Impact of Sarcopenia and Frailty in a Multicenter Cohort of Polypathological Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Reference Population
2.2. Sample Conformation
2.3. Inclusion Criteria
2.4. Development of the Study, Data Collection and Follow-Up
2.5. Sarcopenia Assessment
2.6. Frailty Assessment
2.7. Additional Definitions
2.8. Statistical Analysis
2.9. Ethics Committee Approval
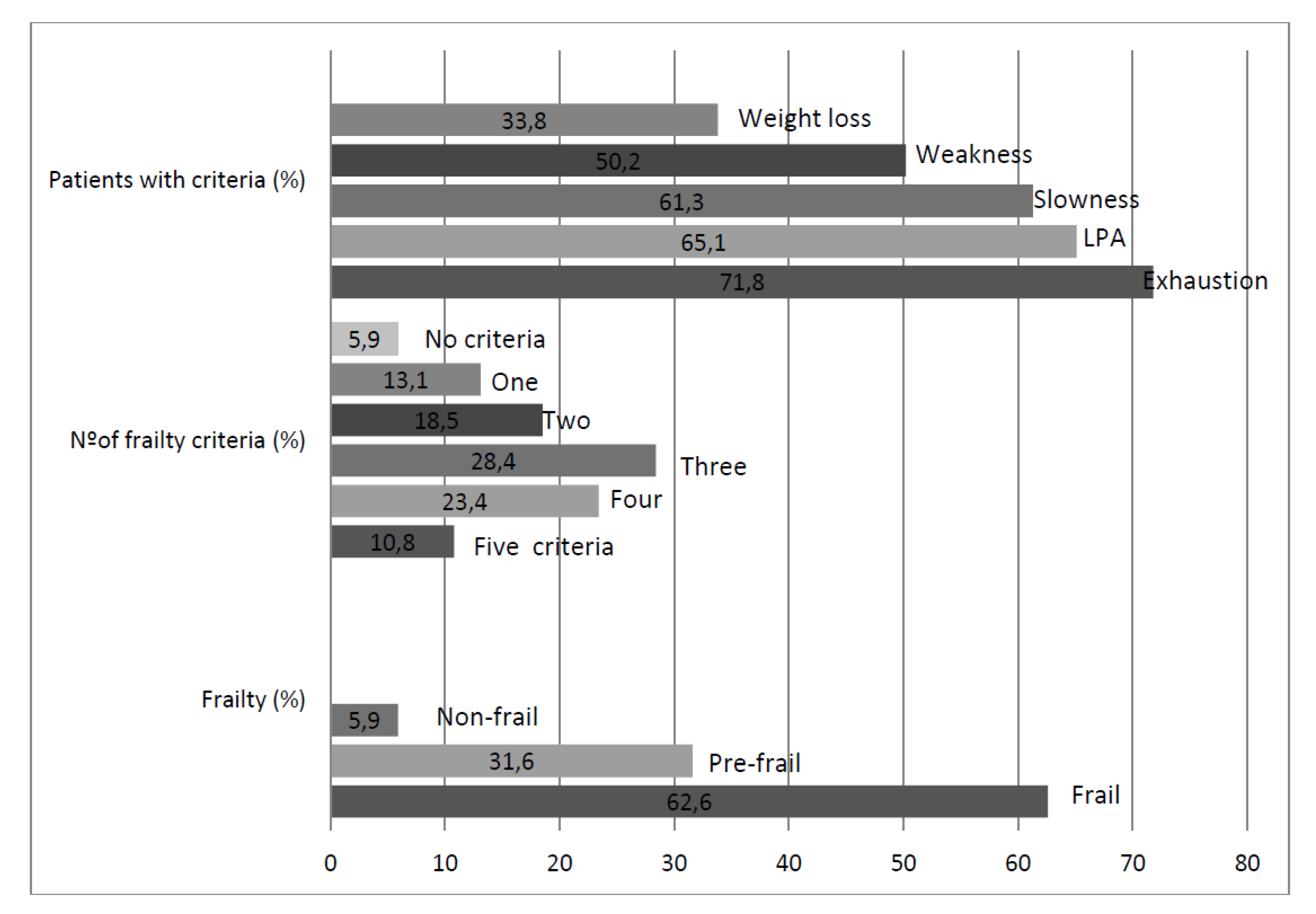
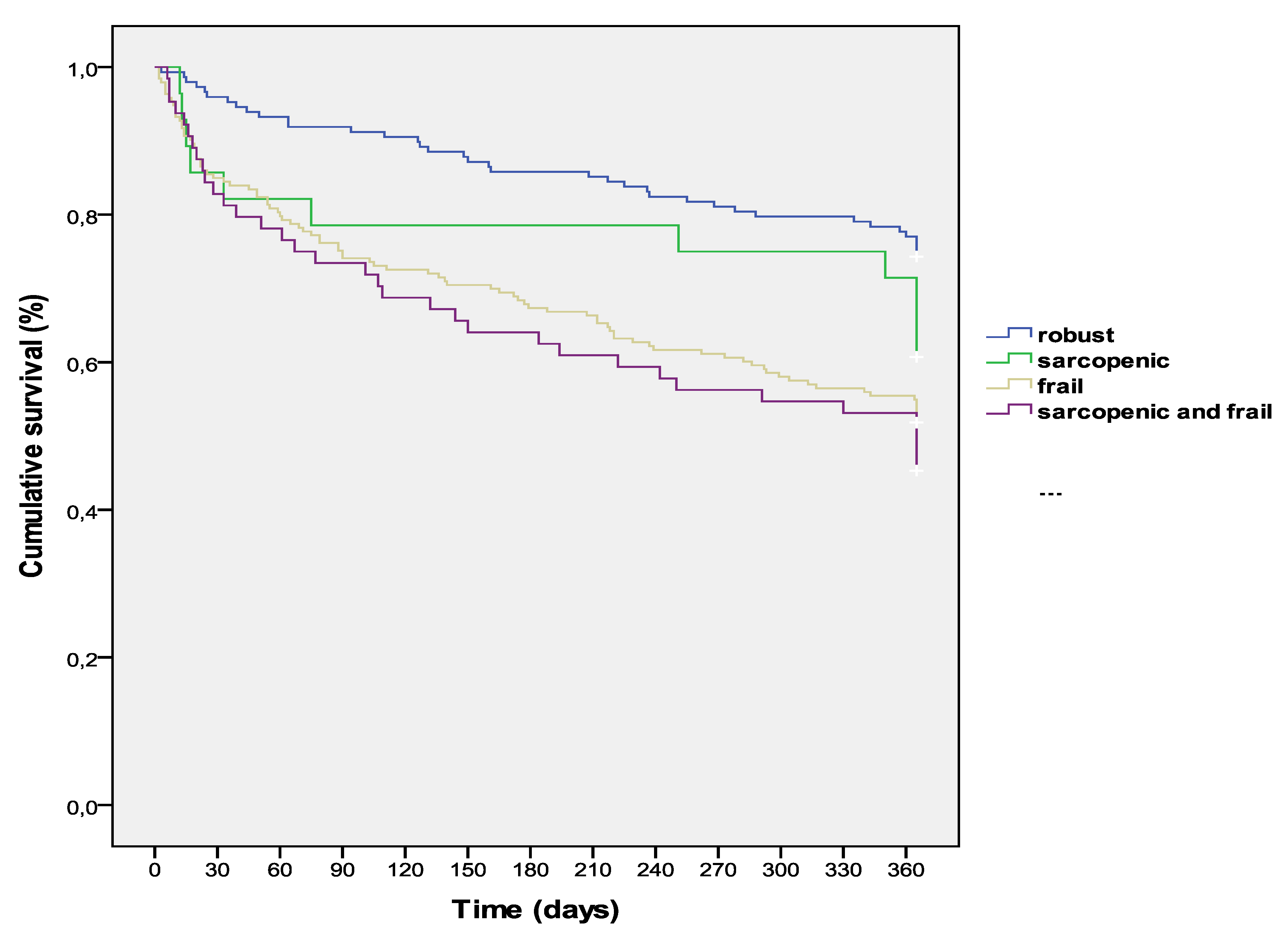
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Eurostat. Population Structure and Ageing. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Population_structure_and_ageing (accessed on 30 October 2016).
