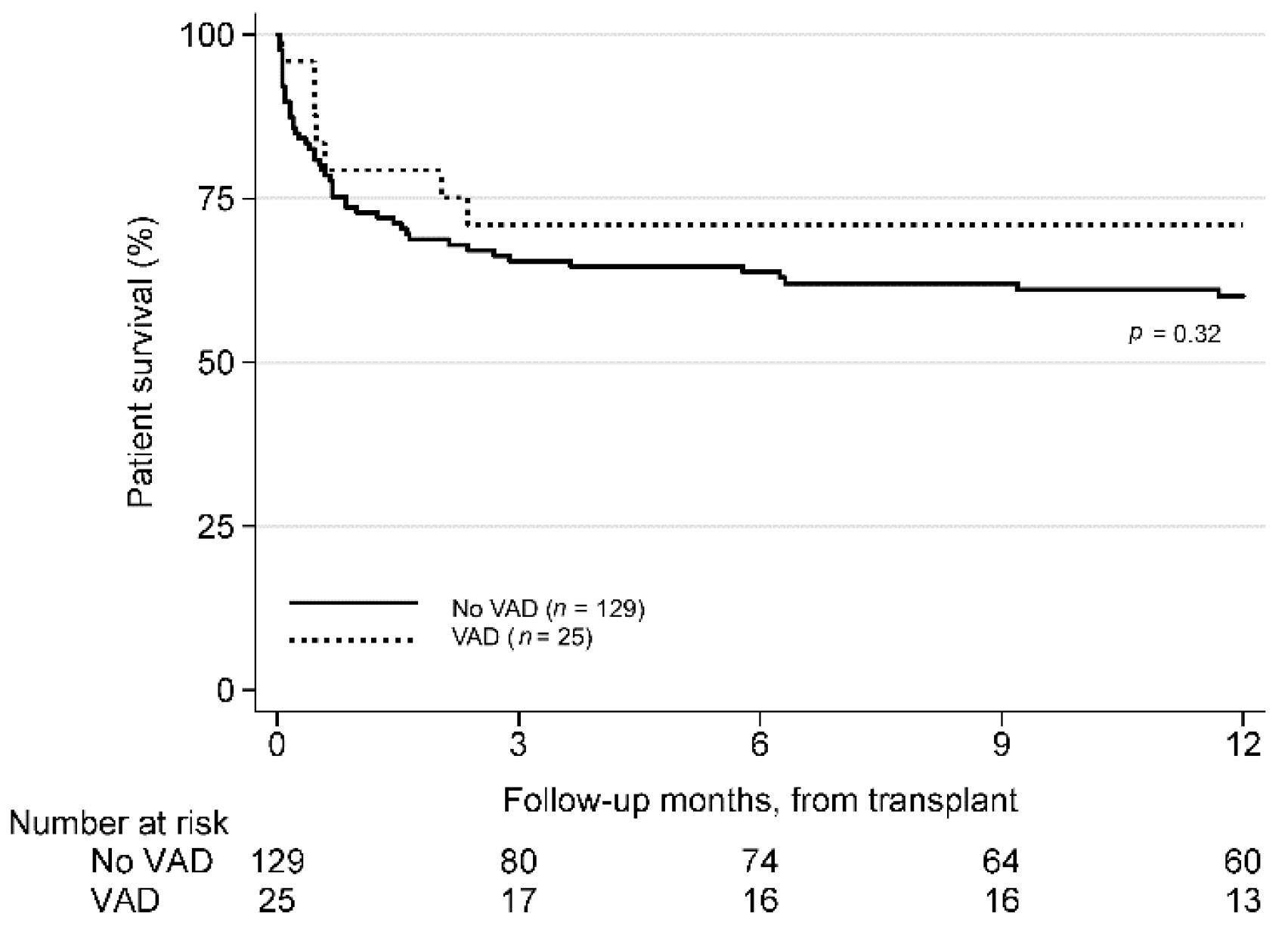
3.3. Post-Transplant Survival
4. Discussion
5. Survival after OHT from VA- ECMO
6. Limitations
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Disclosure
References
- Boyle, A.J.; Ascheim, D.D.; Russo, M.J.; Kormos, R.L.; John, R.; Naka, Y.; Gelijns, A.C.; Hong, K.N.; Teuteberg, J.J. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J. Heart Lung Transplant. 2011, 30, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, D.; Sakaguchi, T.; Saito, S.; Miyagawa, S.; Nishi, H.; Yoshikawa, Y.; Fukushima, S.; Saito, T.; Daimon, T.; Ueno, T.; et al. Predictor of early mortality for severe heart failure patients with left ventricular assist device implantation: significance of INTERMACS level and renal function. Circ. J. 2012, 76, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Barge-Caballero, E.; Segovia-Cubero, J.; Almenar-Bonet, L.; Gonzalez-Vilchez, F.; Villa-Arranz, A.; Delgado-Jimenez, J.; Lage-Galle, E.; Perez-Villa, F.; Lambert-Rodríguez, J.L.; Manito-Lorite, N.; et al. Preoperative INTERMACS profiles determine postoperative outcomes in critically ill patients undergoing emergency heart transplantation: Analysis of the spanish national heart transplant registry. Circ. Heart Fail. 2013, 6, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.; Miranda-Herrera, D.; Gustafson, S.K.; Heubner, B.; Skeans, M.; Wang, X.; Snyder, J.J.; Kasiske, B.L.; Israni, A.K.; Miranda, D. Impact of increased time at the highest urgency category on heart transplant outcomes for candidates with ventricular assist devices. J. Heart Lung Transplant. 2016, 35, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Whitcomb, B.W. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul. Health Metr. 2004, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Schaubel, D.E.; Webb, R.L.; Dickinson, D.M.; Ashby, V.B.; Dykstra, D.M.; Hulbert-Shearon, T.E.; McCullough, K.P.; Hulbert-Shearon, T.E. Analytical approaches for transplant research. Am. J. Transplant. 2004, 4, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B. Seventh INTERMACS annual report: 15,000 patients and counting. J. Heart Lung Transplant. 2015, 34, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Raftery, A.; Hoeting, J.; Volinsky, C.; Painter, I.; Yeung, K.Y. BMA: Bayesian Model Averaging. Available online: https://cran.r-project.org/web/packages/BMA/BMA.pdf (accessed on 1 Apirl 2019).
