Neuroimaging Studies on Disorders of Consciousness: A Meta-Analytic Evaluation
Abstract
:1. Introduction
- active and passive tasks would share brain regions associated with low-level cognitive processing, e.g., auditory processing, while they should be anatomically segregated at the level of higher-order-associative cortices;
- UWS and MCS patients would be characterised by the activation of dissociable neural networks. In particular, we expect that the dorsal-attentional network, responsible for externally directed cognitive processes, would be mainly recruited in MCSs;
- UWS and MCS activations would overlap in regions associated with low-level cognitive tasks, i.e., with passive tasks mainly, while no signs of shared activations should be found in brain regions recruited during active tasks.
2. Experimental Section
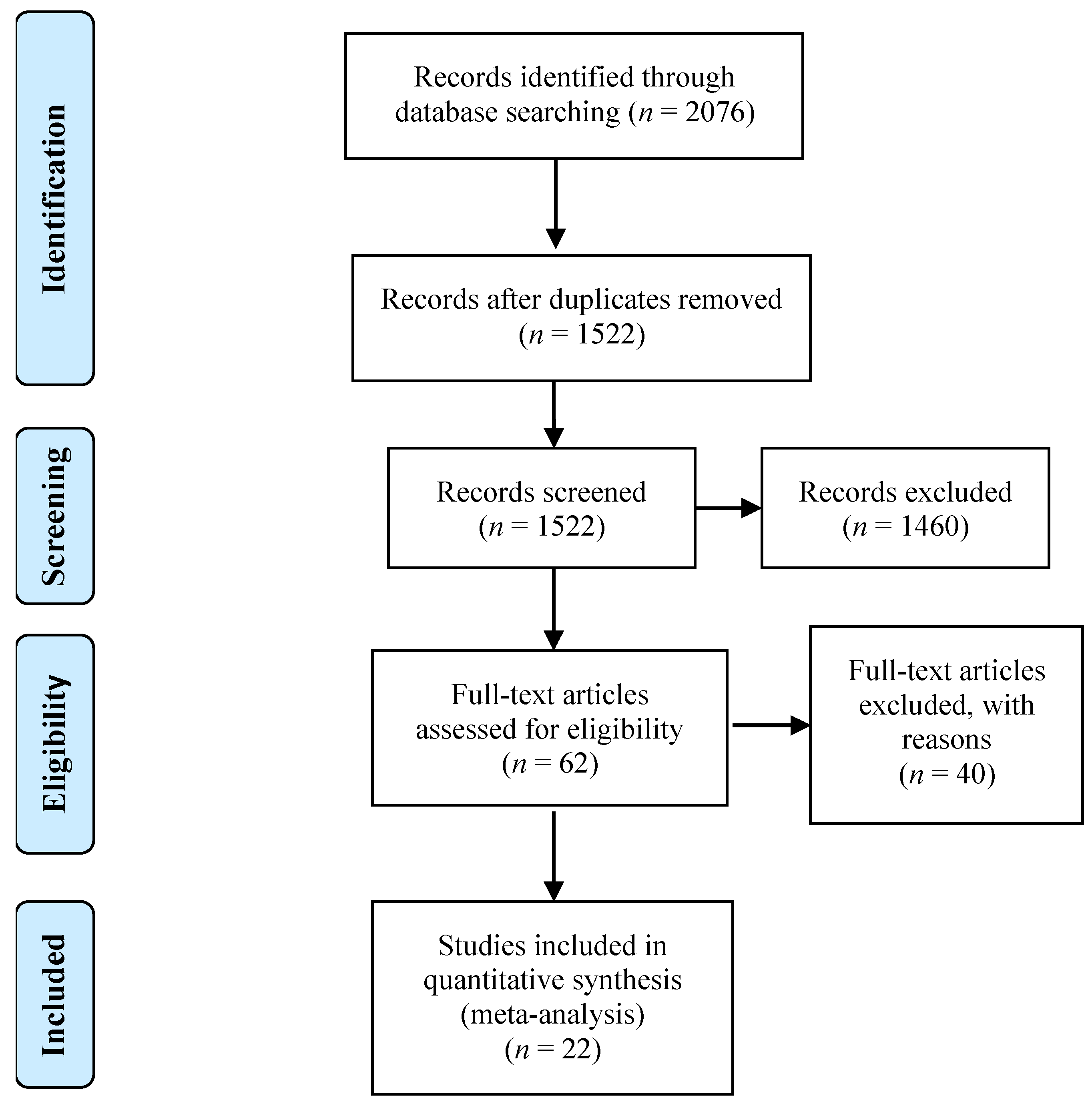
2.1. Study Selection
2.2. Meta-Analytic Procedures
- anatomical masking—we applied a filtering of the coordinates using the “less conservative” mask available in the GingerALE software. After this procedure, 5/441 peaks belonging to the passive tasks and 2/96 belonging to the active tasks fell out of the mask. When running the analyses on the classes of patients, 1/240 activation peak was excluded from the MCS, 6/297 peaks were excluded from the UWS; therefore, the final overall number of foci was 523;
- creation of the ALE maps—for this step we adopted a standard 6-mm full-width at half-maximum (FWHM) to model the Gaussian function around each single coordinate, according to the procedure reported in the methodological paper by Eickhoff [35];
- thresholding procedure—the ALE maps were thresholded at p < 0.001 uncorrected in order to detect also the lowest level of activation across patients (for the sake of completeness, in the supplementary materials Table S2 we report also the results with the most conservative thresholding method available in the literature, i.e., p < 0.05 FWE).
