Bicuspid Aortic Valve Alters Aortic Protein Expression Profile in Neonatal Coarctation Patients
Abstract
1. Introduction
2. Experimental Section
2.1. Patients and Tissue Collection
2.2. Sample Preparation
2.3. Nano-LC Mass Spectrometry
2.4. Data Processing and Analysis
2.5. Elastin Staining Analysis
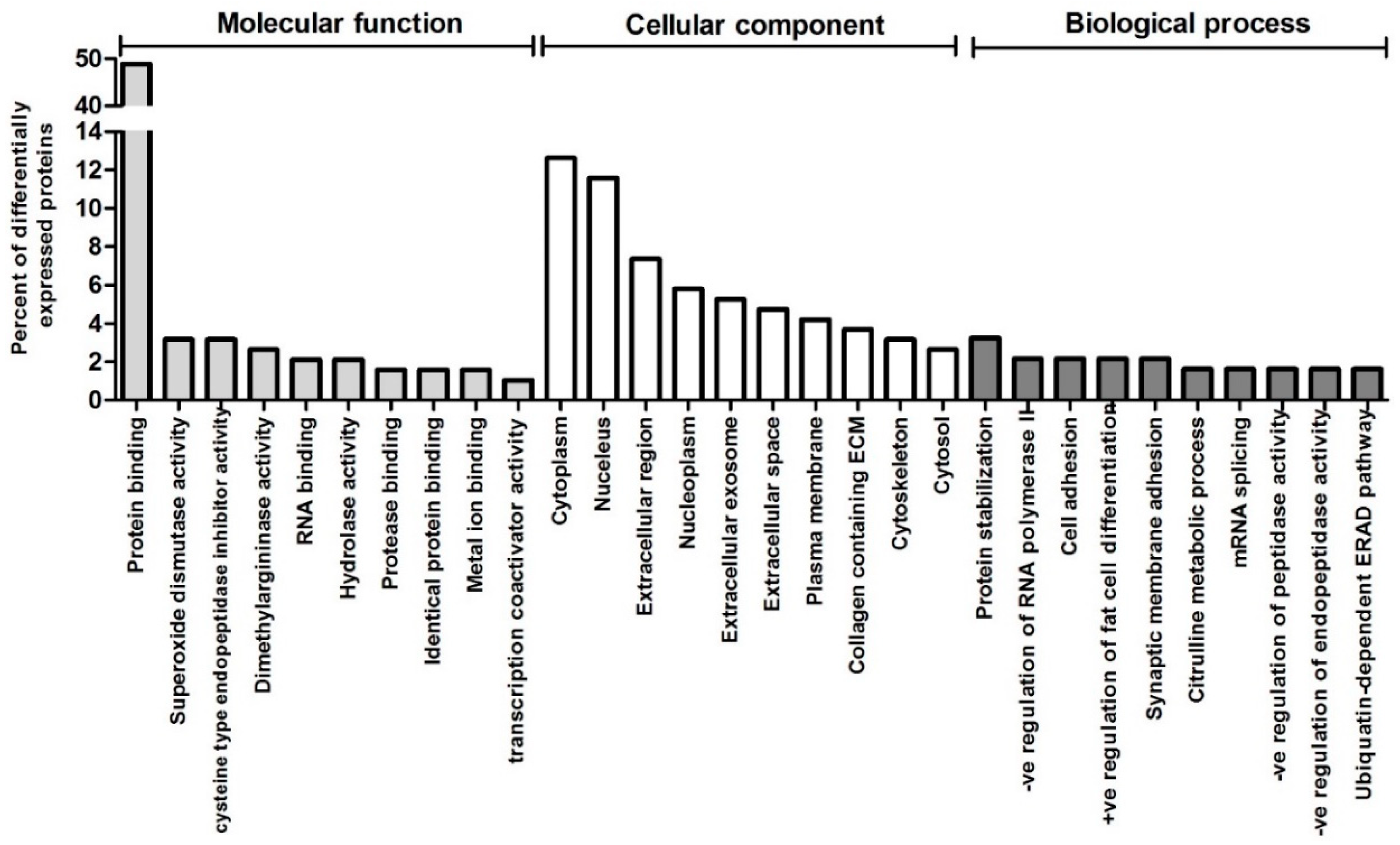
3. Results
4. Discussion
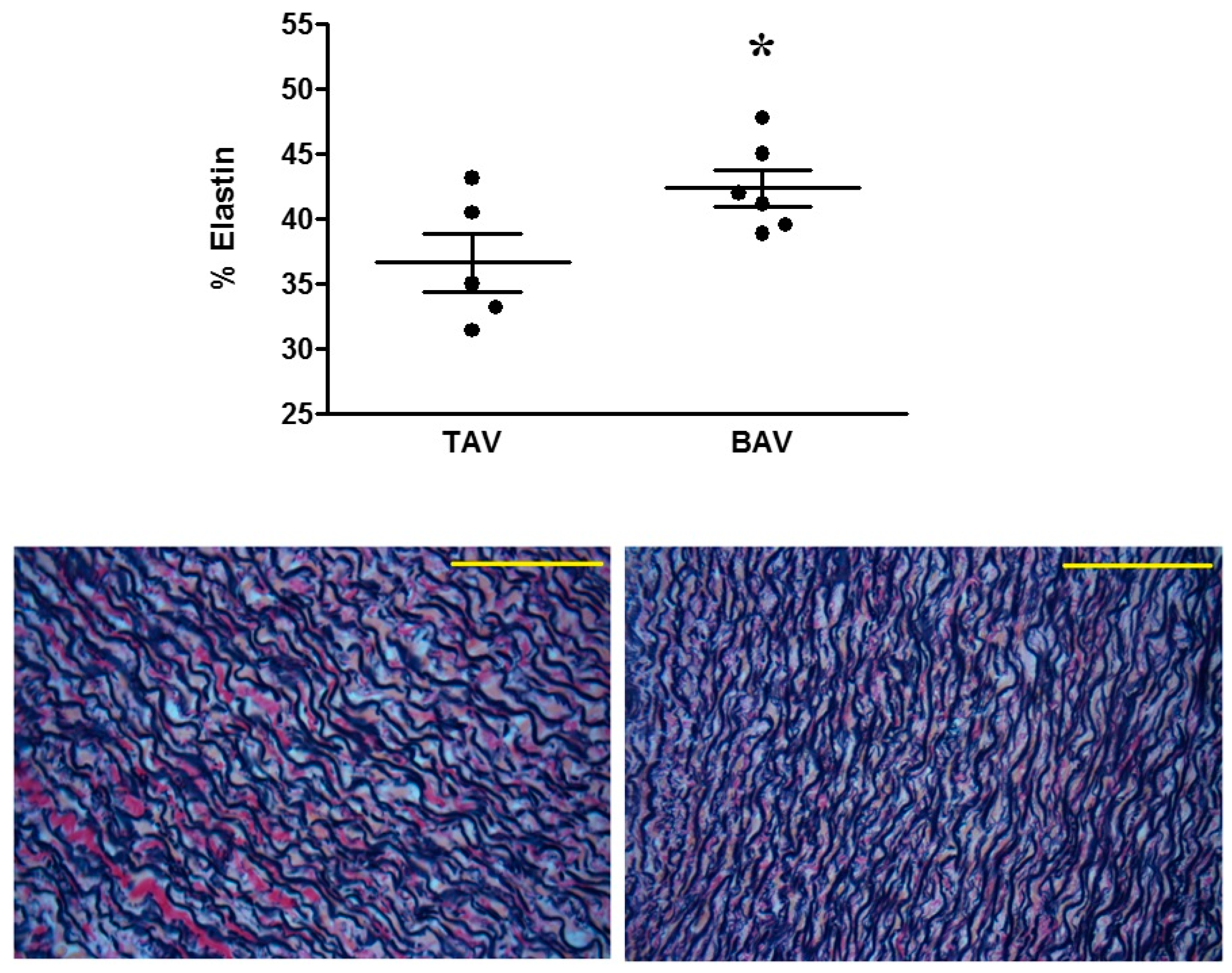
4.1. Proteomic and Structural Analysis Identify Changes in Elastin as the Main Structural Aortic Alteration Associated with BAV
4.2. Inositol and Oxidative Stress Signalling Pathways are Altered in BAV Patients
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teo, L.L.; Cannell, T.; Babu-Narayan, S.V.; Hughes, M.; Mohiaddin, R.H. Prevalence of associated cardiovascular abnormalities in 500 patients with aortic coarctation referred for cardiovascular magnetic resonance imaging to a tertiary center. Pediatr. Cardiol. 2011, 32, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Preventza, O.; Livesay, J.J.; Cooley, D.A.; Krajcer, Z.; Cheong, B.Y.; Coselli, J.S. Coarctation-associated aneurysms: A localized disease or diffuse aortopathy. Ann. Thorac. Surg. 2013, 95, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Yang, J.R.; Wei, Y.S.; Liang, B.L.; Wei, Y.B.; Zhou, K.Q.; Wang, Z.; Yan, B.; Gao, Y.L. Ischemia-reperfusion injury in an aortic dissection patient. Am. J. Emerg. Med. 2015, 33, 987.e5–987.e6. [Google Scholar] [CrossRef]
- Oliver, J.M.; Gallego, P.; Gonzalez, A.; Aroca, A.; Bret, M.; Mesa, J.M. Risk factors for aortic complications in adults with coarctation of the aorta. J. Am. Coll. Cardiol. 2004, 44, 1641–1647. [Google Scholar] [CrossRef]
- Jashari, H.; Lannering, K.; Ibrahimi, P.; Djekic, D.; Mellander, M.; Rydberg, A.; Henein, M.Y. Persistent reduced myocardial deformation in neonates after CoA repair. Int. J. Cardiol. 2016, 221, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Bambul Heck, P.; Pabst von Ohain, J.; Kaemmerer, H.; Ewert, P.; Hager, A. Survival and cardiovascular events after coarctation-repair in long-term follow-up (COAFU): Predictive value of clinical variables. Int. J. Cardiol. 2017, 228, 347–351. [Google Scholar] [CrossRef]
