Low-Dose Propranolol as Secondary Prophylaxis for Varix Bleeding Decreases Mortality and Rebleeding Rate in Patients with Tense Ascites
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design
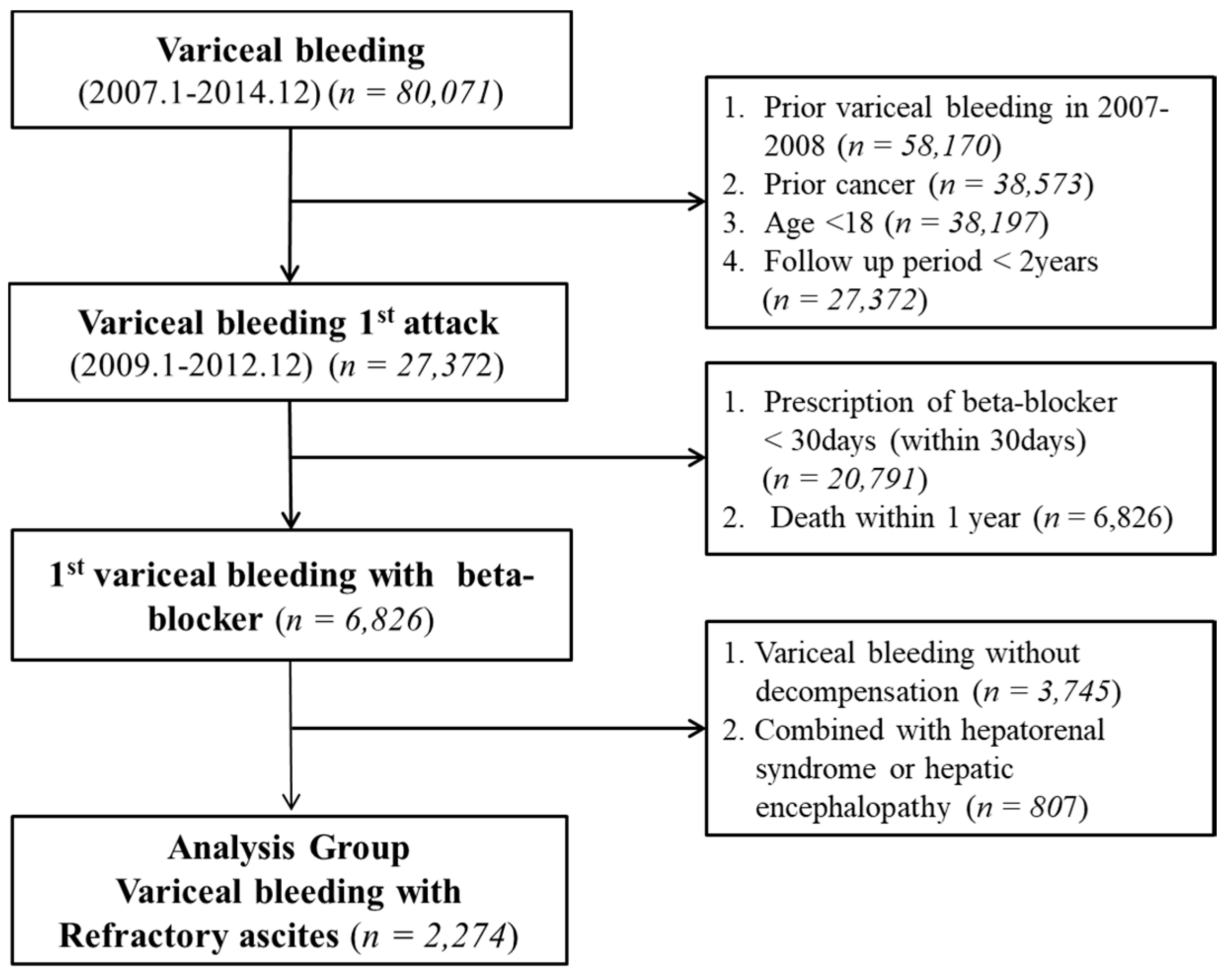
2.3. Study Population
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.6. Baseline Adjustment of Study Population
2.7. Definition of Inadequate User and Medication Compliance
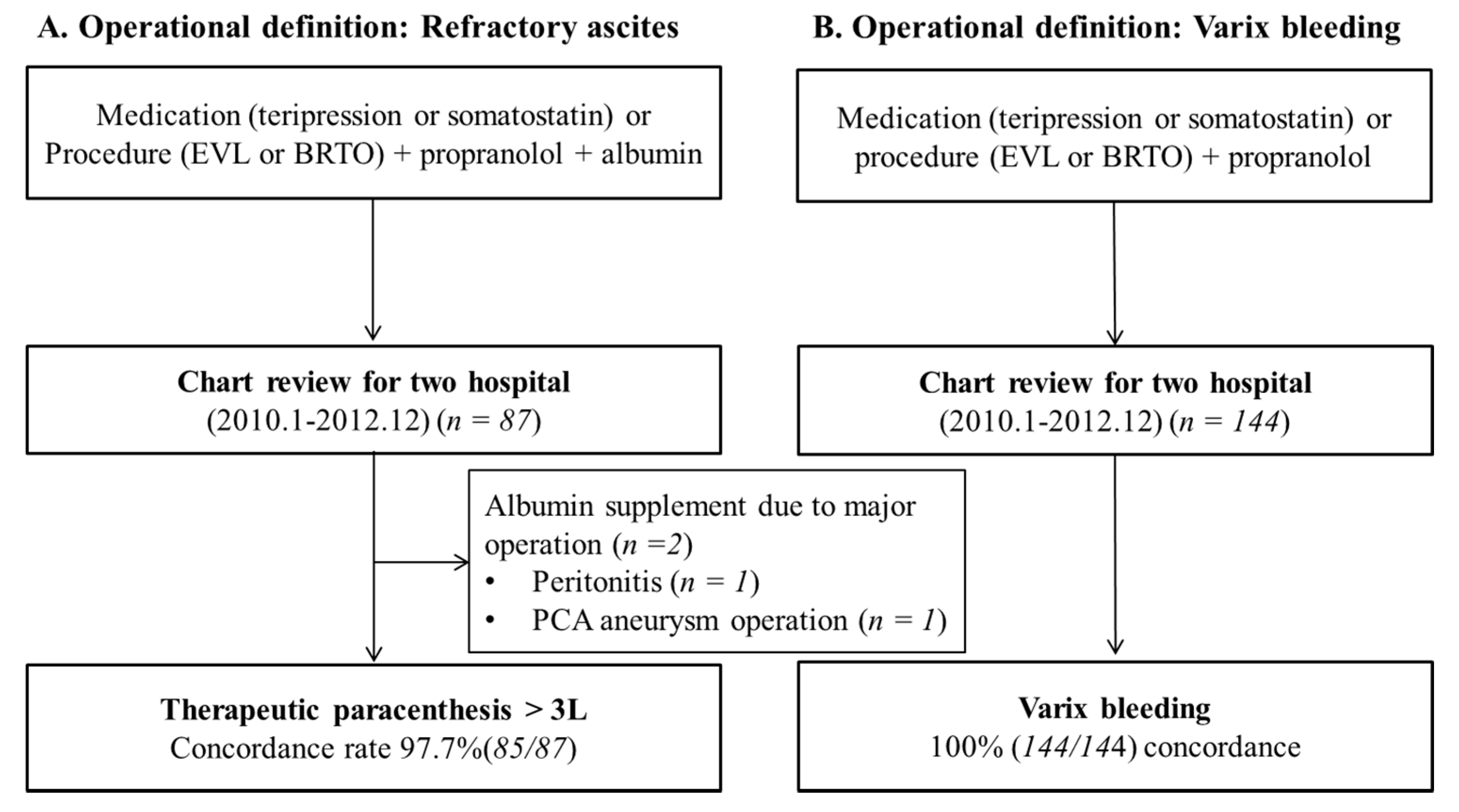
2.8. Operational Definitions of Decompensated Complications
2.9. Validation of Operational Definitions
2.10. Primary and Secondary Endpoint
2.11. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Validation of Operational Definitions
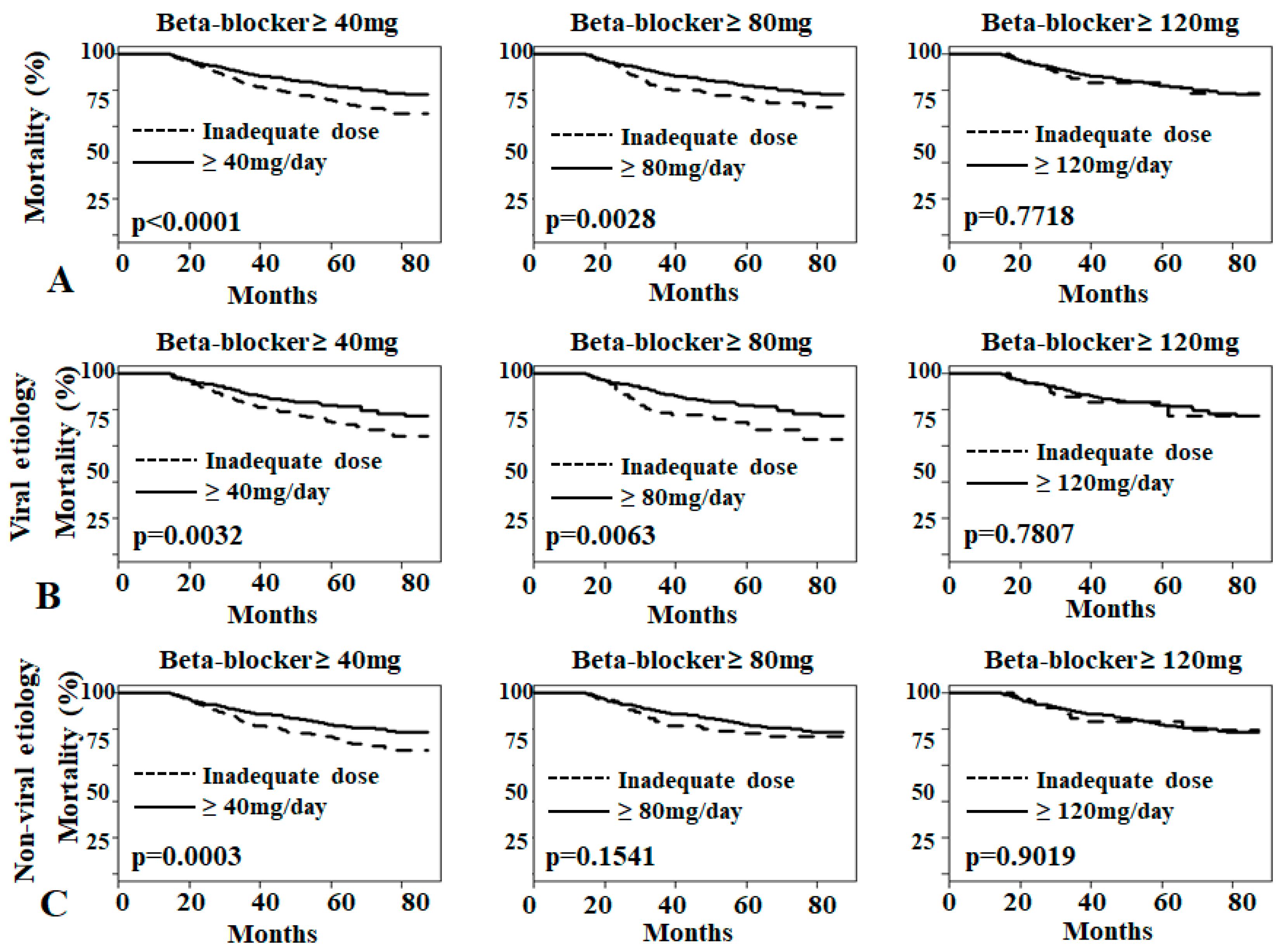
3.3. Effects of Low-Dose Propranolol on Overall Mortality
3.4. Effects of Low-Dose Propranolol on Rebleeding Rate
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Garcia-Tsao, G.; Bosch, J. Management of varices and variceal hemorrhage in cirrhosis. N. Eng. J. Med. 2010, 362, 823–832. [Google Scholar] [CrossRef]
- Poynard, T.; Cales, P.; Pasta, L.; Ideo, G.; Pascal, J.P.; Pagliaro, L.; Lebrec, D. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N. Engl. J. Med. 1991, 324, 1532–1538. [Google Scholar]
- Ideo, G.; Bellati, G.; Fesce, E.; Grimoldi, D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: A prospective, randomized study. Hepatology 1988, 8, 6–9. [Google Scholar] [CrossRef]
- Graham, D.Y.; Smith, J.L. The course of patients after variceal hemorrhage. Gastroenterology 1981, 80, 800–809. [Google Scholar]
- Bosch, J.; Garcia-Pagan, J.C. Prevention of variceal rebleeding. Lancet 2003, 361, 952–954. [Google Scholar] [CrossRef]
