Intra-Vitreal Administration of Microvesicles Derived from Human Adipose-Derived Multipotent Stromal Cells Improves Retinal Functionality in Dogs with Retinal Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. The Preparation of Conditioned Medium from Human Adipose-Derived Multipotent Stromal Cells (CM-ASC) Rich in Microvesicles (MVs)
2.2. Isolation and Characterization of Canine Müller-Like Cells (CaMLC)
2.3. The Evaluation of the Biological Activity of the Microvesicles Derived from hASCs in Cultures with CaMLCs under Oxidative Stress Conditions
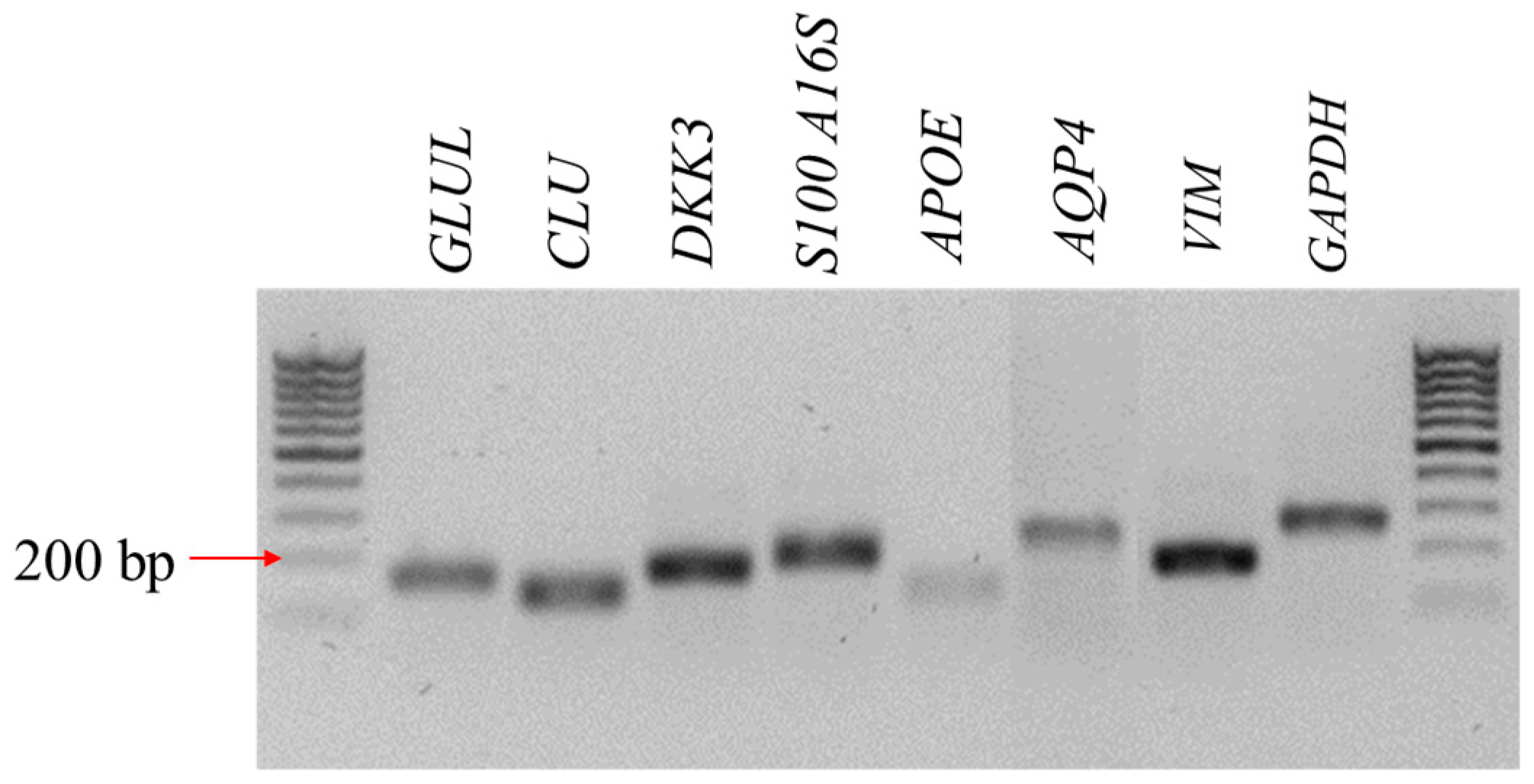
2.4. The Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. The Clinical Cases
2.5.1. Animals
2.5.2. Behavioral Observation
2.5.3. Ophthalmic Examination
2.5.4. Electroretinography
2.5.5. The Administration of hASC-MVs
2.6. Statistical Analysis
3. Results
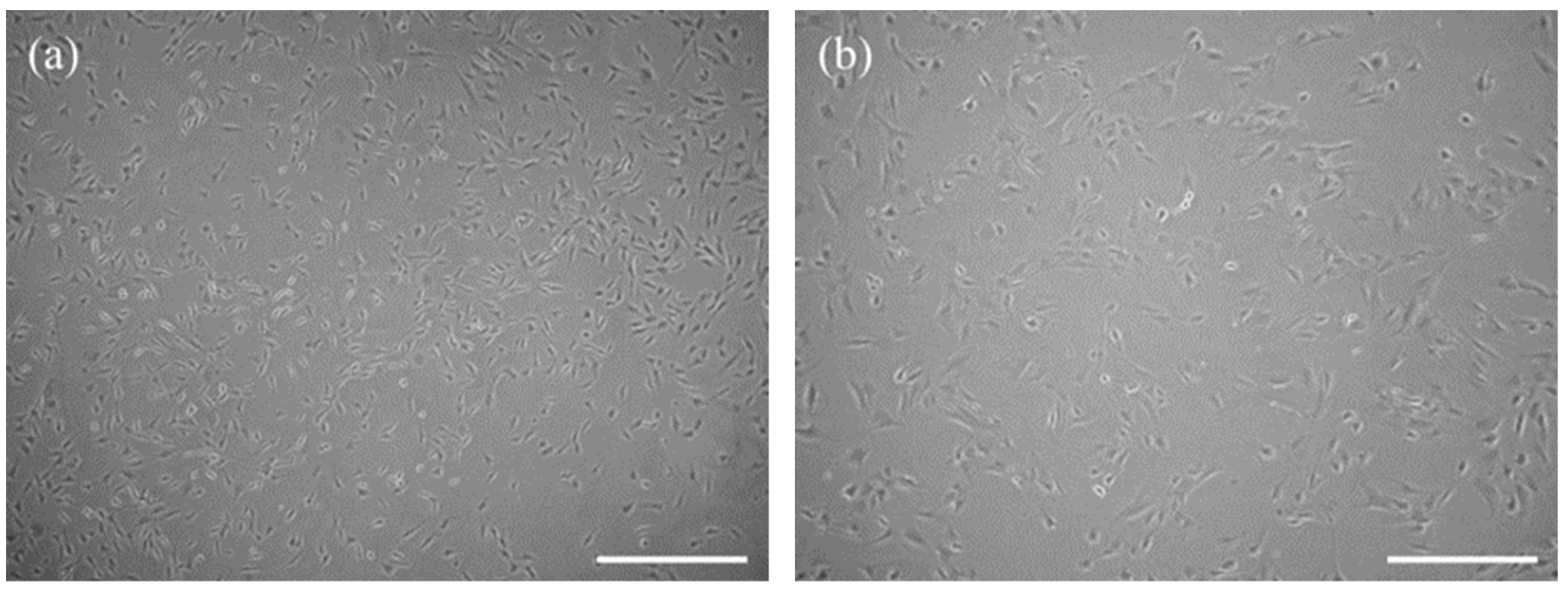
3.1. The Characteristic Features of CaMLCs Used in the Experiment
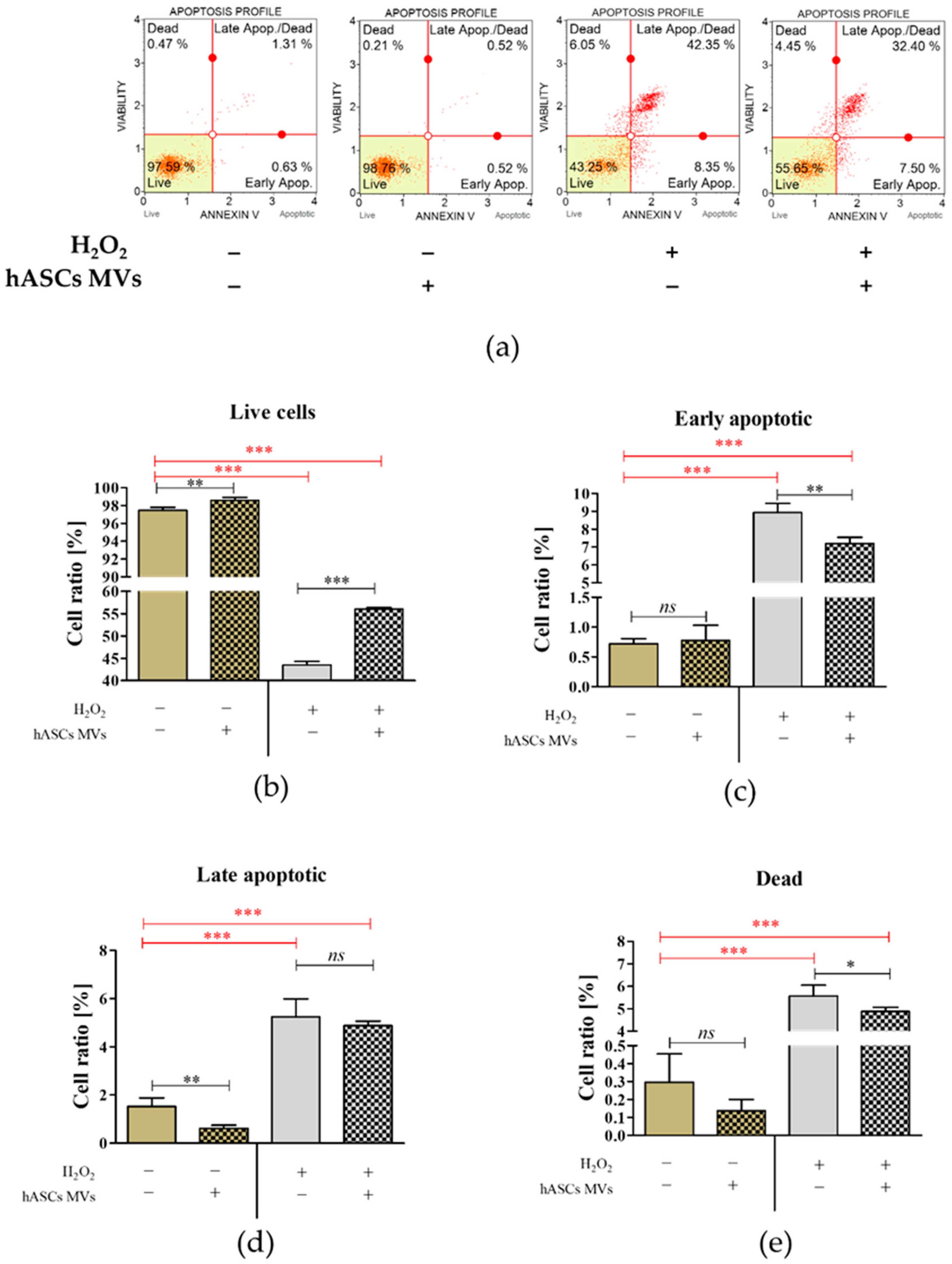
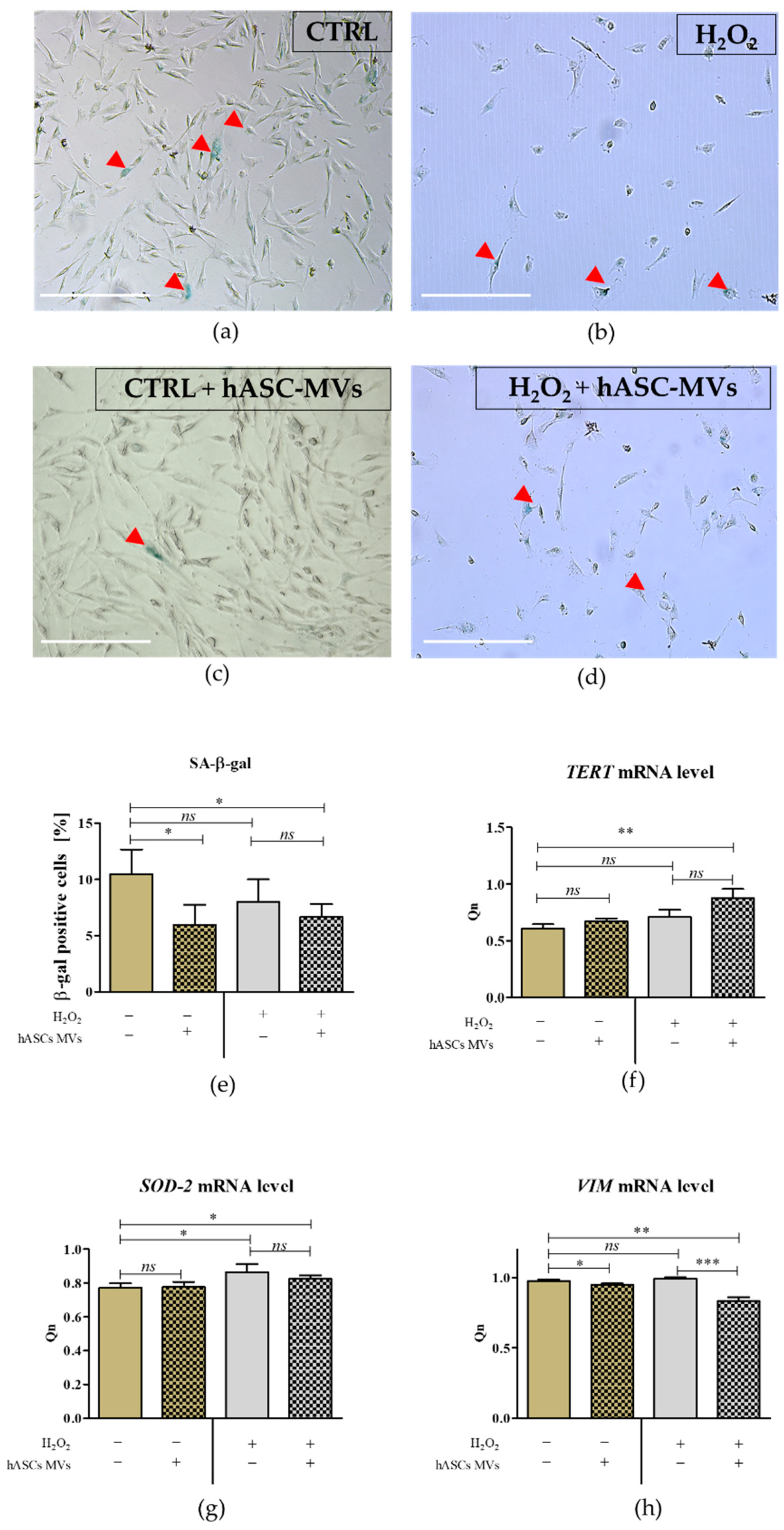
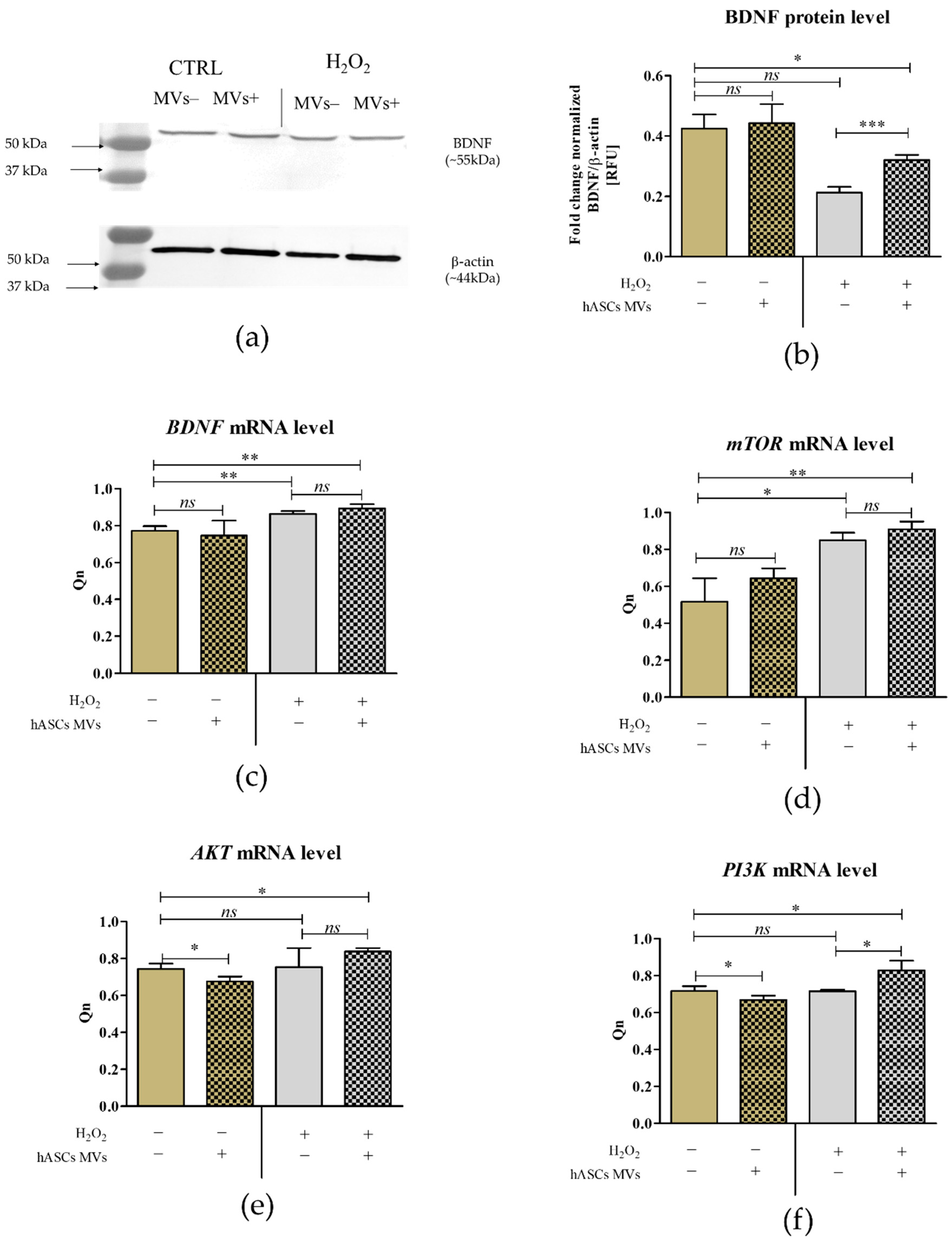
3.2. The Evaluation of Biological Properties of hASC-MVs–In Vitro
3.3. The Evaluation of Biological Properties of hASC-MVs–In Vivo
3.3.1. Behavioral Observations
3.3.2. Ophthalmic Examination
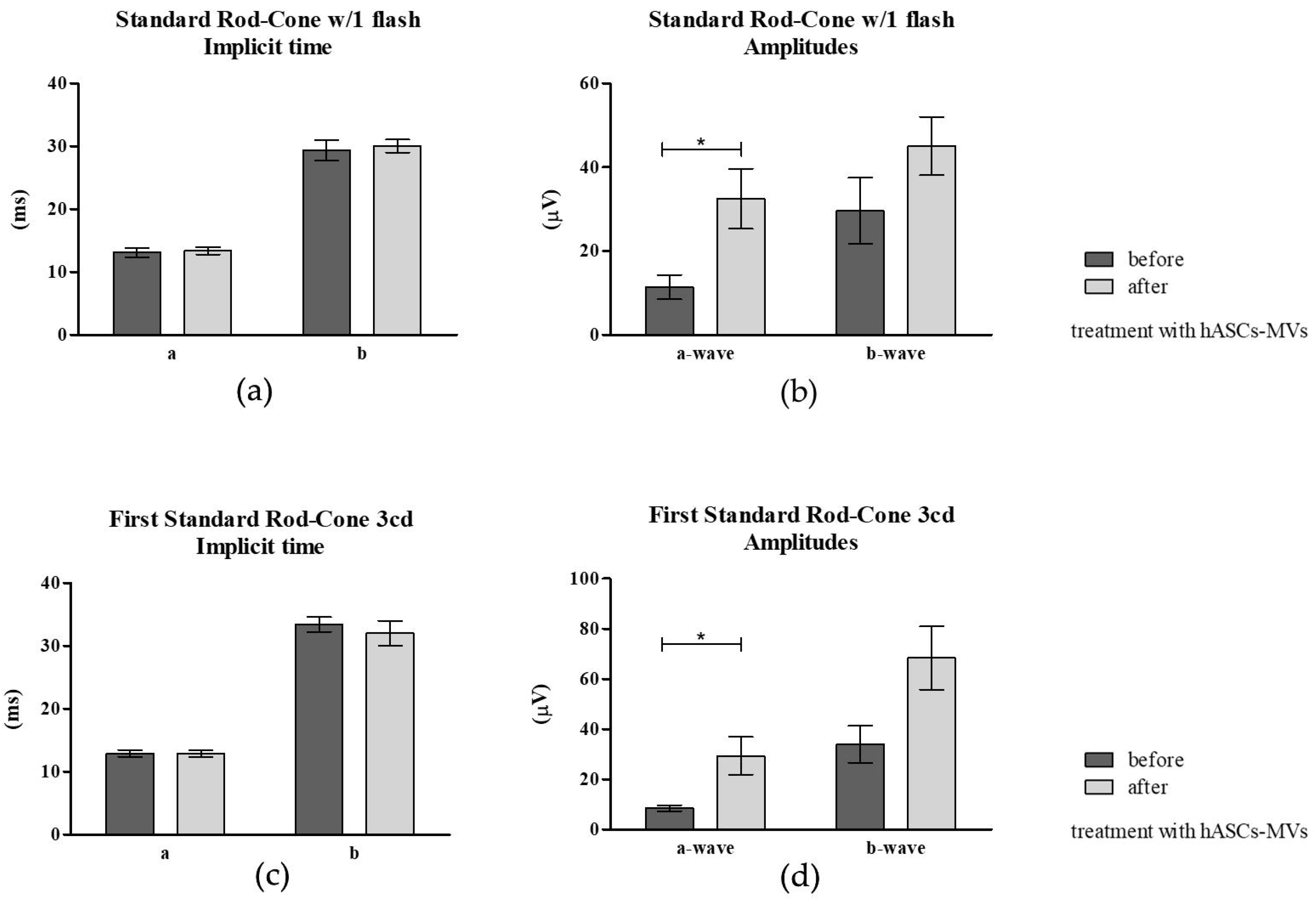
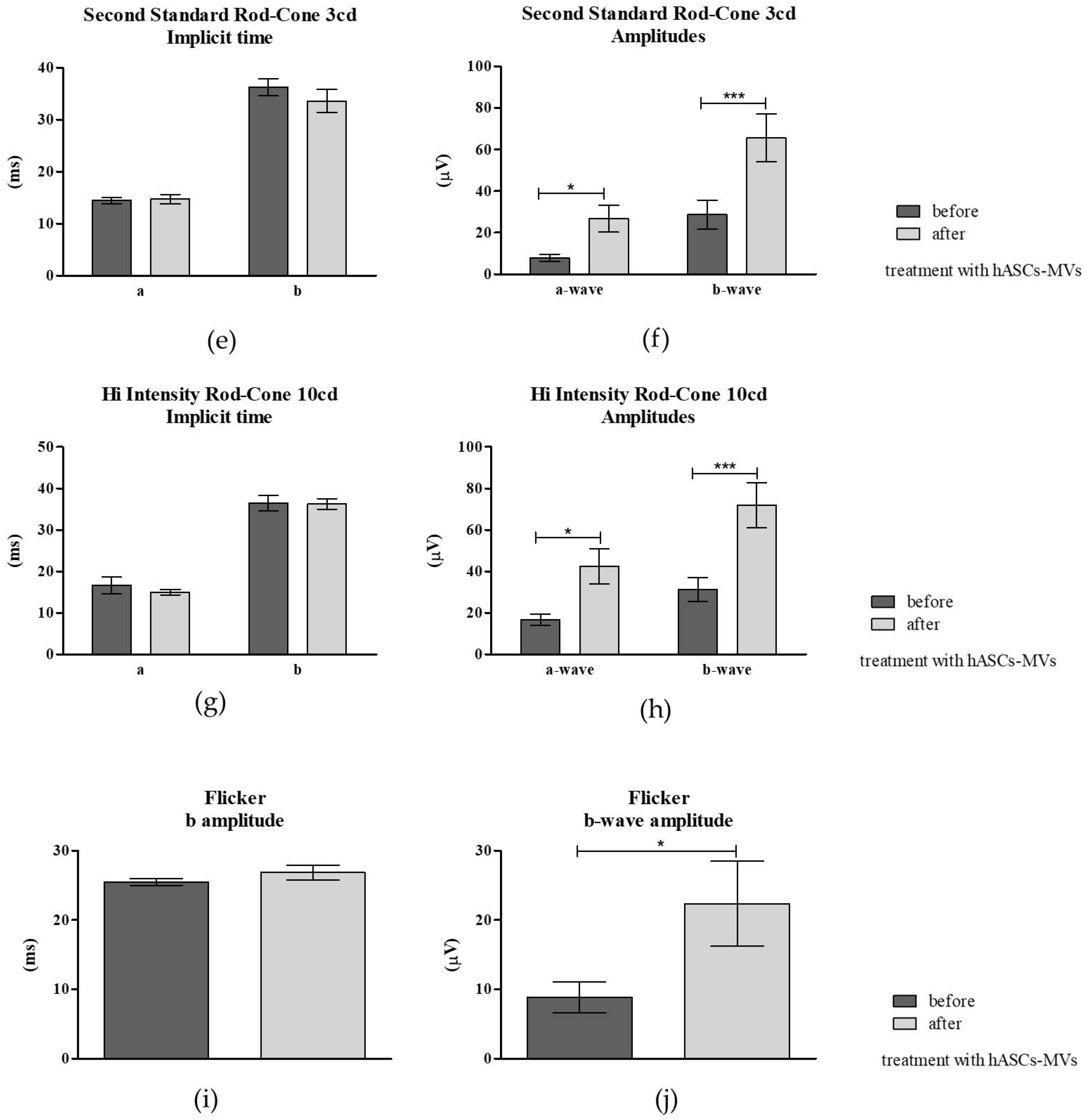
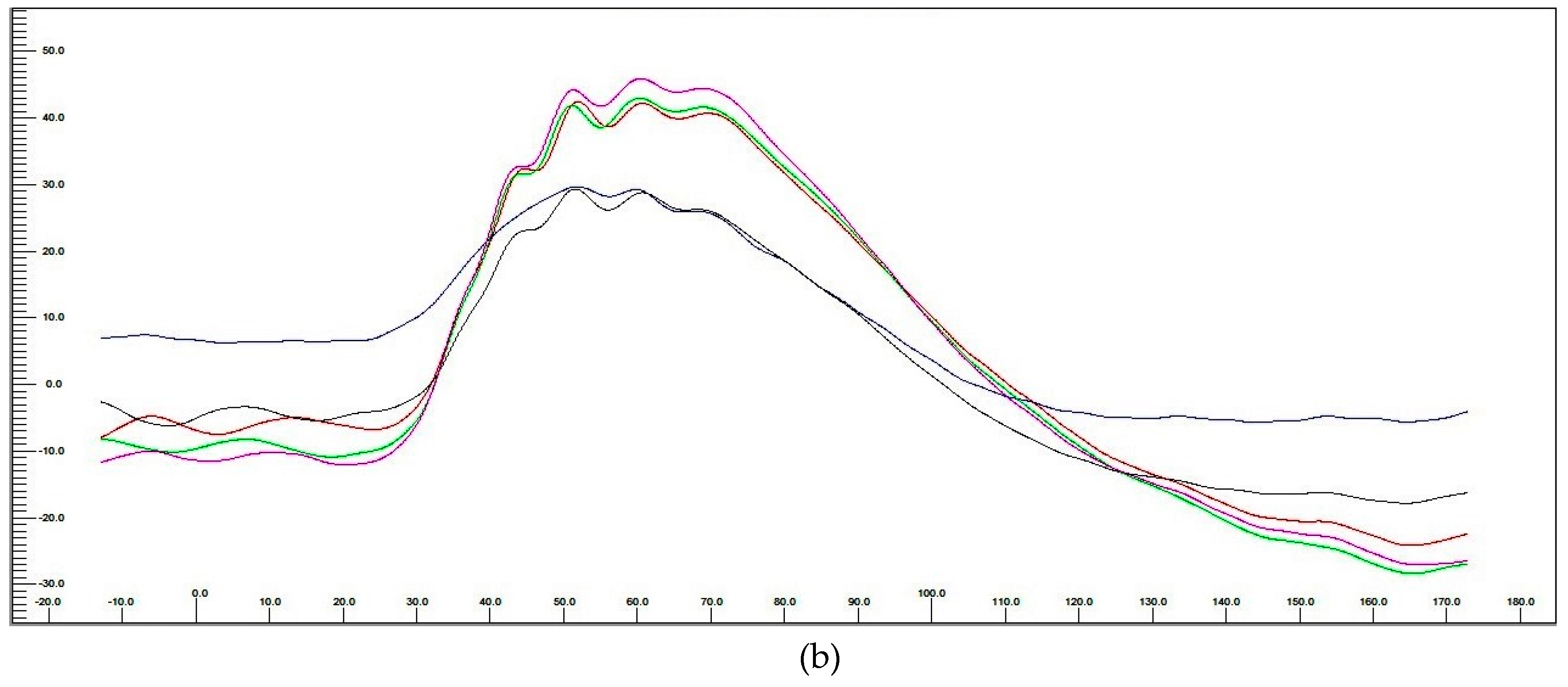
3.3.3. Electroretinography (ERG) Post hASC-MV Administration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kelawala, D.N.; Patil, D.B.; Parikh, P.V.; Sheth, M.J.; Joshi, C.G.; Reddy, B. Clinical studies on progressive retinal atrophy in 31 dogs. Iran J. Vet. Res. 2017, 18, 119–123. [Google Scholar]
- Wiik, A.C.; Ropstad, E.O.; Ekesten, B.; Karlstam, L.; Wade, C.M.; Lingaas, F. Progressive retinal atrophy in Shetland sheepdog is associated with a mutation in the CNGA1 gene. Anim. Genet. 2015, 46, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Karlskov-Mortensen, P.; Proschowsky, H.F.; Gao, F.; Fredholm, M. Identification of the mutation causing progressive retinal atrophy in Old Danish Pointing Dog. Anim. Genet. 2018, 49, 237–241. [Google Scholar] [CrossRef]