- Keshavarz-Motamed, Z.; Garcia, J.; Kadem, L. Fluid dynamics of coarctation of the aorta and effect of bicuspid aortic valve. PLoS One 2013, 8, e72394. [Google Scholar] [CrossRef]
- Pedersen, T.A. Late morbidity after repair of aortic coarctation. Dan. Med. J. 2012, 59, B4436. [Google Scholar] [PubMed]
- Losenno, K.L.; Goodman, R.L.; Chu, M.W. Bicuspid aortic valve disease and ascending aortic aneurysms: Gaps in knowledge. Cardiol. Res. Pract. 2012, 2012, 145202. [Google Scholar] [CrossRef] [PubMed]
- Rinnstrom, D.; Engstrom, K.G.; Johansson, B. Subtypes of bicuspid aortic valves in coarctation of the aorta. Heart Vessels 2014, 29, 354–363. [Google Scholar] [CrossRef]
- Bonachea, E.M.; Chang, S.W.; Zender, G.; LaHaye, S.; Fitzgerald-Butt, S.; McBride, K.L.; Garg, V. Rare GATA5 sequence variants identified in individuals with bicuspid aortic valve. Pediatr. Res. 2014, 76, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Zhao, Y.D.; Courtman, D.W.; Stewart, D.J. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000, 101, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Freylikhman, O.; Tatarinova, T.; Smolina, N.; Zhuk, S.; Klyushina, A.; Kiselev, A.; Moiseeva, O.; Sjoberg, G.; Malashicheva, A.; Kostareva, A. Variants in the NOTCH1 gene in patients with aortic coarctation. Congenit. Heart Dis. 2014, 9, 391–396. [Google Scholar] [CrossRef]
- Kjellqvist, S.; Maleki, S.; Olsson, T.; Chwastyniak, M.; Branca, R.M.; Lehtio, J.; Pinet, F.; Franco-Cereceda, A.; Eriksson, P. A combined proteomic and transcriptomic approach shows diverging molecular mechanisms in thoracic aortic aneurysm development in patients with tricuspid- and bicuspid aortic valve. Mol. Cell Proteom. 2013, 12, 407–425. [Google Scholar] [CrossRef]
