Outcome of Robot-Assisted Bilateral Internal Mammary Artery Grafting via Left Pleura in Coronary Bypass Surgery
Abstract
1. Introduction
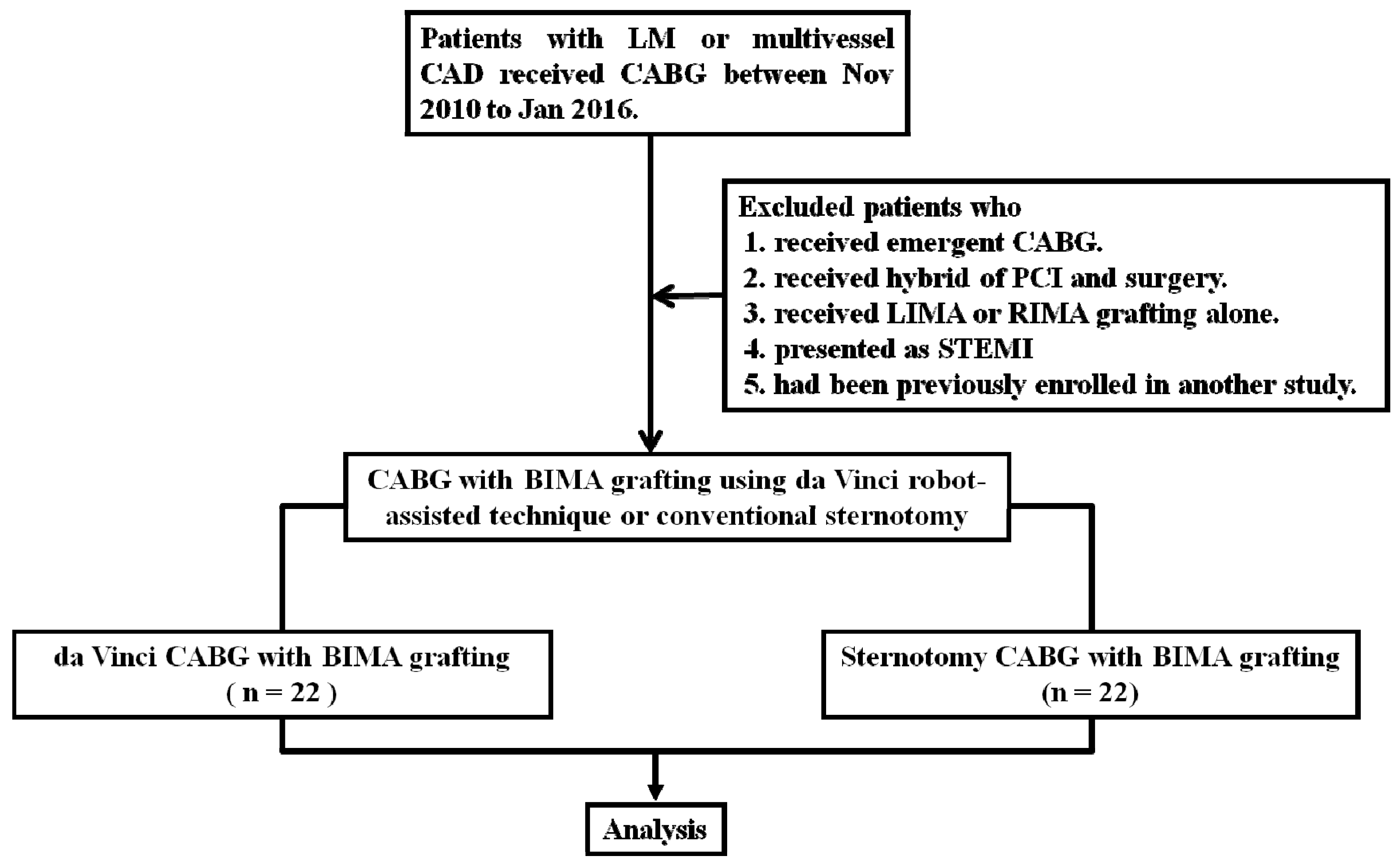
2. Materials and Methods
2.1. Study Design and Patient Selection
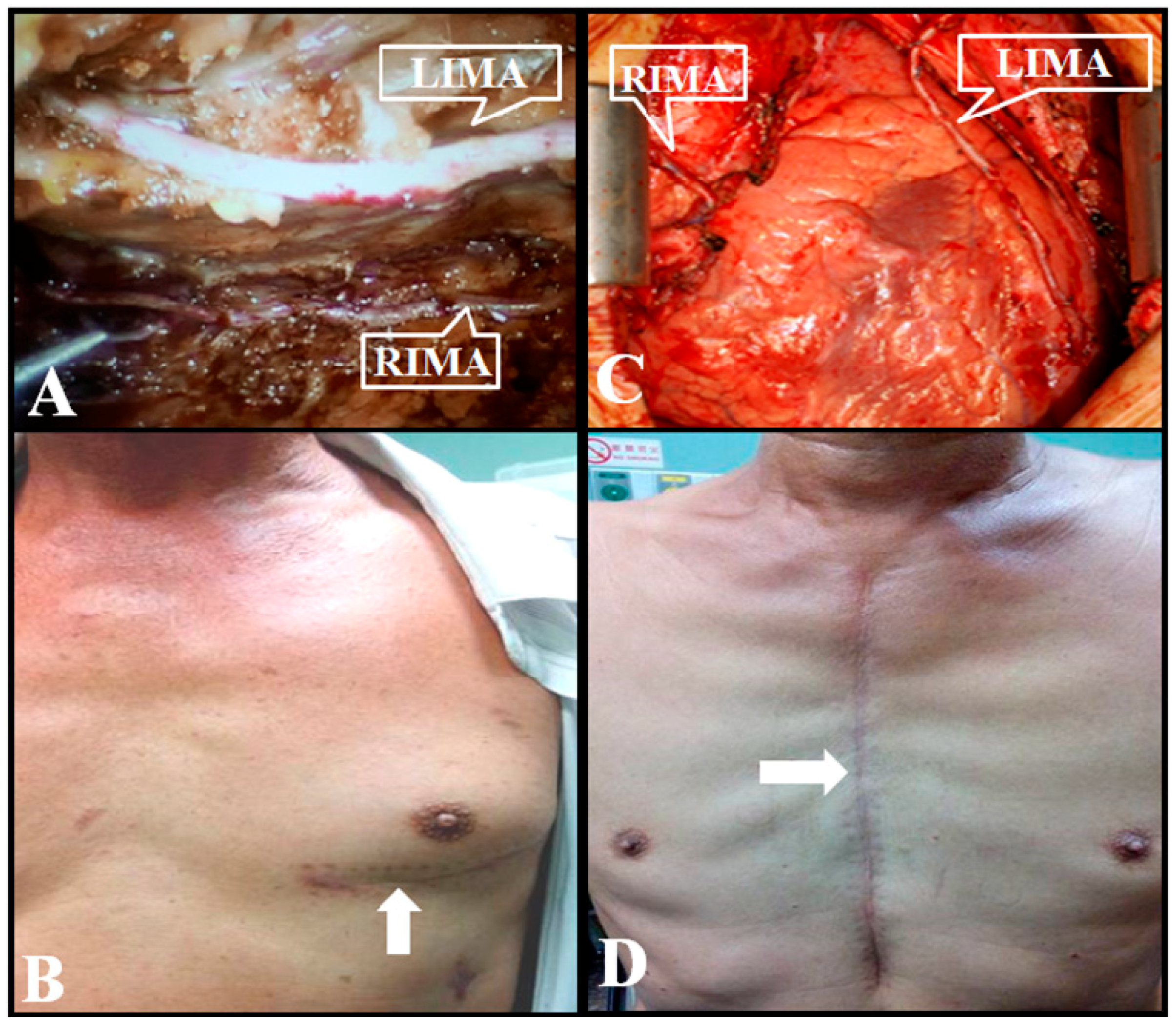
2.2. Da Vinci Robot-Assisted CABG with BIMA Grafting via Left Pleura
2.3. Conventional Sternotomy CABG with BIMA Grafting
2.4. Definitions
2.5. Primary Endpoints at Six Months
2.6. Statistical Analysis
3. Results
3.1. Patient Demographic and Characteristic Data
3.2. In-Hospital Adverse Events
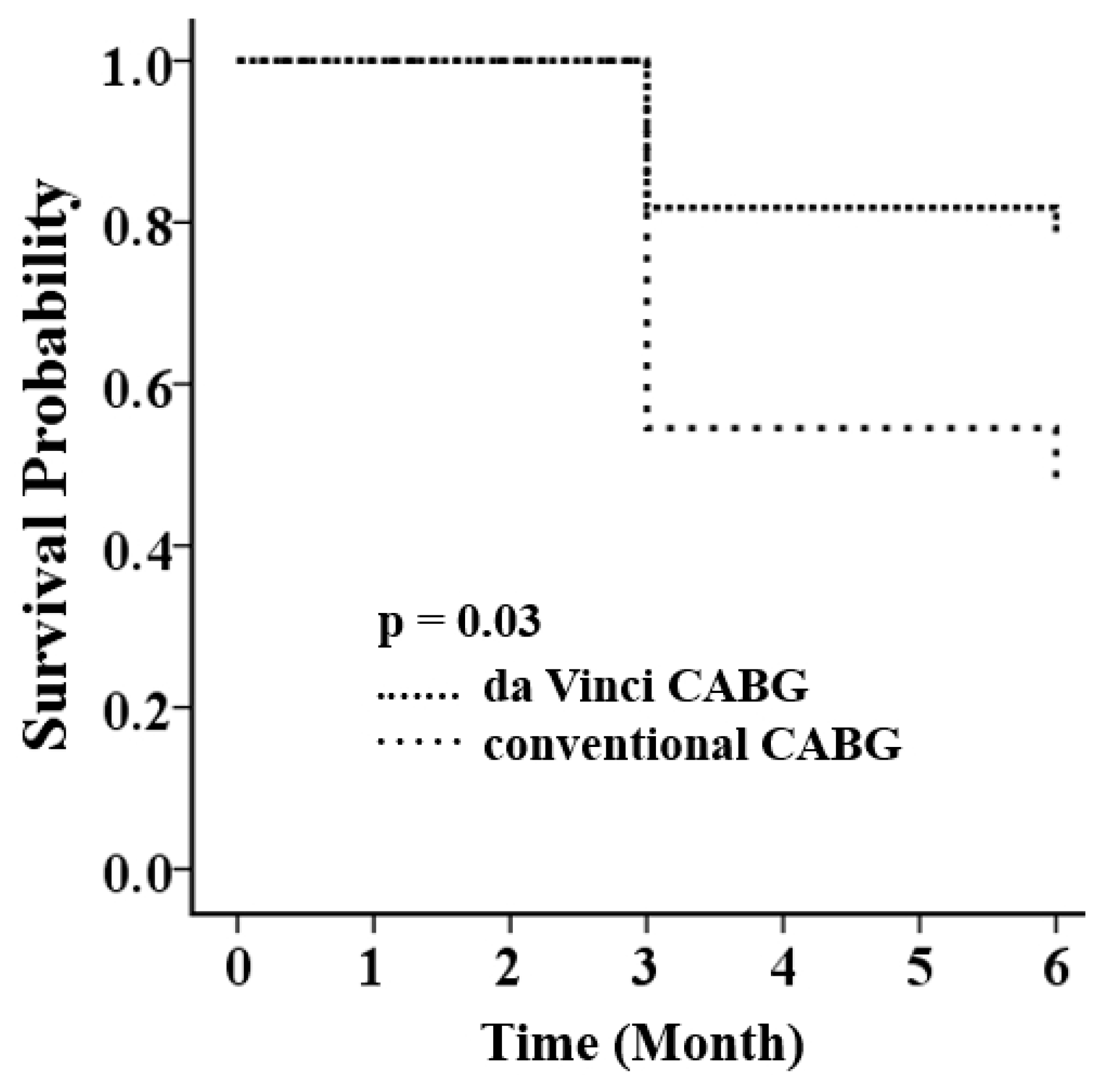
3.3. Primary Endpoints at Six Months
4. Discussion
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Task Force, M.; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European society of cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar]