- Dunson, D.B.; Herring, A.H. Bayesian Model Selection and Averaging in Additive and Proportional Hazards Models. Lifetime Data Anal. 2005, 11, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.; Gray, R. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Makdisi, G.; Wang, I.-W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. 2015, 7, E166–E176. [Google Scholar]
- Kapur, N.K.; Zisa, D.C. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) fails to solve the haemodynamic support equation in cardiogenic shock. EuroIntervention 2016, 11, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Rousse, N.; Juthier, F.; Pinçon, C.; Hysi, I.; Banfi, C.; Robin, E.; Fayad, G.; Jegou, B.; Prat, A.; Vincentelli, A. ECMO as a bridge to decision: Recovery, VAD, or heart transplantation? Int. J. Cardiol. 2015, 187, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Ramzy, D.; Azarbal, B.; Arabia, F.A.; Esmailian, F.; Czer, L.S.; Kobashigawa, J.A.; Moriguchi, J.D. Device strategies for patients in INTERMACS profiles 1 and 2 cardiogenic shock: Double bridge with extracorporeal membrane oxygenation and initial implant of more durable devices. Artif. Organs. 2017, 41, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.C.; Tsai, P.R.; Chou, N.K.; Chi, N.H.; Wang, S.S.; Ko, W.J. Extracorporeal membrane oxygenation bridge to adult heart transplantation. Clin. Transplant. 2010, 24, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Christie, J.D.; Dipchand, A.I.; Dobbels, F.; Goldfarb, S.B.; Levvey, B.J.; Meiser, B.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Official Adult Heart Transplant Report—2014; Focus Theme: Retransplantation. J. Heart Lung Transplant. 2014, 33, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Zalawadiya, S.; Fudim, M.; Bhat, G.; Cotts, W.; Lindenfeld, J. Extracorporeal membrane oxygenation support and post-heart transplant outcomes among United States adults. J. Heart Lung Transplant. 2017, 36, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Smedira, N.G.; Moazami, N.; Golding, C.M.; McCarthy, P.M.; Apperson-Hansen, C.; Blackstone, E.H.; Cosgrove, D.M. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: Survival at five years. J. Thorac. Cardiovasc. Surg. 2001, 122, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.; Smith, J.M.; Skeans, M.A.; Edwards, L.B.; Uccellini, K.; Snyder, J.J.; Israni, A.K.; Kasiske, B.L. OPTN/SRTR 2015 Annual Data Report: Heart. Am. J. Transplant. 2017, 17, 286–356. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.E.; Grimmm, J.C.; Magruder, J.T.; Shah, A.S. Development of a transplantation risk index in patients with mechanical circulatory support: A decision support tool. JACC Heart Fail. 2016, 4, 277–286. [Google Scholar] [CrossRef] [PubMed]