- (a)
- the regions commonly activated by active and passive tasks (active–passive conjunction map) as well as the regions mostly activated by one condition (i.e., active tasks) as compared with the other one (i.e., passive tasks) and vice versa—these computations allowed us to test hypothesis 1;
- (b)
- the regions commonly activated by UWS and MCS (UWS MCS conjunction map), as well as the regions mostly activated by one category of patients (i.e., MCS) as compared with the other one (i.e., UWS) and vice versa—these computations allowed us to test hypotheses 2 and 3.
3. Results
- Passive Task (Table 4): from the set of passive task activation peaks, we found a significant effect in the orbital cortices (BA 47) and in the inferior frontal gyri (pars triangularis; BAs 46, 45), at the border with the middle frontal gyrus (BA 9), in the lateral temporal cortices as well in subcortical regions. In particular, the two subcortical clusters were located (i) between the amygdala, the globus pallidus on the right hemisphere, and (ii) between the amygdala and the parahippocampal gyrus of the left hemisphere;
- Active Task (Table 4): from the pool of active task-related foci, significant clusters were found in the paracentral lobule (BAs 4 and 6), in the inferior parietal lobule (BA 40), in the fusiform gyrus (BA 20), in the lingual gyrus (BA 19), in the middle occipital gyrus (BA 19), in the superior occipital gyrus (BA 18), and in the cuneus (BA 18) of both hemispheres. Concerning the left hemisphere, we found a significant effect in the superior frontal gyrus (BA 8), in the inferior frontal gyrus pars triangularis (at the border with the middle frontal gyrus; BA 9), in the postcentral gyrus (BA 2), and in a left subcortical cluster located between the amygdala and the putamen nucleus. Moreover, we found a significant effect in the right middle frontal gyrus (BAs 8, 9, and 10);
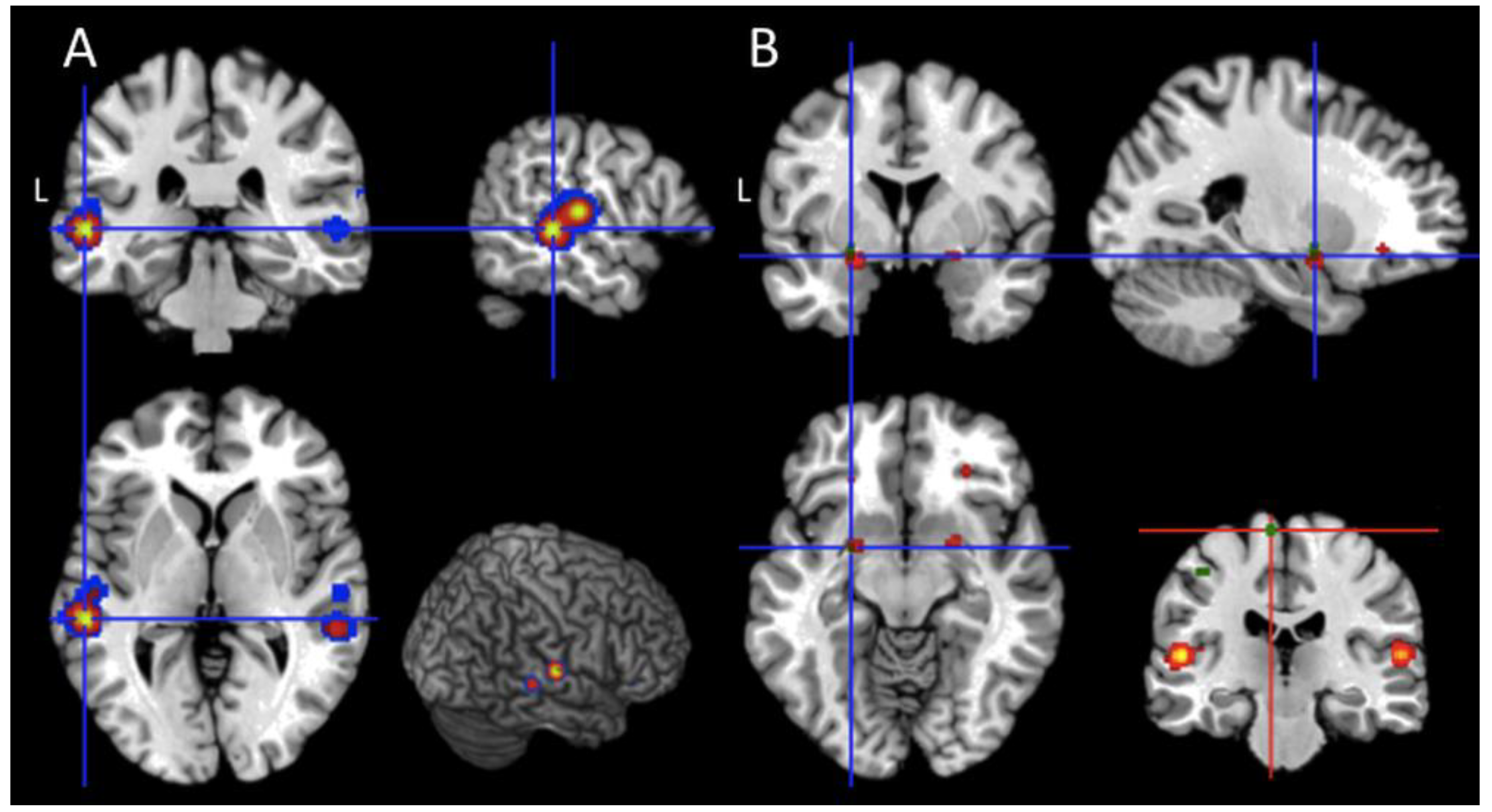
- Passive and Active task - conjunction and contrast analysis: the active and passive map (Table 5) was computed to explicitly test hypothesis 1. As clearly reported in Table 5, the only brain region commonly activated by active and passive paradigm is the subcortical cluster located between the left amygdala and the putamen. Finally, the contrast analyses (p < 0.001 uncorrected) revealed a higher level of activation for the comparison “active > passive tasks” in the left paracentral lobule (x = −6, y = −24, z = 68; BA 6); the reversed contrast, namely “passive > active tasks” did not show any significant effect;