- Valikangas, T.; Suomi, T.; Elo, L.L. A comprehensive evaluation of popular proteomics software workflows for label-free proteome quantification and imputation. Brief Bioinform. 2018, 19, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.R.; Iacobazzi, D.; Abdul-Ghani, S.; Ghorbel, M.T.; Heesom, K.J.; George, S.J.; Caputo, M.; Suleiman, M.S.; Tulloh, R.M. The cardiac proteome in patients with congenital ventricular septal defect: A comparative study between right atria and right ventricles. J. Proteom. 2019, 191, 107–113. [Google Scholar] [CrossRef]
- Akkinapally, S.; Hundalani, S.G.; Kulkarni, M.; Fernandes, C.J.; Cabrera, A.G.; Shivanna, B.; Pammi, M. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst. Rev. 2018, 2, CD011417. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Braghetta, P.; Sabatelli, P.; Mura, I.; Doliana, R.; Colombatti, A.; Volpin, D.; Bonaldo, P.; Bressan, G.M. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell Biol. 2004, 24, 638–650. [Google Scholar] [CrossRef]
- Munjal, C.; Opoka, A.M.; Osinska, H.; James, J.F.; Bressan, G.M.; Hinton, R.B. TGF-beta mediates early angiogenesis and latent fibrosis in an Emilin1-deficient mouse model of aortic valve disease. Dis. Model. Mech. 2014, 7, 987–996. [Google Scholar] [CrossRef]
- Zweers, M.C.; van Vlijmen-Willems, I.M.; van Kuppevelt, T.H.; Mecham, R.P.; Steijlen, P.M.; Bristow, J.; Schalkwijk, J. Deficiency of tenascin-X causes abnormalities in dermal elastic fiber morphology. J. Invest. Dermatol. 2004, 122, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Bristow, J.; Carey, W.; Egging, D.; Schalkwijk, J. Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2005, 139C, 24–30. [Google Scholar] [CrossRef]
- Blaauboer, M.E.; Boeijen, F.R.; Emson, C.L.; Turner, S.M.; Zandieh-Doulabi, B.; Hanemaaijer, R.; Smit, T.H.; Stoop, R.; Everts, V. Extracellular matrix proteins: A positive feedback loop in lung fibrosis? Matrix Biol. 2014, 34, 170–178. [Google Scholar] [CrossRef]
- Bailey, M.; Pillarisetti, S.; Jones, P.; Xiao, H.; Simionescu, D.; Vyavahare, N. Involvement of matrix metalloproteinases and tenascin-C in elastin calcification. Cardiovasc. Pathol. 2004, 13, 146–155. [Google Scholar] [CrossRef]