- Puth, M.-T.; Weckbecker, K.; Schmid, M.; Münster, E. Prevalence of multimorbidity in Germany: Impact of age and educational level in a cross-sectional study on 19,294 adults. BMC Health 2017, 17, 826. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Wittel, M.; Jadad, A.; Moreno-Gaviño, L.; Hernández-Quiles, C.; Toscano, F.; Cassani, M.; Ramírez, N.; Ollero-Baturone, M. Peeking through the cracks: An assessment of the prevalence, clinical characteristics and health-related quality of life of people with polypathology in a hospital setting. Arch. Gerontol. Geriatr. 2010, 51, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Wittel, M.; Barón-Franco, B.; Murcia-Zaragoza, J.; Fuertes-Martín, A.; Ramos-Cantos, C.; Fernández-Moyano, A.; Galindo, F.; Ollero-Baturone, M. A multi-institutional, hospital-based assessment of clinical, functional, sociofamilial and health-care characteristics of polypathological patients (PP). Arch. Gerontol. Geriatr. 2011, 53, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gaviño, L.; Bernabeu-Wittel, M.; Alvarez-Tello, M.; Gómez, M.R.; Colombo, P.B.; Garza, M.C.; Baturone, M.O.; García-Morillo, S. Overload felt by the figure of the main caregiver in a cohort of patients with multiple pathologies. Atención Primaria 2008, 40, 193–198. [Google Scholar] [CrossRef][Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachex. Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Aging, frailty and age-related diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef]
- Wou, F.; Conroy, S. The Frailty Syndrome. Medicine 2013, 41, 13–15. [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Nobili, A.; Vitale, G. Frailty and sarcopenia: From theory to clinical implementation and public health relevance. Eur. J. Intern. Med. 2016, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.A.; Abraham, W.T.; Chin, M.; Chin, M.H.; Cinquegrani, M.P.; Feldmanmd, A.M.; Francis, G.S.; Ganiats, T.G.; Goldstein, S.; Gregoratos, G.; et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. J. Am. Coll. Cardiol. 2005, 46, e1–e82. [Google Scholar] [CrossRef] [PubMed]
- Bestall, J.; A Paul, E.; Garrod, R.; Garnham, R.; Jones, P.W.; A Wedzicha, J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity indexfor use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuck, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Phisiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.G.; Barros, A.J. Bioelectrical impedance analysis in clinical practice: A new perspective on its use beyond body composition equations. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 311–317. [Google Scholar] [CrossRef]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Schuit, A.J.; Schouten, E.G.; Westerterp, K.R.; Saris, W.H. Validity of the physical activity scale for the elderly (PASE): According to energy expenditure assessed by the doubly labeled water method. J. Clin. Epidemiol. 1997, 50, 541–546. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Gutierrez-Avila, G.; Alfaro-Acha, A.; Amor-Andres, M.S.; De Los Angeles De La Torre Lanza, M.; Escribano Aparicio, M.V.; Humanes Aparicio, S.; Larrion Zugasti, J.L.; Gomez-Serranillo Reus, M.; Rodriguez-Artalejo, F.; et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J. Nutr. Health Aging 2011, 15, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Wittel, M.; Ollero-Baturone, M.; Ruiz-Cantero, A.; Moreno-Gaviño, L.; Barón-Franco, B.; Fuertes, A.; Murcia-Zaragoza, J.; Ramos-Cantos, C.; Alemán, A.; on behalf of PROFUND researchers. Functional decline over one-year follow up in a multicenter cohort of polypathological patients: A new approach to functional prognostication. Int. J. Gerontol. 2012, 6, 68–74. [Google Scholar] [CrossRef][Green Version]