- Unresponsive Wakefulness Syndrome (UWS; Table 4): the patients in UWS showed significant activations in the inferior frontal gyrus, both in the pars orbitalis (BA 47) and in the pars triangularis (45, and 46) extending to the border with the middle frontal gyrus (BA 9), in the supplementary motor area (SMA; BA 6), in the lateral temporal cortices, and the amygdala (BA 34) of both hemispheres. Furthermore, a significant effect was found at the subcortical level between the amygdala, the globus pallidus on the right hemisphere and between the amygdala and the parahippocampal gyrus of the left hemisphere;
- Minimally Conscious State (MCS; Table 4): we found significant clusters of activations in the orbital part of the inferior frontal gyrus (BA 47), in the lateral temporal cortices, and in the fusiform gyrus (BA 20) of both hemispheres. Also, in this case, a significant effect was found at the subcortical level between the amygdala, the globus pallidus on the right hemisphere and between the amygdala and the parahippocampal gyrus of the left hemisphere. Furthermore, significant results emerged in the left inferior frontal gyrus at the border with the middle frontal gyrus (pars triangularis; BAs 9 and 45);
- UWS and MCS patients—conjunction and contrast analysis: the activations described across studies and shared by the two classes of patients were located in the orbital portions of the inferior frontal gyrus (BA 47), in the superior temporal gyrus (BA 41), and in the two subcortical clusters located around the amygdala of both hemispheres (Table 5). Furthermore, significant shared activations were found in the dorsal part of the left inferior frontal gyrus (at the border with the middle frontal gyrus; BAs 9 and 45), in the left middle temporal gyrus (BA 22), and in the right heschl gyrus (at the border with the insular cortex; BA 13). The between-groups contrast analyses did not show any significant result.
4. Discussion
4.1. Active and Passive Task-Related Activations in DOC Patients
4.2. Are There Neurofunctional Markers of Consciousness Recovery in the dorsal-attentional Network?
4.3. The Minimum State of the Brain: Shared Activations between UWS and MCS
5. Conclusion: Recommendations for Neuroimaging Studies of DOC Patients and Limitation of the Study
- The GingerALE approach only permits the comparison of two categories and looking at their commonalities. This means that every other higher-order comparison, as well as comparisons with other maps (such as those extracted from Neurosynth) cannot be performed using an inferential approach. This is the reason we adopted a voxel-count approach for our intersection analyses. However, the readers should be aware that these analyses gave just information about the spatial extent (as expressed in terms of voxels) of anatomical overlap.