- Chew, T.; Haase, B.; Bathgate, R.; Willet, C.E.; Kaukonen, M.K.; Mascord, L.J.; Lohi, H.T.; Wade, C.M. A Coding Variant in the Gene Bardet-Biedl Syndrome 4 (BBS4) Is Associated with a Novel Form of Canine Progressive Retinal Atrophy. G3 Genes Genomes Genet. (Bethesda) 2017, 7, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.B.; Park, S.A.; Kim, S.E.; Park, Y.W.; Narfström, K.; Seo, K. Clinical and electroretinographic findings of progressive retinal atrophy in miniature schnauzer dogs of South Korea. J. Vet. Med. Sci. 2013, 75, 1303–1308. [Google Scholar] [CrossRef][Green Version]
- Whatham, A.R.; Nguyen, V.; Zhu, Y.; Hennessy, M.; Kalloniatis, M. The value of clinical electrophysiology in the assessment of the eye and visual system in the era of advanced imaging. Clin. Exp. Optom. 2014, 97, 99–115. [Google Scholar] [CrossRef]
- Usui, S.; Komeima, K.; Lee, S.Y.; Jo, Y.-J.; Ueno, S.; Rogers, B.S.; Wu, Z.; Shen, J.; Lu, L.; Oveson, B.C.; et al. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol. Ther. 2009, 17, 778–786. [Google Scholar] [PubMed]
- Trachsel-Moncho, L.; Benlloch-Navarro, S.; Fernández-Carbonell, Á.; Ramírez-Lamelas, D.T.; Olivar, T.; Silvestre, D.; Poch, E.; Miranda, M. Oxidative stress and autophagy-related changes during retinal degeneration and development. Cell Death Disease 2018, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.M.; Johnson, L.E.; Ahuja, S.; Ekström, P.A.R.; Romero, J.; van Veen, T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 2007, 145, 1120–1129. [Google Scholar] [CrossRef]
- Komeima, K.; Rogers, B.S.; Campochiaro, P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007, 213, 809–815. [Google Scholar] [CrossRef]
- Wang, W.; Hernandez, J.; Moore, C.; Jackson, J.; Narfström, K. Antioxidant supplementation increases retinal responses and decreases refractive error changes in dogs. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef][Green Version]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef]