- Eoh, J.H.; Shen, N.; Burke, J.A.; Hinderer, S.; Xia, Z.; Schenke-Layland, K.; Gerecht, S. Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells. Acta Biomater. 2017, 52, 49–59. [Google Scholar] [CrossRef]
- Wanjare, M.; Agarwal, N.; Gerecht, S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2015, 309, C271–C281. [Google Scholar] [CrossRef]
- Guzzardi, D.G.; Barker, A.J.; van Ooij, P.; Malaisrie, S.C.; Puthumana, J.J.; Belke, D.D.; Mewhort, H.E.; Svystonyuk, D.A.; Kang, S.; Verma, S.; et al. Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights from Wall Shear Stress Mapping. J. Am. Coll. Cardiol. 2015, 66, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Phillippi, J.A.; Kotlarczyk, M.P.; Hill, J.C.; Ellis, B.W.; St Croix, C.M.; Cantu-Medellin, N.; Kelley, E.E.; Gleason, T.G. Elevated oxidative stress in the aortic media of patients with bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 2017, 154, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Birukov, K.G. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid. Redox. Signal. 2009, 11, 1651–1667. [Google Scholar] [CrossRef]
- Arcucci, A.; Ruocco, M.R.; Albano, F.; Granato, G.; Romano, V.; Corso, G.; Bancone, C.; De Vendittis, E.; Della Corte, A.; Montagnani, S. Analysis of extracellular superoxide dismutase and Akt in ascending aortic aneurysm with tricuspid or bicuspid aortic valve. Eur. J. Histochem. 2014, 58, 2383. [Google Scholar] [CrossRef]
- Phillippi, J.A.; Hill, J.C.; Billaud, M.; Green, B.R.; Kotlarczyk, M.P.; Gleason, T.G. Bicuspid Aortic Valve Morphotype Correlates with Regional Antioxidant Gene Expression Profiles in the Proximal Ascending Aorta. Ann. Thorac. Surg. 2017, 104, 79–87. [Google Scholar] [CrossRef][Green Version]