- De Franchis, R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J. Hepatol. 2010, 53, 762–768. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, A.; Angus, P.W.; Baijal, S.S.; Chawla, Y.K.; Dhiman, R.K.; Janaka de Silva, H.; Hamid, S.; Hirota, S.; Hou, M.C.; et al. Primary prophylaxis of gastroesophageal variceal bleeding: Consensus recommendations of the Asian Pacific Association for the Study of the Liver. Hepatol. Int. 2008, 2, 429–439. [Google Scholar]
- Serste, T.; Melot, C.; Francoz, C.; Durand, F.; Rautou, P.E.; Valla, D.; Moreau, R.; Lebrec, D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010, 52, 1017–1022. [Google Scholar] [CrossRef]
- Serste, T.; Francoz, C.; Durand, F.; Rautou, P.E.; Melot, C.; Valla, D.; Moreau, R.; Lebrec, D. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: A cross-over study. J. Hepatol. 2011, 55, 794–799. [Google Scholar] [CrossRef]
- Kimer, N.; Feineis, M.; Moller, S.; Bendtsen, F. Beta-blockers in cirrhosis and refractory ascites: A retrospective cohort study and review of the literature. Scand. J. Gastroenterol. 2015, 50, 129–137. [Google Scholar] [CrossRef]
- Leithead, J.A.; Rajoriya, N.; Tehami, N.; Hodson, J.; Gunson, B.K.; Tripathi, D.; Ferguson, J.W. Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut 2015, 64, 1111–1119. [Google Scholar] [CrossRef]
- Robins, A.E.; Bowden, A.; Watson, W.; Smith, F.; Gelson, W.T.J. GW: Propanolol at modest dose dose not impair survival in patients with cirrhosis and refractory ascites. Gut 2012, 61, A183. [Google Scholar] [CrossRef][Green Version]
- Onali, S.; Kalafateli, M.; Majumdar, A.; Westbrook, R.; O’Beirne, J.; Leandro, G.; Patch, D.; Tsochatzis, E.A. Non-selective beta-blockers are not associated with increased mortality in cirrhotic patients with ascites. Liver Int. 2017, 37, 1334–1344. [Google Scholar] [CrossRef]