- Daly, C.; Ward, R.; Reynolds, A.L.; Galvin, O.; Collery, R.F.; Kennedy, B.N. Brain-Derived Neurotrophic Factor as a Treatment Option for Retinal Degeneration. Adv. Exp. Med. Biol. 2018, 1074, 465–471. [Google Scholar]
- Mey, J.; Thanos, S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993, 602, 304–317. [Google Scholar] [CrossRef]
- Domenici, L.; Origlia, N.; Falsini, B.; Cerri, E.; Barloscio, D.; Fabiani, C.; Sansò, M.; Giovannini, L. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS ONE 2014, 9, e115579. [Google Scholar] [CrossRef][Green Version]
- Xu, W.; Xu, G.-X. Mesenchymal stem cells for retinal diseases. Int. J. Ophthalmol. 2011, 4, 413–421. [Google Scholar]
- Razavi, S.; Razavi, M.R.; Zarkesh Esfahani, H.; Kazemi, M.; Mostafavi, F.S. Comparing brain-derived neurotrophic factor and ciliary neurotrophic factor secretion of induced neurotrophic factor secreting cells from human adipose and bone marrow-derived stem cells. Dev. Growth Differ. 2013, 55, 648–655. [Google Scholar] [CrossRef]
- Mendel, T.A.; Clabough, E.B.D.; Kao, D.S.; Demidova-Rice, T.N.; Durham, J.T.; Zotter, B.C.; Seaman, S.A.; Cronk, S.M.; Rakoczy, E.P.; Katz, A.J.; et al. Pericytes Derived from Adipose-Derived Stem Cells Protect against Retinal Vasculopathy. PLoS ONE 2013, 8, e65691. [Google Scholar] [CrossRef]
- Panagiotou, N.; Wayne Davies, R.; Selman, C.; Shiels, P.G. Microvesicles as Vehicles for Tissue Regeneration: Changing of the Guards. Curr. Pathobiol. Rep. 2016, 4, 181–187. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin. Transl. Med. 2016, 5, 7. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Front Mol Biosci 2017, 4, 50. [Google Scholar] [CrossRef]