- Some of the results reported here may be triggered by the specific set of experimental tasks implemented in the fMRI studies. Future meta-analyses should better evaluate task-specific effect in this literature.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Monti, M.M.; Vanhaudenhuyse, A.; Coleman, M.R.; Boly, M.; Pickard, J.D.; Tshibanda, L.; Owen, A.M.; Laureys, S. Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 2010, 362, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S. The neural correlate of (un)awareness: Lessons from the vegetative state. Trends Cogn. Sci. 2005, 9, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK coma recovery scale-revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice guideline update recommendations summary: Disorders of consciousness. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Coleman, M.R.; Boly, M.; Davis, M.H.; Laureys, S.; Pickard, J.D. Detecting awareness in the vegetative state. Science 2006, 313, 1402. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eden, G.F.; Jones, K.M.; Zeffiro, T.A. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage 2002, 16, 765–780. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D.; Rodriguez-Moreno, D.; Kamal, A.; Kim, K.H.S.; Giacino, J.T.; Plum, F.; Hirsch, J.; Zhu, Y.H.; Zhang, S.Z.; Chen, Y.Z. fMRI reveals large-scale network activation in minimally conscious patients. Neurology 2005, 64, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, X.; Gao, L.; Mao, Y.; Zhong, P.; Tang, W.; Zhou, L. cortical activity after emotional visual stimulation in minimally conscious state patients. J. Neurotrauma 2009, 26, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Di, H.; Hu, X.; Jing, S.; Thibaut, A.; Di Perri, C.; Huang, W.; Nie, Y.; Schnakers, C.; Laureys, S. Cerebral response to subject’s own name showed high prognostic value in traumatic vegetative state. BMC Med. 2015, 13, 83. [Google Scholar] [CrossRef]
- Liang, X.; Kuhlmann, L.; Johnston, L.A.; Grayden, D.B.; Vogrin, S.; Crossley, R.; Fuller, K.; Lourensz, M.; Cook, M.J. Extending communication for patients with disorders of consciousness. J. Neuroimaging 2014, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.M.; Pickard, J.D.; Owen, A.M. Visual cognition in disorders of consciousness: From V1 to top-down attention. Hum. Brain Mapp. 2013, 34, 1245–1253. [Google Scholar] [CrossRef]
- Hampshire, A.; Parkin, B.L.; Cusack, R.; Espejo, D.F.; Allanson, J.; Kamau, E.; Pickard, J.D.; Owen, A.M. Assessing residual reasoning ability in overtly non-communicative patients using fMRI. NeuroImage Clin. 2013, 2, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Crone, J.S.; Ladurner, G.; Höller, Y.; Golaszewski, S.; Trinka, E.; Kronbichler, M. Deactivation of the default mode network as a marker of impaired consciousness: An fMRI study. PLoS ONE 2011, 6, e26373. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, T.A.; Manes, F.F.; Villarreal, M.; Owen, A.M.; Della-Maggiore, V. Functional imaging reveals movement preparatory activity in the vegetative state. Front. Hum. Neurosci. 2011, 5, 5. [Google Scholar] [CrossRef]
- Moreno, D.R.; Schiff, N.D.; Giacino, J.; Kalmar, K.; Hirsch, J. A network approach to assessing cognition in disorders of consciousness. Neurology 2010, 75, 1871–1878. [Google Scholar] [CrossRef]
- Fernández-Espejo, D.; Junque, C.; Cruse, D.; Bernabeu, M.; Roig-Rovira, T.; Fábregas, N.; Rivas, E.; Mercader, J.M. Combination of diffusion tensor and functional magnetic resonance imaging during recovery from the vegetative state. BMC Neurol. 2010, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Di, H.; Liu, Y.; Yu, S.; Gong, Q.; Duncan, N.; Weng, X.; Laureys, S.; Northoff, G. Anterior cingulate activity and the self in disorders of consciousness. Hum. Brain Mapp. 2010, 31, 1993–2002. [Google Scholar] [CrossRef]