| VAD Brand | Non-Transplanted | Transplanted | Total |
|---|---|---|---|
| HeartMate II | 2 | 6 | 8 |
| Heartware HVAD | 0 | 5 | 5 |
| Abiomed BVS 5000 | 0 | 2 | 2 |
| HeartMate XVE | 0 | 1 | 1 |
| Impella Recover 2.5 | 1 | 0 | 1 |
| Levitronix Centrimag | 0 | 1 | 1 |
| Syncardia CardioWest | 0 | 1 | 1 |
| Thoratec | 1 | 2 | 3 |
| Unknown | 4 | 7 | 11 |
| Total | 8 | 25 | 33 |
| Total (n = 355) | Alive or Transplanted (n = 229) | Dead (n = 126) | Unadjusted HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Demographics and past medical history | |||||
| Age | 50 (38–60) | 50 (37–59) | 52.5 (41–60) | 1.01 (1.00–1.02) | 0.16 |
| Male | 236 (66.5) | 151 (65.9) | 85 (67.5) | 1.01 (0.70–1.47) | 0.95 |
| Non-white | 97 (27.3) | 56 (24.5) | 41 (32.5) | 1.41 (0.97–2.05) | 0.07 |
| BMI | 26.3 (23.8–30.0) | 25.5 (23.0–29.2) | 27.7 (24.6–32.6) | 1.04 (1.01–1.07) | 0.01 |
| Non-O ABO blood type | 209 (58.9) | 143 (62.4) | 66 (52.4) | 0.79 (0.56–1.12) | 0.19 |
| Smoking | 123 (34.6) | 74 (32.3) | 49 (38.9) | 1.31 (0.91–1.87) | 0.14 |
| Diabetes | 81 (22.8) | 40 (17.5) | 41 (32.5) | 1.94 (1.33–2.82) | 0.001 |
| Cardiac surgery | 160 (45.1) | 101 (44.1) | 59 (46.8) | 1.16 (0.82–1.66) | 0.40 |
| NICMP | 97 (27.3) | 77 (33.6) | 20 (15.9) | 0.42 (0.26–0.68) | <0.001 |
| Characteristics at time of listing | |||||
| Mechanical Ventilation | 157 (44.2) | 92 (40.2) | 65 (51.6) | 1.54 (1.09–2.19) | 0.02 |
| Dialysis | 42 (11.8) | 21 (9.2) | 21 (16.7) | 1.80 (1.12–2.87) | 0.02 |
| eGFR < 60 | 181 (51) | 107 (46.7) | 74 (58.7) | 1.50 (1.05–2.14) | 0.03 |
| Inotrope | 146 (41.1) | 94 (41.0) | 52 (41.3) | 1.01 (0.71–1.44) | 0.97 |
| Mean PAP | 28.0 (22.0–37.0) | 28.0 (21.5–38.5) | 31.0 (22.5–35.0) | 1.00 (0.98–1.02) | 0.93 |
| Mean PCWP | 20.0 (13.0–26.0) | 20.0 (14.0–27.0) | 20.0 (12.0–24.0) | 0.98 (0.95–1.01) | 0.21 |
| Cardiac output | 3.9 (2.9–4.8) | 3.9 (2.9–4.7) | 3.7 (2.8–4.9) | 1.00 (0.84–1.18) | 0.96 |
| No VAD implant | 332 (90.7) | 197 (86.0) | 125 (99.2) | 19.26 (2.69 -137.90) | 0.003 |
| No VAD (n = 322) | VAD (n = 33) | p-Value | |
|---|---|---|---|
| Age | 51 (37–60) | 48 (42–59) | 0.85 |
| Male | 209 (64.9) | 27 (81.8) | 0.05 |
| Non-AA | 52 (16.2) | 6 (18.2) | 0.76 |