- Katsman, D.; Stackpole, E.J.; Domin, D.R.; Farber, D.B. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS ONE 2012, 7, e50417. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Cepko, C.L. A common progenitor for neurons and glia persists in rat retina late in development. Nature 1987, 328, 131–136. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Roesch, K.; Cepko, C.L. Development and neurogenic potential of Müller glial cells in the vertebrate retina. Prog. Retin. Eye Res. 2009, 28, 249–262. [Google Scholar] [CrossRef]
- Ooto, S.; Akagi, T.; Kageyama, R.; Akita, J.; Mandai, M.; Honda, Y.; Takahashi, M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 2004, 101, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Oh, I.-H. Regeneration of the retina: Toward stem cell therapy for degenerative retinal diseases. BMB Rep. 2015, 48, 193–199. [Google Scholar] [CrossRef]
- Kornicka, K.; Marycz, K.; Tomaszewski, K.A.; Marędziak, M.; Śmieszek, A. The Effect of Age on Osteogenic and Adipogenic Differentiation Potential of Human Adipose Derived Stromal Stem Cells (hASCs) and the Impact of Stress Factors in the Course of the Differentiation Process. Oxid. Med. Cell. Longev. 2015, 2015, 309169. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Śmieszek, A.; Jarmoluk, A.; Nowak, U.; Marycz, K.; Zimoch-Korzycka, A.; Śmieszek, A.; Jarmoluk, A.; Nowak, U.; Marycz, K. Potential Biomedical Application of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges—Biocompatible Scaffolds Inducing Chondrogenic Differentiation of Human Adipose Derived Multipotent Stromal Cells. Polymers 2016, 8, 320. [Google Scholar] [CrossRef]
- Śmieszek, A.; Stręk, Z.; Kornicka, K.; Grzesiak, J.; Weiss, C.; Marycz, K. Antioxidant and Anti-Senescence Effect of Metformin on Mouse Olfactory Ensheathing Cells (mOECs) May Be Associated with Increased Brain-Derived Neurotrophic Factor Levels—An Ex Vivo Study. Int. J. Mol. Sci. 2017, 18, 872. [Google Scholar] [CrossRef]
- Śmieszek, A.; Giezek, E.; Chrapiec, M.; Murat, M.; Mucha, A.; Michalak, I.; Marycz, K. The Influence of Spirulina platensis Filtrates on Caco-2 Proliferative Activity and Expression of Apoptosis-Related microRNAs and mRNA. Mar Drugs 2017, 15, 65. [Google Scholar] [CrossRef]