- Ghosh, A.; Lu, G.; Su, G.; McEvoy, B.; Sadiq, O.; DiMusto, P.D.; Laser, A.; Futchko, J.S.; Henke, P.K.; Eliason, J.L.; et al. Phosphorylation of AKT and abdominal aortic aneurysm formation. Am. J. Pathol. 2014, 184, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Stroud, R.E.; Kaplan, B.S.; Leone, A.M.; Bavaria, J.E.; Gorman, J.H., 3rd; Gorman, R.C.; Ikonomidis, J.S. Differential protein kinase C isoform abundance in ascending aortic aneurysms from patients with bicuspid versus tricuspid aortic valves. Circulation 2007, 116, I144–I149. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Accession # | Gene ID | Description | Mean | SEM | Fold Change (BAV/TAV) | p-Value | log2 (Fold Change) | −log10 (p-Value) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BAV | TAV | BAV | TAV | |||||||
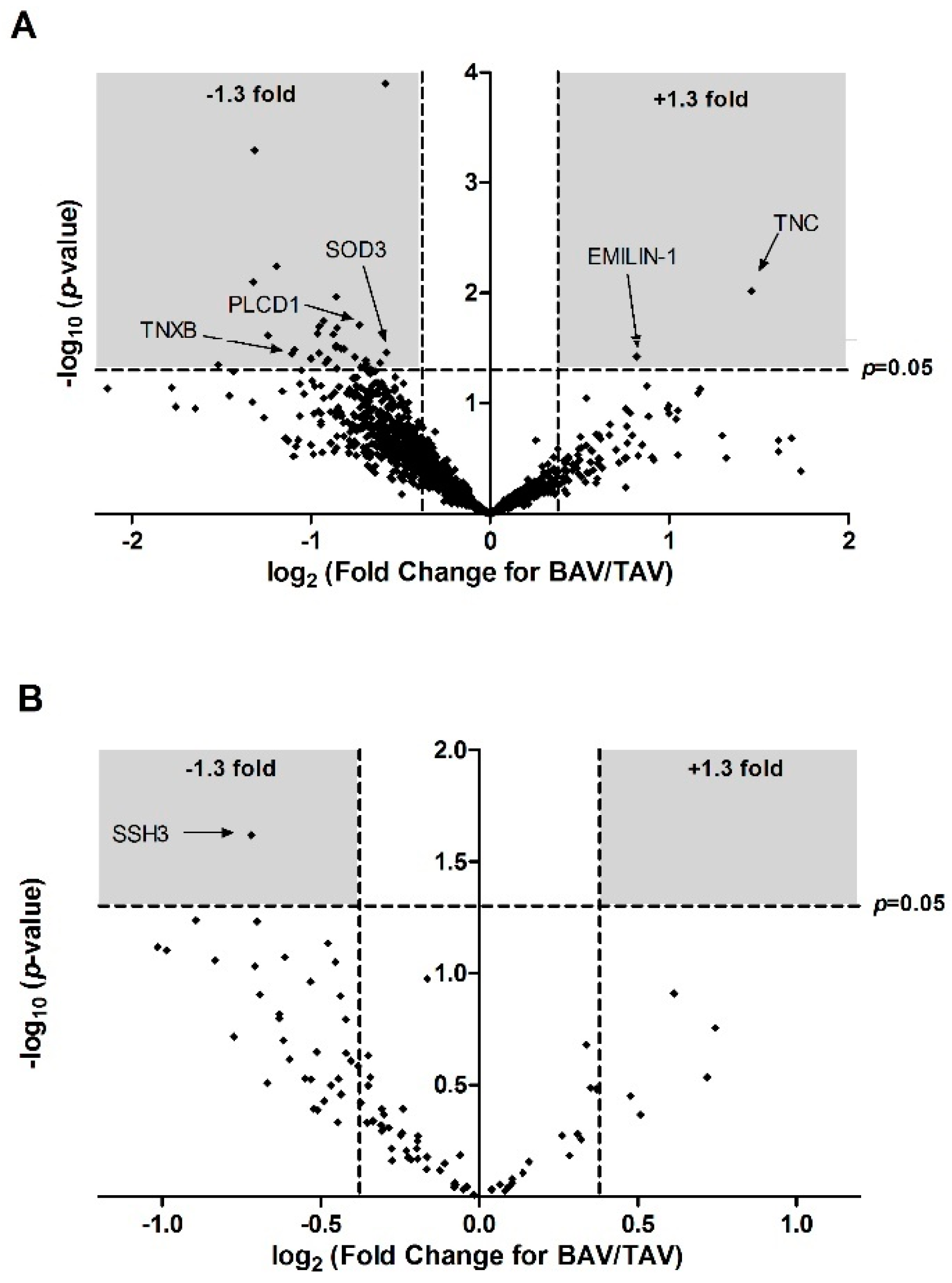
| F5H7R9 | PTMS | Parathymosin (Fragment) | 0.52 | 1.49 | 0.13 | 0.47 | 0.35 | 0.045 | −1.52 | 1.35 |
| A0A024R1R8 | hCG_2014768 | HCG2014768, isoform CRA_a | 1.79 | 4.47 | 0.4 | 0.79 | 0.40 | 0.008 | −1.32 | 2.10 |
| Q15847 | ADIRF | Adipogenesis regulatory factor | 0.50 | 1.24 | 0.09 | 0.13 | 0.40 | 0.001 | −1.31 | 3.29 |
| A0A087WY58 | NEK3 | Serine/threonine-protein kinase Nek3 | 4.09 | 9.67 | 1.67 | 0.79 | 0.42 | 0.024 | −1.24 | 1.61 |
| P23468 | PTPRD | Receptor-type tyrosine-protein phosphatase delta | 2.63 | 6.00 | 0.59 | 0.78 | 0.44 | 0.006 | −1.19 | 2.24 |
| A0A087WWA5 | TNXB | Tenascin-X | 0.73 | 1.58 | 0.11 | 0.39 | 0.46 | 0.036 | −1.11 | 1.45 |
| A0A087WYN4 | TTC25 | Tetratricopeptide repeat protein 25 | 0.49 | 1.05 | 0.11 | 0.22 | 0.47 | 0.033 | −1.09 | 1.48 |
| B4DVR4 | - | cDNA, FLJ60912, highly similar to Vinexin | 1.91 | 3.81 | 0.35 | 0.82 | 0.50 | 0.039 | −1.00 | 1.41 |
| Q96CN7 | ISOC1 | Isochorismatase domain-containing protein 1 | 1.26 | 2.45 | 0.29 | 0.34 | 0.51 | 0.023 | −0.96 | 1.63 |
| P51610 | HCFC1 | Host cell factor 1 | 0.99 | 1.91 | 0.17 | 0.32 | 0.52 | 0.020 | −0.95 | 1.70 |
| O76070 | SNCG | Gamma-synuclein | 0.67 | 1.30 | 0.18 | 0.18 | 0.52 | 0.035 | −0.95 | 1.46 |
| E9PNK6 | TPD52L1 | Tumour protein D53 | 1.43 | 2.72 | 0.29 | 0.35 | 0.53 | 0.018 | −0.93 | 1.75 |
| B7Z650 | - | cDNA, FLJ58685, highly similar to Homo sapiens echinoderm microtubule associated protein like 1 | 1.15 | 2.17 | 0.25 | 0.40 | 0.53 | 0.043 | −0.92 | 1.37 |