- Díez-Manglano, J.; on behalf of the PLUPAR Study Researchers; Giménez-López, M.; Garcés-Horna, V.; Sevil-Puras, M.; Castellar-Otín, E.; González-García, P.; Fiteni-Mera, I.; Morlanes-Navarro, T. Excessive polypharmacy and survival in polypathological patients. Eur. J. Clin. Pharmacol. 2015, 71, 733–739. [Google Scholar]
- Lozano-Montoya, I.; Correa-Pérez, A.; Abraha, I.; Soiza, R.L.; Cherubini, A.; O’Mahony, D.; Cruz-Jentoft, A.J. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: A systematic overview—The SENATOR Project ONTOP Series. Clin. Interv. Aging 2017, 12, 721–740. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Dedeyne, L.; Deschodt, M.; Verschueren, S.; Tournoy, J.; Gielen, E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: A systematic review. Clin. Interv. 2017, 12, 873–896. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.-A.; Richardson, J.; Giangregorio, A.; Negm, A.M.; Kennedy, C.C.; Thabane, L.; Adachi, J.D.; Cameron, I.D.; Papaioannou, A. Management of frailty: A protocol of a network meta-analysis of randomized controlled trials. Syst. Rev. 2017, 6, 130. [Google Scholar]
- Lee, W.J.; Liu, L.K.; Peng, L.N.; Lin, M.H.; Chen, L.K.; ILAS Research Group. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: Results from the I-Lan longitudinal aging study. J. Am. Med. Dir. Assoc. 2013, 14, 528. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Metab. Disord. 2017, 16, 755. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Dam, T.-T.; Peters, K.W.; Fragala, M.; Cawthon, P.M.; Harris, T.B.; McLean, R.; Shardell, M.; Alley, D.E.; Kenny, A.; Ferrucci, L.; et al. An Evidence-Based Comparison of Operational Criteria for the Presence of Sarcopenia. J. Gerontol. Ser. A 2014, 69, 584–590. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R.; Kiel, D.P. Developing Consensus Criteria for Sarcopenia: An Update. J. Bone Miner. Res. 2015, 30, 588–592. [Google Scholar] [CrossRef]
- Buckinx, F.; Reginster, J.-Y.; Brunois, T.; Lenaerts, C.; Beaudart, C.; Croisier, J.-L.; Petermans, J.; Bruyère, O. Prevalence of sarcopenia in a population of nursing home residents according to their frailty status: Results of the SENIOR cohort. J. Musculoskelet. Neuronal Interact. 2017, 17, 209–217. [Google Scholar]
- Marty, E.; Liu, Y.; Samuel, A.; Or, O.; Lane, J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone 2017, 105, 276–286. [Google Scholar] [CrossRef]
- Kim, H.; Hirano, H.; Edahiro, A.; Ohara, Y.; Watanabe, Y.; Kojima, N.; Kim, M.; Hosoi, E.; Yoshida, Y.; Yoshida, H.; et al. Sarcopenia: Prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr. Gerontol. Int. 2016, 16 (Suppl. 1), 110–122. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kojima, G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J. Am. Med Dir. Assoc. 2015, 16, 940–945. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef]