- Śmieszek, A.; Basińska, K.; Chrząstek, K.; Marycz, K. In Vitro and In Vivo Effects of Metformin on Osteopontin Expression in Mice Adipose-Derived Multipotent Stromal Cells and Adipose Tissue. Available online: https://www.hindawi.com/journals/jdr/2015/814896/ (accessed on 2 September 2018).
- Marycz, K.; Kornicka, K.; Irwin-Houston, J.M.; Weiss, C. Combination of resveratrol and 5-azacytydine improves osteogenesis of metabolic syndrome mesenchymal stem cells. J. Cell. Mol. Med. 2018, 22, 4771–4793. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Szlapka-Kosarzewska, J.; Weiss, C. Excessive Endoplasmic Reticulum Stress Correlates with Impaired Mitochondrial Dynamics, Mitophagy and Apoptosis, in Liver and Adipose Tissue, but Not in Muscles in EMS Horses. Int. J. Mol. Sci. 2018, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Narfström, K.; Ekesten, B.; Rosolen, S.G.; Spiess, B.M.; Percicot, C.L.; Ofri, R. Committee for a Harmonized ERG Protocol, European College of Veterinary Ophthalmology Guidelines for clinical electroretinography in the dog. Doc. Ophthalmol. 2002, 105, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, L.; Liu, Y. Mouse Müller Cell Isolation and Culture. Bio Protoc. 2017, 7, e2429. [Google Scholar] [CrossRef]
- Roesch, K.; Jadhav, A.P.; Trimarchi, J.M.; Stadler, M.B.; Roska, B.; Sun, B.B.; Cepko, C.L. The transcriptome of retinal Müller glial cells. J. Comp. Neurol. 2008, 509, 225–238. [Google Scholar] [CrossRef]
- Deeg, C.A.; Amann, B.; Lutz, K.; Hirmer, S.; Lutterberg, K.; Kremmer, E.; Hauck, S.M. Aquaporin 11, a regulator of water efflux at retinal Müller glial cell surface decreases concomitant with immune-mediated gliosis. J. Neuroinflamm. 2016, 13, 89. [Google Scholar] [CrossRef]
- Eldh, M.; Ekström, K.; Valadi, H.; Sjöstrand, M.; Olsson, B.; Jernås, M.; Lötvall, J. Exosomes Communicate Protective Messages during Oxidative Stress; Possible Role of Exosomal Shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Miao, S.; Chen, J.; Du, T.; Zhong, L.; Ju, G.; Liu, G.; Zhu, Y. The Anti-Oxidative Role of Micro-Vesicles Derived from Human Wharton-Jelly Mesenchymal Stromal Cells through NOX2/gp91(phox) Suppression in Alleviating Renal Ischemia-Reperfusion Injury in Rats. PLoS ONE 2014, 9, e92129. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes Secreted from GATA-4 Overexpressing Mesenchymal Stem Cells Serve as a Reservoir of Anti-Apoptotic microRNAs for Cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, R.; Wang, P.; Zhang, H.; Xiang, M.; Meng, D.; Sun, N.; Chen, A.F.; Chen, S. Protective effects of human induced pluripotent stem cell-derived exosomes on high glucose-induced injury in human endothelial cells. Exp. Ther. Med. 2018, 15, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, T.; Kalinina, N.; Karagyaur, M.; Stambolsky, D.; Rubina, K.; Revischin, A.; Pavlova, G.; Parfyonova, Y.; Tkachuk, V. Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 2011, 6, e17899. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci. Rep. 2017, 7, 4153. [Google Scholar] [CrossRef]
- De Jong, O.G.; Van Balkom, B.W.M.; Schiffelers, R.M.; Bouten, C.V.C.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Kornicka, K.; Śmieszek, A.; Węgrzyn, A.S.; Röcken, M.; Marycz, K. Immunomodulatory Properties of Adipose-Derived Stem Cells Treated with 5-Azacytydine and Resveratrol on Peripheral Blood Mononuclear Cells and Macrophages in Metabolic Syndrome Animals. J. Clin. Med. 2018, 7, 383. [Google Scholar] [CrossRef]
- Cislo-Pakuluk, A.; Marycz, K. A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications. Stem Cell Rev. 2017, 13, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Chang, B. Mouse models for studies of retinal degeneration and diseases. Methods Mol. Biol. 2013, 935, 27–39. [Google Scholar] [PubMed]




| Animal | Sex | Age (Years) | Breed/Type | Retinopathy |
|---|---|---|---|---|
| 1 | female | 12 | Crossbred | Suspected progressive retinal atrophy (PRA) |
| 2 | male | 5 | Crossbred | Suspected progressive retinal atrophy (PRA) |