- Bang, U.C.; Benfield, T.; Hyldstrup, L.; Jensen, J.E.; Bendtsen, F. Effect of propranolol on survival in patients with decompensated cirrhosis: A nationwide study based Danish patient registers. Liver Int. 2016, 36, 1304–1312. [Google Scholar] [CrossRef]
- Bossen, L.; Krag, A.; Vilstrup, H.; Watson, H.; Jepsen, P. Nonselective beta-blockers do not affect mortality in cirrhosis patients with ascites: Post Hoc analysis of three randomized controlled trials with 1198 patients. Hepatology 2016, 63, 1968–1976. [Google Scholar] [CrossRef]
- Mandorfer, M.; Bota, S.; Schwabl, P.; Bucsics, T.; Pfisterer, N.; Kruzik, M.; Hagmann, M.; Blacky, A.; Ferlitsch, A.; Sieghart, W.; et al. Nonselective beta-blockers Increase Risk for Hepatorenal Syndrome and Death in Patients With Cirrhosis and Spontaneous Bacterial Peritonitis. Gastroenterology 2014, 146, 1680–1690. [Google Scholar] [CrossRef]
- Bhutta, A.Q.; Garcia-Tsao, G.; Reddy, K.R.; Tandon, P.; Wong, F.; O’Leary, J.G.; Acharya, C.; Banerjee, D.; Abraldes, J.G.; Jones, T.M.; et al. Beta-blockers in hospitalised patients with cirrhosis and ascites: Mortality and factors determining discontinuation and reinitiation. Aliment. Pharmacol. Therap. 2018, 47, 78–85. [Google Scholar] [CrossRef]
- Lebrec, D. Pharmacological treatment of portal hypertension: Present and future. J. Hepatol. 1998, 28, 896–907. [Google Scholar] [CrossRef]
- Feu, F.; Garcia-Pagan, J.C.; Bosch, J.; Luca, A.; Teres, J.; Escorsell, A.; Rodes, J. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995, 346, 1056–1059. [Google Scholar] [CrossRef]
- Abraldes, J.G.; Tarantino, I.; Turnes, J.; Garcia-Pagan, J.C.; Rodes, J.; Bosch, J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 2003, 37, 902–908. [Google Scholar] [CrossRef]