- Reijnierse, E.M.; Trappenburg, M.C.; Blauw, G.J.; Verlaan, S.; de van der Schueren, M.A.; Meskers, C.G.; Maier, A.B. Common Ground? The Concordance of Sarcopenia and Frailty Definitions. J. Am. Med. Dir. Assoc. 2015, 17, 371. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T. Overlaps between Frailty and Sarcopenia Definitions. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 65–69. [Google Scholar]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- Carmeli, E. Frailty and Primary Sarcopenia: A Review. Adv. Exp. Med. Biol. 2017, 1020, 53–68. [Google Scholar] [PubMed]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Ollero-Baturone, M.; Moreno-Gaviño, L.; Barón-Franco, B.; Fuertes, A.; Murcia-Zaragoza, J.; Ramos-Cantos, C.; Alemán, A.; Fernández-Moyano, A. Development of a new predictive model for polypathological patients. The PROFUND index. Eur. J. Intern. Med. 2011, 22, 311–317. [Google Scholar] [CrossRef] [PubMed]
| Category A |
| A.1 Chronic heart failure with past/present stage II dyspnea of NYHA 1 A.2 Coronary heart disease |
| Category B |
| B.1 Vasculitides and/or systemic autoimmune diseases B.2 Chronic renal disease (creatininaemia >1.4/1.3 mg/dL in men/women or proteinuria 2, during ≥3 months |
| Category C |
| Chronic lung disease with past/present stage 2 dyspnea of MRC 3, or FEV1 < 65%, or basal SatO2 ≤ 90% |
| Category D |
| D.1 Chronic inflammatory bowel disease D.2 Chronic liver disease with evidence of portal hypertension 4 |
| Category E |
| E.1 Stroke E.2 Neurological disease with permanent motor deficit, leading to severe impairment of basic activities of daily living (Barthel’s index < 60). E.3 Neurological disease with permanent moderate-severe cognitive impairment (Pfeiffer’s test with ≥5 errors). |
| Category F |
| F.1 Symptomatic peripheral artery disease F.2 Diabetes mellitus with proliferate retinopathy or symptomatic neuropathy |
| Category G |
| G.1 Chronic anemia (Hb < 10g/dL during ≥3 months) due to digestive-tract losses or acquired haemopathy not tributary of treatment with curative intention G.2 Solid-organ or Hematological active neoplasia not tributary of treatment with curative intention |
| Category H |
| Chronic osteoarticular disease, leading to severe impairment of basic activities of daily living (Barthel’s index < 60) |
| Clinical Features | Mean (SD)/Median [IQR]/N (%) |
|---|---|
| Number of defining categories per patient | 2.5 (0.8) |
| Patients with ≥ 3 categories | 175 (39.5%) |
| Prevalence of defining categories in recruited polypathological patients | |
| Category A (heart diseases) | 374 (84.6%) |
| Category B (kidney/autoimmune diseases) | 202 (45.7%) |
| Category C (lung diseases) | 183 (41.4%) |
| Category E (neurological diseases) | 133 (30.1%) |
| Category F (peripheral arterial disease/diabetes with neuropathy) | 80 (18.1%) |
| Category G (chronic neoplasia/anemia) | 70 (15.8%), |
| Category H (degenerative osteoarticular disease) | 43 (9.7%) |
| Category D (liver disease) | 28 (6.3%) |
| Number of other comorbidities per patient | 5.9 (2.3) |
| Cardiovascular | 1.8 (0.9) |
| Endocrine and metabolic | 1.6 (1) |
| Respiratory | 0.75 (0.9) |
| Most frequent comorbidities | |
| Hypertension | 380 (86%) |
| Dyslipemia | 232 (52.5%) |