- Heelmann, V.; Lippert-Grüner, M.; Rommel, T.; Wedekind, C. Abnormal functional MRI BOLD contrast in the vegetative state after severe traumatic brain injury. Int. J. Rehabil. Res. 2010, 33, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espejo, D.; Junqué, C.; Vendrell, P.; Bernabeu, M.; Roig, T.; Bargalló, N.; Mercader, J.M. Cerebral response to speech in vegetative and minimally conscious states after traumatic brain injury. Brain Inj. 2008, 22, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Di, H.B.; Yu, S.M.; Weng, X.C.; Laureys, S.; Yu, D.; Li, J.Q.; Qin, P.M.; Zhu, Y.H.; Zhang, S.Z.; Chen, Y.Z. Cerebral response to patient’s own name in the vegetative and minimally conscious states. Neurology 2007, 68, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Staffen, W.; Kronbichler, M.; Aichhorn, M.; Mair, A.; Ladurner, G. Selective brain activity in response to one’s own name in the persistent vegetative state. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1383–1384. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, T.; Tiberti, C.; Niklison, J.; Tamashiro, M.; Ron, M.; Carpintiero, S.; Villarreal, M.; Forcato, C.; Leiguarda, R.; Manes, F. Assessing level of consciousness and cognitive changes from vegetative state to full recovery. Neuropsychol. Rehabil. 2005, 15, 307–322. [Google Scholar] [CrossRef]
- Owen, A.; Coleman, M.; Menon, D.; Johnsrude, I.; Rodd, J.; Davis, M.; Taylor, K.; Pickard, J. Residual auditory function in persistent vegetative state: A combined pet and fMRI study. Neuropsychol. Rehabil. 2005, 15, 290–306. [Google Scholar] [CrossRef]
- Owen, A.M.; Menon, D.K.; Johnsrude, I.S.; Bor, D.; Scott, S.K.; Manly, T.; Williams, E.J.; Mummery, C.; Pickard, J.D. Detecting residual cognitive function in persistent vegetative state. Neurocase 2002, 8, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Sharon, H.; Pasternak, Y.; Ben Simon, E.; Gruberger, M.; Giladi, N.; Krimchanski, B.Z.; Hassin, D.; Hendler, T. Emotional processing of personally familiar faces in the vegetative state. PLoS ONE 2013, 8, e74711. [Google Scholar] [CrossRef]
- Nigri, A.; Catricalà, E.; Ferraro, S.; Bruzzone, M.G.; D’Incerti, L.; Sattin, D.; Sebastiano, D.R.; Franceschetti, S.; Marotta, G.; Benti, R.; et al. The neural correlates of lexical processing in disorders of consciousness. Brain Imaging Behav. 2017, 11, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Nigri, A.; Ferraro, S.; Bruzzone, M.G.; Nava, S.; D’Incerti, L.; Bertolino, N.; Sattin, D.; Leonardi, M.; Lundström, J.N. Central olfactory processing in patients with disorders of consciousness. Eur. J. Neurol. 2016, 23, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, F.; Cecchetti, L.; Gibson, R.M.; Logi, F.; Owen, A.M.; Malasoma, F.; Cozza, S.; Pietrini, P.; Ricciardi, E. Progression from vegetative to minimally conscious state is associated with changes in brain neural response to passive tasks: A longitudinal single-case functional MRI study. J. Int. Neuropsychol. Soc. 2016, 22, 620–630. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Yu, T.; Mueller, F.; Vogel, D.; Veser, S.; Lang, S. True or false? Activations of language-related areas in patients with disorders of consciousness. Curr. Pharm. Des. 2014, 20, 4239–4247. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within–experiment and within–group effects in activation likelihood estimation meta–analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta–analysis revisited. Neuroimage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate–based activation likelihood estimation meta–analysis of neuroimaging data: A random–effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Rorden, C.; Brett, M. Stereotaxic display of brain lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef]
- Posner, M.I. Attentional networks and consciousness. Front. Psychol. 2012, 3, 64. [Google Scholar] [CrossRef]
- Koch, C.; Tsuchiya, N. Attention and consciousness: Two distinct brain processes. Trends Cogn. Sci. 2007, 11, 16–22. [Google Scholar] [CrossRef]
- Spreng, R.N.; Sepulcre, J.; Turner, G.R.; Stevens, W.D.; Schacter, D.L. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J. Cogn. Neurosci. 2013, 25, 74–86. [Google Scholar] [CrossRef]
- Dehaene, S.; Changeux, J.-P.; Naccache, L.; Sackur, J.; Sergent, C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn. Sci. 2006, 10, 204–211. [Google Scholar] [CrossRef]
- Tononi, G.; Koch, C. Consciousness: Here, there and everywhere? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140167. [Google Scholar] [CrossRef]