| H7C4C5 | MAP4 | Microtubule-associated protein (Fragment) | 0.87 | 1.63 | 0.20 | 0.26 | 0.53 | 0.040 | −0.90 | 1.40 |
| O76024 | WFS1 | Wolframin | 1.29 | 2.36 | 0.25 | 0.33 | 0.54 | 0.024 | −0.88 | 1.62 |
| Q4ZG81 | FLJ20701 | Putative uncharacterized protein FLJ20701 (Fragment) | 0.92 | 1.66 | 0.22 | 0.16 | 0.55 | 0.031 | −0.86 | 1.51 |
| Q9Y4G6 | TLN2 | Talin-2 | 1.04 | 1.88 | 0.20 | 0.28 | 0.55 | 0.030 | −0.86 | 1.52 |
| A0A024RAR8 | ARTS-1 | Type 1 tumour necrosis factor receptor shedding aminopeptidase regulator, isoform CRA-a | 0.87 | 1.58 | 0.14 | 0.18 | 0.55 | 0.011 | −0.86 | 1.97 |
| Q59GL1 | - | Synaptotagmin binding, cytoplasmic RNA interacting protein variant (Fragment) | 2.65 | 4.8 | 0.59 | 0.78 | 0.55 | 0.048 | −0.86 | 1.31 |
| A8K8F9 | PLCD1 | Phosphoinositide phospholipase C | 0.81 | 1.47 | 0.15 | 0.18 | 0.55 | 0.021 | −0.85 | 1.69 |
| B1AJY5 | PSMD10 | 26S proteasome non-ATPase regulatory subunit 10 | 1.62 | 2.88 | 0.35 | 0.35 | 0.56 | 0.032 | −0.83 | 1.50 |
| BD4L66 | - | cDNA FLJ56297, highly similar to Rattus norvegicus ubiquitin-conjugating enzyme E2Z (PUTATIVE) (Ube2z) | 2.04 | 3.60 | 0.36 | 0.54 | 0.57 | 0.032 | −0.82 | 1.49 |
| F2Z2V0 | CPNE1 | Copine-1 (Fragment) | 0.86 | 1.45 | 0.11 | 0.25 | 0.59 | 0.038 | −0.75 | 1.42 |
| Q9H7C9 | AAMDC | Mth938 domain-containing protein | 1.39 | 2.31 | 0.25 | 0.16 | 0.60 | 0.020 | −0.73 | 1.71 |
| O95865 | DDAH2 | N(G), N(G)-dimethylarginine dimethylaminohydrolase 2 | 1.57 | 2.60 | 0.32 | 0.29 | 0.61 | 0.047 | −0.72 | 1.33 |
| Q5T6V5 | C9orf64 | UPF0553 protein C9orf64 | 1.11 | 1.80 | 0.19 | 0.24 | 0.62 | 0.047 | −0.70 | 1.33 |
| P01034 | CST3 | Cystatin-C | 1.55 | 2.51 | 0.27 | 0.31 | 0.62 | 0.041 | −0.69 | 1.39 |
| Q7Z4V5 | HDGFRP2 | Hepatoma-derived growth factor-related protein 2 | 1.11 | 1.78 | 0.20 | 0.21 | 0.62 | 0.044 | −0.69 | 1.36 |
| B3KM48 | - | cDNA, FLJ10286fis, clone HEMBB1001384, highly similar to COP9 signalosome complex subunit 4 | 1.52 | 2.41 | 0.25 | 0.32 | 0.63 | 0.049 | −0.67 | 1.31 |