| Diabetes with no visceral involvement | 216 (49%) |
| Atrial fibrillation | 178 (40%) |
| Obesity | 159 (36%) |
| Anxiety and depressive disorders | 74 (17%) |
| Benign prostate hyperplasia | 64 (14.5%) |
| Osteoporosis | 42 (9.5%) |
| Frequent symptoms | |
| Fatigue | 304 (70%) |
| Anorexia | 212 (48%) |
| Insomnia | 194 (44%) |
| Chronic pain | 178 (40%) |
| Cough | 158 (36%) |
| Patients with basal III-IV class of NYHA // III-IV class of mMRC | 128 (29%) |
| Patients with delirium in last hospital admission | 76 (17%) |
| Nausea/Vomiting | 37 (8.5%) |
| Pressure ulcer(s) | 35 (8%) |
| PROFUND index | 6 [6] |
| Number of prescribed drugs at inclusion / Patients with polypharmacy | 10 (4)/429 (96.5%) |
| Patients with home oxygen therapy | 74 (17%) |
| Hospitalizations in last 3 months / total days in hospital in last 3 months | 0.6 (0.8)/5 (9) |
| Basal Barthel´s Index | 66 (30) |
| Biological and Anthropometric Features | Mean (SD)/Median [IQR] |
|---|---|
| Main biological parameters | |
| Hemoglobin (d/dL) | 11.3 (2) |
| Creatinin (mg/dL) | 1.26 (1) |
| Albumin (g/dL) | 3.2 (0.9) |
| Bilirrubin (mg/dL) | 0.47 [0.6] |
| Sodium (mEq/L) | 139 (8) |
| Calcium (mg/dL) | 8.7 (0.7) |
| Cholesterol (mg/dL) | 151 (42) |
| Triglicerydes (mg/dL) | 116 (80) |
| Ferritin (ng/mL) | 105 (211) |
| Vitamin D (ng/mL) | 11 (17) |
| Leucocytes (number/µL) | 8000 (4000) |
| Lymphocytes (number/µL) | 1200 (400) |
| Anthropometric features BMI (kg/m2) | 30 (6.6%) |
| Dominant hand strength (kg) | 18 (16) |
| Men | 27 (16) |
| Women | 14 (10) |
| Patients with dominant hand strength below 50 percentile | 223 (50.5%) |
| Men | 112 (46%) |
| Women | 111 (56%) |
| Skeletal muscle mass index (kg/m2) | 11.9 (4.8) |
| Men | 12.9 (5) |
| Women | 10,9 (4) |
| Total body water (L) | 42 (10) |
| Men | 46 (109 |
| Women | 37 (8) |
| Total Fat mass (kg) | 26 (13) |
| Men | 23.1 (12) |
| Women | 29 (14) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabeu-Wittel, M.; González-Molina, Á.; Fernández-Ojeda, R.; Díez-Manglano, J.; Salgado, F.; Soto-Martín, M.; Muniesa, M.; Ollero-Baturone, M.; Gómez-Salgado, J. Impact of Sarcopenia and Frailty in a Multicenter Cohort of Polypathological Patients. J. Clin. Med. 2019, 8, 535. https://doi.org/10.3390/jcm8040535
Bernabeu-Wittel M, González-Molina Á, Fernández-Ojeda R, Díez-Manglano J, Salgado F, Soto-Martín M, Muniesa M, Ollero-Baturone M, Gómez-Salgado J. Impact of Sarcopenia and Frailty in a Multicenter Cohort of Polypathological Patients. Journal of Clinical Medicine. 2019; 8(4):535. https://doi.org/10.3390/jcm8040535
Chicago/Turabian StyleBernabeu-Wittel, Máximo, Álvaro González-Molina, Rocío Fernández-Ojeda, Jesús Díez-Manglano, Fernando Salgado, María Soto-Martín, Marta Muniesa, Manuel Ollero-Baturone, and Juan Gómez-Salgado. 2019. "Impact of Sarcopenia and Frailty in a Multicenter Cohort of Polypathological Patients" Journal of Clinical Medicine 8, no. 4: 535. https://doi.org/10.3390/jcm8040535
APA StyleBernabeu-Wittel, M., González-Molina, Á., Fernández-Ojeda, R., Díez-Manglano, J., Salgado, F., Soto-Martín, M., Muniesa, M., Ollero-Baturone, M., & Gómez-Salgado, J. (2019). Impact of Sarcopenia and Frailty in a Multicenter Cohort of Polypathological Patients. Journal of Clinical Medicine, 8(4), 535. https://doi.org/10.3390/jcm8040535