| Obese (BMI ≥ 30) | 81 (25.2) | 2 (6.1) | 0.01 |
| Non-O ABO | 190 (59.0) | 19 (57.6) | 0.87 |
| Smoking | 114 (35.4) | 9 (27.3) | 0.35 |
| Diabetes | 77 (23.9) | 4 (12.1) | 0.12 |
| Cardiac surgery | 148 (46.0) | 12 (36.4) | 0.29 |
| Prior transplant | 100 (31.1) | 1 (3.0) | 0.001 |
| NICMP | 81 (25.2) | 16 (48.5) | 0.004 |
| Ventilation * | 146 (45.3) | 11 (33.3) | 0.19 |
| Dialysis * | 38 (11.8) | 4 (12.1) | 0.96 |
| Inotrope * | 134 (41.6) | 12 (36.4) | 0.56 |
| Days on listing | 14 (5–52) | 189 (77–283) | <0.001 |
| Days in 1A status | 5 (2–11) | 37 (19–58) | <0.001 |
| Transplant after listing | 129 (40.1) | 25 (75.8) | <0.001 |
| No VAD Prior to OHT (n = 129) | VAD Prior to OHT (n = 25) | p-Value | |
|---|---|---|---|
| Age | 50 (37–60) | 55 (45–63) | 0.13 |
| Male | 83 (64.3) | 21 (84.0) | 0.06 |
| Non-AA | 111 (86.1) | 22 (88.0) | 0.79 |
| Obese (BMI ≥ 30) | 25 (19.4) | 4 (16.0) | 0.69 |
| Non-O ABO | 81 (62.8) | 159 (60.0) | 0.79 |
| Smoking | 38 (29.5) | 7 (28.0) | 0.88 |
| Diabetes | 24 (18.6) | 4 (16.0) | 0.76 |
| Cardiac surgery | 68 (52.7) | 9 (36.0) | 0.13 |
| Prior transplant | 32 (24.8) | 1 (4.0) | 0.02 |
| NICMP | 40 (31.0) | 12 (48.0) | 0.10 |
| Ventilation * | 47 (36.4) | 5 (20.0) | 0.11 |
| Dialysis * | 14 (10.9) | 3 (12.0) | 0.87 |
| Inotrope * | 55 (42.6) | 8 (32.0) | 0.32 |
| Days on listing | 12 (4–57) | 152 (66–259) | <0.001 |
| Days in 1A status | 5 (2–20) | 35 (22–49) | <0.001 |
| Variable | Total (n = 154) | Post-Transplant Mortality | |||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Recipient’s Demographics and Past Medical History | |||||
| Age | 50 (38–60) | 1.01 (0.99–1.02) | 0.48 | 1.01 (0.99–1.03) | 0.297 |
| Male | 104 (67.5) | 0.78 (0.45–1.34) | 0.37 | ||
| Non-White | 32 (20.8) | 2.42 (1.40–4.19) | 0.002 | 1.60 (0.86–2.99) | 0.140 |
| BMI * | 25.3 (22.2–28.4) | 1.06 (1.01–1.11) | 0.02 | 1.03 (0.98–1.09) | 0.218 |
| Non-O ABO | 96 (62.3) | 1.54 (0.87–2.72) | 0.14 | ||
| Ischemic time ≥ 4 | 33 (22.3) | 1.17 (0.62–2.19) | 0.63 | ||
| PRA I ≥ 20% | 34 (22.1) | 2.13 (1.21–3.74) | 0.008 | 2.36 (1.29–4.29) | 0.005 |
| PRA II ≥ 20% | 34 (22.1) | 0.90 (0.47–1.74) | 0.76 | ||
| Heart re-transplant | 33 (21.4) | 1.59 (0.88–2.87) | 0.13 | ||
| Smoking history | 45 (29.2) | 1.71 (1.00–2.92) | 0.051 | 1.92 (1.06–3.48) | 0.032 |
| Diabetes | 28 (18.2) | 1.48 (0.81–2.72) | 0.20 | ||
| Creatinine * | 1.2 (0.9–1.6) | 1.64 (1.21–2.22) | 0.001 | 1.76 (1.26–2.47) | 0.001 |