- Bernard, B.; Lebrec, D.; Mathurin, P.; Opolon, P.; Poynard, T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: A meta-analysis. Hepatology 1997, 25, 63–70. [Google Scholar] [CrossRef]
- Garcia-Tsao, G. Beta-blockers in cirrhosis: The window re-opens. J. Hepatol. 2016, 64, 532–534. [Google Scholar] [CrossRef]
- Ge, P.S.; Runyon, B.A. The changing role of beta-blocker therapy in patients with cirrhosis. J. Hepatol. 2014, 60, 643–653. [Google Scholar] [CrossRef]
- Brito-Azevedo, A. Carvedilol and survival in cirrhosis with ascites: A cognitive bias? J. Hepatol. 2017, 67, 425–426. [Google Scholar] [CrossRef]
- Reiberger, T.; Mandorfer, M. Beta adrenergic blockade and decompensated cirrhosis. J. Hepatol. 2017, 66, 849–859. [Google Scholar] [CrossRef]
- Suk, K.T.; Kim, M.Y.; Park, D.H.; Kim, K.H.; Jo, K.W.; Hong, J.H.; Kim, J.W.; Kim, H.S.; Kwon, S.O.; Baik, S.K. Effect of propranolol on portal pressure and systemic hemodynamics in patients with liver cirrhosis and portal hypertension: A prospective study. Gut Liver 2007, 1, 159–164. [Google Scholar] [CrossRef]

| Variables | All (n = 2274) | Inadequate (n = 1388) | Propranolol 40–80 mg (n = 608) | Propranolol 80–120 mg (n = 177) | Propranolol >120 mg (n = 101) | * p-Value |
|---|---|---|---|---|---|---|
| Age | 52.60 ± 11.74 | 52.94 ± 12.07 | 52.59 ± 11.04 | 51.85 ± 11.78 | 49.33 ± 10.71 | <0.0001 |
| Follow up period (month) | 43.74 ± 15.82 | 44.26 ± 15.49 | 43.15 ± 16.39 | 42.04 ± 16.35 | 43.12 ± 15.81 | 0.2091 |
| Rebleeding days (month) | 22.84 ± 21.02 | 25.35 ± 21.88 | 19.40 ± 19.17 | 17.36 ± 8.76 | 18.75 ± 18.04 | <0.0001 |
| Dosage of β-blocker (mg) | 74 ± 45.6 | 31.6 ± 41.6 | 76.4 ± 32.4 | 109.6 ± 29.6 | 169.6 ± 42.4 | <0.0001 |