| P62312 | LSM6 | U6 snRNA-associated Sm-like protein LSm6 | 1.24 | 1.89 | 0.22 | 0.13 | 0.65 | 0.043 | −0.61 | 1.37 |
| A0A087WYV5 | SLIT2 | Slit homolog 2 protein | 1.25 | 1.88 | 0.06 | 0.09 | 0.67 | <0.0002 | −0.58 | 3.90 |
| P08294 | SOD3 | Extracellular superoxide dismutase (Cu-Zn) | 0.87 | 1.3 | 0.08 | 0.18 | 0.67 | 0.035 | −0.58 | 1.46 |
| A0A024R884 | TNC | Tenascin C (Hexabrachion), isoform CRA_a | 1.88 | 0.68 | 0.29 | 0.15 | 2.75 | 0.010 | 1.46 | 2.02 |
| A0A0C4DFX3 | EMILIN1 | elastin microfibril interfacer 1 | 2.90 | 1.64 | 0.39 | 0.27 | 1.76 | 0.038 | 0.82 | 1.42 |
| IPA Canonical Pathway | p-Value | Molecule |
|---|---|---|
| Superoxide radicals degradation | 1.16 × 10−2 | SOD3 |
| D-myo-inositol (1,4,5) Trisphosphate Biosynthesis | 3.85 × 10−2 | PLCD1 |
| IPA Disease or Function | p-Value | Molecules | # Molecules |
|---|---|---|---|
| Overall | |||
| Morphology of hippocampal neurons | 9.13 × 10−5 | CST3, SYNCRIP | 2 |
| Formation of elastin fibres | 1.12 × 10−4 | EMILIN1, TNXB | 2 |
| Secondary Tumour | 1.41 × 10−4 | ADIRF, CST3, MAP4, PSMD10, PTPRD, SLIT2, SNCG, SOD3, TNC | 9 |
| Migration of oligodendrocyte precursor cells | 1.58 × 10−4 | SLIT2, TNC | 2 |
| Morphogenesis of aortic valve | 3.44 × 10−4 | EMILIN1, SLIT2 | 2 |
| Cardiovascular System Development and Function | |||
| Morphogenesis of aortic valve | 3.44 × 10−4 | EMILIN1, SLIT2 | 2 |
| Relaxation of carotid artery | 1.01 × 10−2 | SOD3 | 1 |
| Angiogenesis | 1.58 × 10−2 | CST3, EMILIN1, ERAP1, PLCD1, SLIT2, TNC | 6 |
| Relaxation of aorta | 2.02 × 10−2 | SOD3 | 1 |
| Abnormal morphology of tunica media | 2.16 × 10−2 | CST3 | 1 |
| Vasculogenesis | 2.43 × 10−2 | CST3, ERAP1, PLCD1, SLIT2, TNC | 5 |
| Vascularization of placenta | 2.44 × 10−2 | PLCD1 | 1 |
| Abnormal morphology of aortic arch | 4.27 × 10−2 | CST3 | 1 |
| Accession # | Gene ID | Protein Name | Protein Phosphosite | Mean | SEM | Fold Change (BAV/TAV) | p-Value | log2 (Fold Change) | −log10 (p-Value) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BAV | TAV | BAV | TAV | ||||||||