- Gaillard, R.; Dehaene, S.; Adam, C.; Clémenceau, S.; Hasboun, D.; Baulac, M.; Cohen, L.; Naccache, L. Converging intracranial markers of conscious access. PLoS Biol. 2009, 7, e1000061. [Google Scholar] [CrossRef]
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state. N. Engl. J. Med. 1994, 330, 1499–1508. [Google Scholar] [CrossRef]
- Working Party of the Royal College of Physicians. The vegetative state: Guidance on diagnosis and management. Clin. Med. 2003, 3, 249–254. [Google Scholar] [CrossRef]
- Wannez, S.; Heine, L.; Thonnard, M.; Gosseries, O.; Laureys, S. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann. Neurol. 2017, 81, 883–889. [Google Scholar] [CrossRef]
| Study ID | Authors | Year | Technique | Sample Size | Task Category | Task Type |
|---|---|---|---|---|---|---|
| 1 | Wang et al. ∞ [13] | 2005 | fMRI | 66 (n = 39 UWS; n = 25 MCS; n = 2 EMCS) | passive | Spoken own name by a familiar voice |
| 2 | Liang et al. [14] | 2014 | fMRI | 5 (n = 2 UWS; n = 3 MCS) | passive and active | Spoken sentences and motor/mental imagery |
| 3 | Monti et al. [15] | 2013 | fMRI | 1 (MCS) | passive | Visual stimulation |
| 4 | Hampshire et al. χ [16] | 2013 | fMRI | 1(UWS) | active | Visual imagery |
| 5 | Crone et al. [17] | 2011 | fMRI | 25 (n = 17 UWS; n = 8 MCS) | passive | Sentences listening |
| 6 | Bekinschtein et al. α [18] | 2011 | fMRI | 5 (all UWS) | passive | Hand movement verbal command |
| 7 | Moreno et al. Δ [19] | 2010 | fMRI | 10 (n = 3 UWS; n = 5 MCS; n = 1 EMC; n = 1 LIS) | active | Visual naming |
| 8 | Fernández-Espejo et al. £ [20] | 2010 | fMRI | 1 (UWS) | passive | Sentences listening |
| 9 | Qin et al. [21] | 2010 | fMRI | 11 (n = 7 UWS; n = 4 MCS) | passive | Spoken own names by familiar voice |
| 10 | Monti et al. • [1] | 2010 | fMRI | 54 (n = 23 UWS; n = 31 MCS) | active | Motor imagery |
| 11 | Heelmann et al. [22] | 2010 | fMRI | 6 (all UWS) | passive | Visual and sensory stimulations |
| 12 | Fernández-Espejo et al. [23] | 2008 | fMRI | 7 (n = 3 UWS; n = 4 MCS) | passive | Narratives listening |
| 13 | Di et al. * [24] | 2007 | fMRI | 11 (n = 7 UWS; n = 4 MCS) | passive | Spoken own name by a familiar voice |
| 14 | Staffen et al. [25] | 2006 | fMRI | 1 (UWS) | passive | Spoken own name by a familiar voice |
| 15 | Bekinschtein et al. × [26] | 2005 | fMRI | 1 (UWS) | passive | Sentences listening |
| 16 | Owen et al. µ [27] | 2005 | fMRI | 1 (UWS) | passive | Sentences listening |
| 17 | Owen et al. [28] | 2002 | fMRI | 3 (all UWS) | passive | Visual stimulation, familiar face perception, and speech perception |
| 18 | Sharon et al. [29] | 2013 | fMRI | 4 (all UWS) | passive and active | Face perception and visual imagery |
| 19 | Nigri et al. ¥ [30] | 2017 | fMRI | 14 (n = 4 UWS; n = 10 MCS) | passive | Words listening |
| 20 | Nigri et al. [31] | 2016 | fMRI | 33 (n = 26 UWS; n = 7 MCS) | passive | Olfactory stimulation |
| 21 | Tomaiuolo et al. [32] | 2016 | fMRI | 1 (tested twice both in UWS and MCS) | passive | Sentences listening |
| 22 | Kotchoubey et al. + [33] | 2014 | fMRI | 55 (n = 29 UWS; n = 26 MCS) | passive | Sentences listening |
| Brain Regions | Study | Left Hemisphere | Study | Right Hemisphere | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| Precentral gyrus | χ; α | −39 | −6 | 51 | ||||
| Superior frontal gyrus | Δ | −18 | 35 | 42 | ||||
| Middle frontal gyrus | Δ | −33 | 33 | 35 | Δ | 38 | 33 | 34 |
| Δ | 22 | 31 | 44 | |||||
| Inferior frontal gyrus | + | −46 | 30 | 14 | + | 50 | 30 | 14 |
| + | −36 | 31 | −12 | ¥; + | 41 | 32 | −12 | |
| ¥ | −36 | 31 | 12 | |||||
| Supplementary Motor Area | •; α | −5 | 5 | 61 | α | 9 | 0 | 62 |
| Inferior parietal | Δ | −43 | −46 | 47 | Δ | 46 | −46 | 50 |
| Paracentral lobule | Δ | −8 | −25 | 70 | Δ | 7 | −32 | 68 |
| Postcentral gyrus | Δ | −42 | −23 | 49 | ||||
| Heschl gyrus | *; ∞; × | −42 | −19 | 10 | *; ∞ | 46 | −17 | 10 |
| Superior temporal gyrus | * | −56 | −38 | 18 | * | 56 | −38 | 18 |
| * | −41 | −39 | 18 | * | 53 | −21 | 3 | |
| * | −53 | −21 | 3 | +; ¥; ∞; α; µ | 58 | −22 | 7 | |
| +; ¥; ∞; α; £; ×; µ | −53 | −21 | 7 | |||||
| Middle temporal gyrus | *; +; ¥; α; £; µ | −56 | −34 | −2 | *; +; ¥; α; µ | 57 | −37 | −1 |
| Insula | χ | 39 | 6 | 2 | ||||
| Parahippocampal | • | −21 | −16 | −21 | •; χ | 25 | −15 | −20 |