| 3 | female | 7 | Yorkshire Terrier Cross | Suspected progressive retinal atrophy (PRA) |
| 4 | male | 15 | Crossbred | Associated with Borrelia sp. infection |
| 5 | female | 2 | Border Collie | Associated with systemic infection of unknown etiology |
| 6 | female | 10 | Crossbred | Suspected progressive retinal atrophy (PRA) |
| 7 | female | 7 | Welsh Terrier | Suspected progressive retinal atrophy (PRA) |
| 8 | male | 7 | German Shepherd Dog | Suspected progressive retinal atrophy (PRA) |
| 9 | male | 12 | Continental Spaniel | Suspected progressive retinal atrophy (PRA) |
| 10 | female | 3 | Australian Kelpie | Unknown |
| Features | Before Therapy | After Therapy | p Value |
|---|---|---|---|
| General behavior | Anxiety, reluctance towards with the handler, aggression with other dogs, problems negotiating obstacles, difficulties in dark rooms, visible stress and frustration. | Calmer, more confident and more tolerant with other dogs. Still a need to adapt after entering dark room but the time needed was shorter. Training was easier on days without very bright light. | p < 0.05 |
| Tracking | Lack of motivation, problems with changes in direction and easily distracted. | Calmer, very precise, focused on the line of scent, no frustration when the track changed, low stress. | p < 0.05 |
| Other relevant comments | Problems with catching objects and judging distances | Slow improvement in catching objects, more dynamic but still problems in dim lighting at night. | p < 0.05 |
| Parameter | Before hASC-MVs Treatment | After hASC-MVs Treatment | |
|---|---|---|---|
| Rod1 | a (ms) | 5.6 | 3.9 |
| b (ms) | 56.7 | 51.8 | |
| a-wave (µV) | 3.3 | 0.7 | |
| b-wave (µV) | 12.8 | 23.5 | |
| Rod2 | a (ms) | 14.4 | 16.5 |
| b (ms) | 55 | 51.9 | |
| a-wave (µV) | 0.6 | 1.3 | |
| b-wave (µV) | 20.3 | 49.9 | |
| Rod3 | a (ms) | 20 | 3 |
| b (ms) | 56.7 | 51.9 | |
| a-wave (µV) | 0.8 | 2.2 | |
| b-wave (µV) | 23.3 | 49.9 | |
| Rod4 | a (ms) | 13.9 | 18.4 |
| b (ms) | 56.4 | 51 | |
| a-wave (µV) | 0.6 | 0.4 | |
| b-wave (µV) | 23.4 | 52.8 | |