| Men (%) | 1810 (79.60) | 1094 (78.82) | 491 (80.76) | 142 (80.23) | 83 (82.18) | 0.6872 |
| Etiology (%) | ||||||
| HBV | 673 (29.60) | 373 (26.87) | 201 (33.06) | 66 (37.29) | 33 (32.67) | 0.0030 |
| HCV | 186 (8.18) | 100 (7.20) | 57 (9.38) | 23 (12.99) | 6 (5.94) | 0.0286 |
| Non-viral | 1415 (62.22) | 915 (65.93) | 350 (57.56) | 88 (49.72) | 62 (61.39) | 0.0002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Jun, D.W.; Choi, J.; Koh, D.H.; Yoon, J.H.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; Choi, H.S. Low-Dose Propranolol as Secondary Prophylaxis for Varix Bleeding Decreases Mortality and Rebleeding Rate in Patients with Tense Ascites. J. Clin. Med. 2019, 8, 573. https://doi.org/10.3390/jcm8050573
Park JH, Jun DW, Choi J, Koh DH, Yoon JH, Lee KN, Lee HL, Lee OY, Yoon BC, Choi HS. Low-Dose Propranolol as Secondary Prophylaxis for Varix Bleeding Decreases Mortality and Rebleeding Rate in Patients with Tense Ascites. Journal of Clinical Medicine. 2019; 8(5):573. https://doi.org/10.3390/jcm8050573
Chicago/Turabian StylePark, Jin Hwa, Dae Won Jun, Jun Choi, Dong Hee Koh, Jai Hoon Yoon, Kang Nyeong Lee, Hang Lak Lee, Oh Young Lee, Byung Chul Yoon, and Ho Soon Choi. 2019. "Low-Dose Propranolol as Secondary Prophylaxis for Varix Bleeding Decreases Mortality and Rebleeding Rate in Patients with Tense Ascites" Journal of Clinical Medicine 8, no. 5: 573. https://doi.org/10.3390/jcm8050573
APA StylePark, J. H., Jun, D. W., Choi, J., Koh, D. H., Yoon, J. H., Lee, K. N., Lee, H. L., Lee, O. Y., Yoon, B. C., & Choi, H. S. (2019). Low-Dose Propranolol as Secondary Prophylaxis for Varix Bleeding Decreases Mortality and Rebleeding Rate in Patients with Tense Ascites. Journal of Clinical Medicine, 8(5), 573. https://doi.org/10.3390/jcm8050573