| Superior occipital gyrus | Δ; χ | −17 | −84 | 28 | Δ | 24 | −81 | 31 |
| Middle occipital gyrus | Δ | −32 | −81 | 16 | Δ | 37 | −80 | 19 |
| Fusiform | Δ | −31 | −40 | −20 | Δ | 34 | −39 | −20 |
| Lingual | Δ | −15 | −68 | −5 | Δ | 16 | −67 | −4 |
| Cuneus | Δ | −6 | −80 | 27 | Δ | 14 | −79 | 28 |
| Patients Category | Total | |||
|---|---|---|---|---|
| UWS | MCS | |||
| Tasks Category | active | 38 | 58 | 96 |
| passive | 259 | 182 | 441 | |
| Total | 297 | 240 | 537 | |
| Cluster’ Number | Area | x | y | z | Brodmann Area | Volume (mm3) |
|---|---|---|---|---|---|---|
| Passive Tasks | ||||||
| 1 | Left Inferior Frontal Gyrus—triangular part/Middle Frontal Gyrus | −50 | 16 | 30 | 9 | 56 |
| 2 | Right Inferior Frontal Gyrus—triangular part/Middle Frontal Gyrus | 49.8 | 30.2 | 14.4 | 46 | 72 |
| 3 | Left Inferior orbito-frontal | −24 | 30 | −10 | 47 | 56 |
| 4 | Left Inferior orbito-frontal | −36 | 31 | −12 | 47 | 16 |
| 5 | Left Inferior Frontal Gyrus—triangular part | −44.3 | 25.8 | 12.9 | 45 | 384 |
| 6 | Right Inferior orbito-frontal | 40.9 | 32.1 | −12 | 47 | 496 |
| 7 | Right Inferior orbito-frontal | 28 | 34 | −12 | 47 | 56 |
| 8 | Left Superior Temporal Gyrus | −54.8 | −25.7 | 3.8 | 41 | 8912 |
| Left Middle Temporal Gyrus | −56 | −34 | −2 | |||
| Left Heschl Gyrus/Insula | −42 | −20 | 10 | 13 | ||
| 9 | Right Superior Temporal Gyrus | 56.7 | −20.5 | 8.2 | 41 | 4192 |
| Right Heschl Gyrus/Insula | 46 | −18 | 10 | 13 | ||
| Right Thalamus/Superior Temporal Gyrus | 8 | −8 | 4 | 22 | ||
| Right Superior Temporal Gyrus | 68 | −28 | 16 | 42 | ||
| 10 | Right Middle Temporal Gyrus | 68.5 | −18 | −8.8 | 21 | 120 |
| 11 | Right Middle Temporal Gyrus | 57.2 | −36.8 | −1 | 22 | 1712 |
| 12 | Left Amygdala/Parahippocampal Gyrus | −22.3 | −0.3 | −14.7 | 34 | 296 |
| 13 | Right Amygdala/Globus Pallidus | 22.5 | 1.8 | −12.6 | 152 | |
| Active Tasks | ||||||
| 1 | Right Middle Frontal Gyrus/Superior Frontal Gyrus | 33 | 53 | 11 | 10 | 64 |
| 2 | Right Middle Frontal Gyrus/Superior Frontal Gyrus | 20.6 | 32 | 43 | 8 | 208 |
| 3 | Left Inferior Frontal Gyrus—triangular part/Middle Frontal Gyrus | −33 | 33 | 35 | 9 | 64 |
| 4 | Right Middle Frontal Gyrus | 38 | 33 | 34 | 9 | 80 |
| 5 | Left Superior Frontal Gyrus | −18 | 35 | 42 | 8 | 80 |
| 6 | Left Paracentral Lobule | −7.2 | −24.6 | 70 | 6 | 136 |
| 7 | Right Paracentral Lobule | 6.9 | −31.5 | 68.1 | 4 | 104 |
| 8 | Left Postcentral Gyrus | −42 | −23 | 49 | 2 | 96 |
| 9 | Left Inferior Parietal Lobule | −43.8 | −45.5 | 45.3 | 40 | 200 |
| 10 | Right Inferior Parietal Lobule | 46 | −46 | 50 | 40 | 56 |
| 11 | Left Fusiform Gyrus | −31 | −40 | −20 | 20 | 80 |
| 12 | Right Fusiform Gyrus | 31.7 | −38.9 | −21.1 | 20 | 1224 |
| 13 | Left Lingual Gyrus | −15 | −68 | −5 | 19 | 96 |
| 14 | Left Middle Occipital Gyrus | −32 | −81 | 16 | 19 | 80 |
| 15 | Right Middle Occipital Gyrus | 37 | −80 | 19 | 19 | 96 |
| 16 | Left Superior Occipital Gyrus/Cuneus | −17 | −84 | 28 | 18 | 224 |
| 17 | Left Cuneus | −6.5 | −80 | 27 | 18 | 112 |
| 18 | Right Superior Occipital Gyrus/Cuneus | 24 | −81 | 31 | 18 | 96 |
| 19 | Right Cuneus | 14 | −79 | 28 | 18 | 80 |
| 20 | Left Amygdala/Putamen | −24.4 | −1 | −10.8 | 80 | |
| 21 | Right Lingual Gyrus | 16 | −67 | −4 | 80 | |
| Unresponsive Wakefulness Syndrome | ||||||
| 1 | Left Inferior Frontal Gyrus—triangular part/Middle Frontal Gyrus | −50 | 16 | 30 | 9 | 56 |
| 2 | Right Inferior Frontal Gyrus—triangular part/Middle Frontal Gyrus | 49.8 | 30.2 | 14.4 | 46 | 72 |
| 3 | Left Inferior orbito-frontal Gyrus | −24 | 30 | −10 | 47 | 56 |
| 4 | Left Inferior orbito-frontal Gyrus | −36 | 31 | −12 | 47 | 16 |
| 5 | Left Inferior Frontal Gyrus—triangular part | −44.3 | 25.8 | 12.9 | 45 | 384 |
| 6 | Right Inferior orbito-frontal Gyrus | 40.9 | 32 | −12 | 47 | 488 |
| 7 | Right Inferior orbito-frontal Gyrus | 28 | 34 | −12 | 47 | 56 |
| 8 | Left Supplementary Motor Area | −4.3 | 4.5 | 60.5 | 6 | 160 |
| 9 | Right Supplementary Motor Area | 7.7 | 0.6 | 63.3 | 6 | 184 |
| 10 | Left Superior Temporal Gyrus | −54.4 | −25.3 | 4.3 | 41 | 7560 |
| Left Middle Temporal Gyrus | −56 | −34 | −2 | |||
| Left Heschl Gyrus/Insula | −42 | −20 | 10 | 13 | ||