| Rod5 | a (ms) | 2.2 | 20.2 |
| b (ms) | 56.2 | 51.9 | |
| a-wave (µV) | 1.3 | 1.4 | |
| b-wave (µV) | 31.1 | 56 | |
| Standard rod and cone responses | a (ms) | 13.3 | 12.1 |
| b (ms) | 33.2 | 30.9 | |
| a-wave (µV) | 33.9 | 43.1 | |
| b-wave (µV) | 79.9 | 105 | |
| High intensity rod and cone response | a (ms) | 12.8 | 11.2 |
| b (ms) | 32.8 | 30.5 | |
| a-wave (µV) | 41.6 | 48 | |
| b-wave (µV) | 87.1 | 101.9 | |
| Cone response | a (ms) | 9.1 | 7.7 |
| b (ms) | 26.1 | 25.5 | |
| a-wave (µV) | 5.6 | 4.4 | |
| b-wave (µV) | 17.2 | 13 | |
| Cone flicker response | b (ms) | 25.2 | 25.2 |
| b-wave (µV) | 34.2 | 29.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cislo-Pakuluk, A.; Smieszek, A.; Kucharczyk, N.; Bedford, P.G.C.; Marycz, K. Intra-Vitreal Administration of Microvesicles Derived from Human Adipose-Derived Multipotent Stromal Cells Improves Retinal Functionality in Dogs with Retinal Degeneration. J. Clin. Med. 2019, 8, 510. https://doi.org/10.3390/jcm8040510
Cislo-Pakuluk A, Smieszek A, Kucharczyk N, Bedford PGC, Marycz K. Intra-Vitreal Administration of Microvesicles Derived from Human Adipose-Derived Multipotent Stromal Cells Improves Retinal Functionality in Dogs with Retinal Degeneration. Journal of Clinical Medicine. 2019; 8(4):510. https://doi.org/10.3390/jcm8040510
Chicago/Turabian StyleCislo-Pakuluk, Anna, Agnieszka Smieszek, Natalia Kucharczyk, Peter G.C. Bedford, and Krzysztof Marycz. 2019. "Intra-Vitreal Administration of Microvesicles Derived from Human Adipose-Derived Multipotent Stromal Cells Improves Retinal Functionality in Dogs with Retinal Degeneration" Journal of Clinical Medicine 8, no. 4: 510. https://doi.org/10.3390/jcm8040510
APA StyleCislo-Pakuluk, A., Smieszek, A., Kucharczyk, N., Bedford, P. G. C., & Marycz, K. (2019). Intra-Vitreal Administration of Microvesicles Derived from Human Adipose-Derived Multipotent Stromal Cells Improves Retinal Functionality in Dogs with Retinal Degeneration. Journal of Clinical Medicine, 8(4), 510. https://doi.org/10.3390/jcm8040510