| Total bilirubin * | 1.2 (0.6–2.0) | 1.05 (1.01–1.09) | 0.01 | 1.05 (1.0–1.10) | 0.022 |
| Mean PAP * | 26.0 (20.0–35.0) | 1.00 (0.97–1.03) | 0.98 | ||
| Mean PCWP * | 17.0 (13.0–26.0) | 1.00 (0.96–1.04) | 0.99 | ||
| Cardiac output * | 4.0 (3.1–5.2) | 1.14 (0.94–1.39) | 0.19 | ||
| High risk CMV | 39 (27.7) | 0.48 (0.20–1.12) | 0.09 | ||
| Dialysis | 29 (19.0) | 1.43 (0.77–2.66) | 0.26 | ||
| Gender mismatch | 49 (31.8) | 2.33 (1.38–3.95) | 0.002 | 1.64 (0.94–2.87) | 0.080 |
| NICMP | 52 (33.8) | 0.62 (0.34–1.14) | 0.13 | ||
| No VAD | 129 (83.8) | 0.67 (0.31–1.49) | 0.33 | 1.85 (0.81–4.21) | 0.145 |
| Donor’s Demographics and Past Medical History | |||||
| Age ≥ 50 | 20 (13.0) | 0.69 (0.27–1.73) | 0.43 | ||
| BMI ≥ 30 | 31 (20.1) | 0.69 (0.34–1.41) | 0.31 | ||
| Smoking history | 27 (17.8) | 0.92 (0.44–1.95) | 0.83 | ||
| Diabetes | 5 (3.3) | 0.70 (0.10–5.04) | 0.72 | ||
| Hypertension | 21 (13.7) | 0.64 (0.26–1.60) | 0.34 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guha, A.; Hannawi, B.; Cruz-Solbes, A.S.; Nguyen, D.T.; Bruckner, B.A.; Trachtenberg, B.; Graviss, E.A.; Bhimaraj, A.; Park, M.; Hussain, I.; et al. Implication of Ventricular Assist Devices in Extracorporeal Membranous Oxygenation Patients Listed for Heart Transplantation. J. Clin. Med. 2019, 8, 572. https://doi.org/10.3390/jcm8050572
Guha A, Hannawi B, Cruz-Solbes AS, Nguyen DT, Bruckner BA, Trachtenberg B, Graviss EA, Bhimaraj A, Park M, Hussain I, et al. Implication of Ventricular Assist Devices in Extracorporeal Membranous Oxygenation Patients Listed for Heart Transplantation. Journal of Clinical Medicine. 2019; 8(5):572. https://doi.org/10.3390/jcm8050572
Chicago/Turabian StyleGuha, Ashrith, Bashar Hannawi, Ana S. Cruz-Solbes, Duc T. Nguyen, Brian A. Bruckner, Barry Trachtenberg, Edward A. Graviss, Arvind Bhimaraj, Myung Park, Imad Hussain, and et al. 2019. "Implication of Ventricular Assist Devices in Extracorporeal Membranous Oxygenation Patients Listed for Heart Transplantation" Journal of Clinical Medicine 8, no. 5: 572. https://doi.org/10.3390/jcm8050572
APA StyleGuha, A., Hannawi, B., Cruz-Solbes, A. S., Nguyen, D. T., Bruckner, B. A., Trachtenberg, B., Graviss, E. A., Bhimaraj, A., Park, M., Hussain, I., MacGillivray, T. E., Suarez, E. E., & Estep, J. D. (2019). Implication of Ventricular Assist Devices in Extracorporeal Membranous Oxygenation Patients Listed for Heart Transplantation. Journal of Clinical Medicine, 8(5), 572. https://doi.org/10.3390/jcm8050572