| 11 | Right Superior Temporal Gyrus | 55.6 | −20.7 | 8 | 41 | 3096 |
| Right Heschl Gyrus/Insula | 46 | −18 | 10 | 13 | ||
| 12 | Right Middle Temporal Gyrus | 68.4 | −17.8 | −8.7 | 21 | 112 |
| 13 | Right Middle Temporal Gyrus | 57.5 | −36.9 | −1 | 22 | 1552 |
| 14 | Left Amygdala/Parahippocampal Gyrus | −22.7 | −0.3 | −13.8 | 34 | 536 |
| 15 | Right Amygdala/Globus Pallidus | 22.6 | 1.9 | −12.8 | 144 | |
| Minimally Conscious State | ||||||
| 1 | Left Inferior Frontal Gyrus—triangular part/Middle Frontal Gyrus | −50 | 16 | 30 | 9 | 56 |
| 2 | Left Inferior orbito-frontal Gyrus | −24 | 30 | −10 | 47 | 56 |
| 3 | Left Inferior Frontal Gyrus—triangular part | −43.6 | 21.9 | 12 | 45 | 128 |
| 4 | Right Inferior orbito-frontal Gyrus | 28.2 | 34.6 | −12.2 | 47 | 96 |
| 5 | Left Fusiform Gyrus | −30.8 | −40.2 | −19.8 | 20 | 248 |
| 6 | Right Fusiform Gyrus | 31.8 | −39.1 | −21 | 20 | 624 |
| 7 | Left Superior Temporal Gyrus | −53.8 | −26.3 | 3.1 | 41 | 4576 |
| Left Middle Temporal Gyrus | −56 | −34 | −2 | |||
| Left Heschl Gyrus/Insula | −42 | −20 | 10 | 13 | ||
| 8 | Right Superior Temporal Gyrus | 58.3 | −22 | 7.4 | 41 | 1248 |
| 9 | Right Middle Temporal Gyrus | 56.7 | −37 | −1 | 22 | 488 |
| 10 | Right Heschl Gyrus/Insula | 46.2 | −17.2 | 10.1 | 13 | 592 |
| 11 | Left Amygdala/Parahippocampal Gyrus | −22.4 | −0.4 | −14.8 | 34 | 360 |
| 12 | Right Amygdala/Globus Pallidus | 22.4 | 2 | −12.6 | 192 | |
| Cluster Number | Area | x | y | z | Brodmann Area | Volume (mm3) |
|---|---|---|---|---|---|---|
| Active and Passive | ||||||
| 1 | Left Amygdala/Putamen | −24 | −1 | −13 | 32 | |
| UWS and MCS | ||||||
| 1 | Left Inferior orbito-frontal | −24 | 30 | −10 | 47 | 56 |
| 2 | Right Inferior orbito-frontal | 28 | 34 | −12 | 47 | 56 |
| 3 | Left Inferior Frontal Gyrus—triangular part/Middle Frontal Gyrus | −50 | 16 | 30 | 9 | 56 |
| 4 | Left Inferior Frontal Gyrus—triangular part | −43.7 | 22.1 | 12.1 | 45 | 112 |
| 5 | Left Superior Temporal Gyrus | −53.7 | −26.3 | 3.3 | 41 | 4432 |
| Left Middle Temporal Gyrus | −56 | −34 | −2 | |||
| Left Heschl Gyrus/Insula | −42 | −20 | 10 | 13 | ||
| 6 | Right Superior Temporal Gyrus | 58.3 | −22 | 7.4 | 41 | 1248 |
| 7 | Right Heschl Gyrus/Insula | 46.2 | −17.2 | 10.1 | 13 | 592 |
| 8 | Left Middle Temporal Gyrus | −56.7 | −37 | −1 | 22 | 488 |
| 9 | Left Amygdala/Parahippocampal Gyrus | −22.4 | −0.4 | −14.8 | 34 | 360 |
| 10 | Right Amygdala/Globus Pallidus | 22.6 | 1.9 | −12.8 | 144 | |
| Brain Region | Voxel Count | |
|---|---|---|
| UWS | MCS | |
| Left frontal inferior triangular part | 41 | 41 |
| Left Inferior orbito-frontal | 4 | 0 |
| Right Inferior orbito-frontal | 10 | 10 |
| Left frontal inferior operculum | 2 | 2 |
| Right Rolandic operculum | 25 | 8 |
| Left supplementary motor area | 124 | 0 |
| Left precentral gyrus | 1 | 1 |
| Left Heschl gyrus | 46 | 30 |
| Right Heschl gyrus | 19 | 13 |
| Left superior temporal | 67 | 35 |
| Right superior temporal | 362 | 225 |
| Left middle temporal | 97 | 53 |
| Left fusiform gyrus | 0 | 90 |
| Right fusiform gyrus | 0 | 6 |
| Patients’ Category | Total | |||
|---|---|---|---|---|
| UWS | MCS | |||
| DAN | Yes | 798 | 514 | 1312 |
| No | 13,662 | 8178 | 21,840 | |
| Total | 14,460 | 8692 | 23,152 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlingeri, M.; Magnani, F.G.; Salvato, G.; Rosanova, M.; Bottini, G. Neuroimaging Studies on Disorders of Consciousness: A Meta-Analytic Evaluation. J. Clin. Med. 2019, 8, 516. https://doi.org/10.3390/jcm8040516
Berlingeri M, Magnani FG, Salvato G, Rosanova M, Bottini G. Neuroimaging Studies on Disorders of Consciousness: A Meta-Analytic Evaluation. Journal of Clinical Medicine. 2019; 8(4):516. https://doi.org/10.3390/jcm8040516
Chicago/Turabian StyleBerlingeri, Manuela, Francesca Giulia Magnani, Gerardo Salvato, Mario Rosanova, and Gabriella Bottini. 2019. "Neuroimaging Studies on Disorders of Consciousness: A Meta-Analytic Evaluation" Journal of Clinical Medicine 8, no. 4: 516. https://doi.org/10.3390/jcm8040516
APA StyleBerlingeri, M., Magnani, F. G., Salvato, G., Rosanova, M., & Bottini, G. (2019). Neuroimaging Studies on Disorders of Consciousness: A Meta-Analytic Evaluation. Journal of Clinical Medicine, 8(4), 516. https://doi.org/10.3390/jcm8040516