| C9JUG3 | SSH3 | Protein phosphatase slingshot homolog 3 | Serine 3 | 1.11 | 1.82 | 0.18 | 0.2 | 0.61 | 2.4 × 10−2 | −0.72 | 1.619 |
| IPA Canonical Pathway | p-Value |
|---|---|
| D-myo-inositol (1,4,5,6)-tetrakisphosphate Biosynthesis | 6.54 × 10−3 |
| D-myo-inositol (3,4,5,6)-tetrakisphosphate Biosynthesis | 6.54 × 10−3 |
| 3-phosphoinositide Degradation | 7.17 × 10−3 |
| D-myo-inositol-5-phosphate Metabolism | 7.35 × 10−3 |
| 3-phosphoinositide Biosynthesis | 9.17 × 10−3 |
| Actin Cytoskeleton Signalling | 1.02 × 10−2 |
| Superpathway of Inositol Phosphate Compounds | 1.08 × 10−2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skeffington, K.L.; Bond, A.R.; Abdul-Ghani, S.; Iacobazzi, D.; Kang, S.-L.; Heesom, K.J.; Wilson, M.C.; Ghorbel, M.; Stoica, S.; Martin, R.; et al. Bicuspid Aortic Valve Alters Aortic Protein Expression Profile in Neonatal Coarctation Patients. J. Clin. Med. 2019, 8, 517. https://doi.org/10.3390/jcm8040517
Skeffington KL, Bond AR, Abdul-Ghani S, Iacobazzi D, Kang S-L, Heesom KJ, Wilson MC, Ghorbel M, Stoica S, Martin R, et al. Bicuspid Aortic Valve Alters Aortic Protein Expression Profile in Neonatal Coarctation Patients. Journal of Clinical Medicine. 2019; 8(4):517. https://doi.org/10.3390/jcm8040517
Chicago/Turabian StyleSkeffington, Katie L., Andrew R. Bond, Safa Abdul-Ghani, Dominga Iacobazzi, Sok-Leng Kang, Kate J. Heesom, Marieangela C. Wilson, Mohamed Ghorbel, Serban Stoica, Robin Martin, and et al. 2019. "Bicuspid Aortic Valve Alters Aortic Protein Expression Profile in Neonatal Coarctation Patients" Journal of Clinical Medicine 8, no. 4: 517. https://doi.org/10.3390/jcm8040517
APA StyleSkeffington, K. L., Bond, A. R., Abdul-Ghani, S., Iacobazzi, D., Kang, S.-L., Heesom, K. J., Wilson, M. C., Ghorbel, M., Stoica, S., Martin, R., Suleiman, M. S., & Caputo, M. (2019). Bicuspid Aortic Valve Alters Aortic Protein Expression Profile in Neonatal Coarctation Patients. Journal of Clinical Medicine, 8(4), 517. https://doi.org/10.3390/jcm8040517