- Yates, M.T.; Soppa, G.K.; Valencia, O.; Jones, S.; Firoozi, S.; Jahangiri, M. Impact of European Society of Cardiology and European Association for Cardiothoracic Surgery Guidelines on Myocardial Revascularization on the activity of percutaneous coronary intervention and coronary artery bypass graft surgery for stable coronary artery disease. J. Thorac. Cardiovasc. Surg. 2014, 147, 606–610. [Google Scholar]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines, and the american association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J. Thorac. Cardiovasc. Surg. 2015, 149, e5–e23. [Google Scholar] [PubMed]
- He, G.W.; Ryan, W.H.; Acuff, T.E.; Bowman, R.T.; Douthit, M.B.; Yang, C.Q.; Mack, M.J. Risk factors for operative mortality and sternal wound infection in bilateral internal mammary artery grafting. J. Thorac. Cardiovasc. Surg. 1994, 107, 196–202. [Google Scholar] [PubMed]
- Deo, S.V.; Shah, I.K.; Dunlay, S.M.; Erwin, P.J.; Locker, C.; Altarabsheh, S.E.; Boilson, B.A.; Park, S.J.; Joyce, L.D. Bilateral Internal Thoracic Artery Harvest and Deep Sternal Wound Infection in Diabetic Patients. Ann. Thorac. Surg. 2013, 95, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lu, Z.; Zhu, H.; Xue, S.; Lian, F. Bilateral Internal Mammary Artery Grafting and Risk of Sternal Wound Infection: Evidence from Observational Studies. Ann. Thorac. Surg. 2013, 95, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.J.; Zhao, S.; Tian, D.H.; Taggart, D.P.; Yan, T.D. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 390–400. [Google Scholar] [PubMed]
- Glineur, D.; Papadatos, S.; Grau, J.B.; Shaw, R.E.; Kuschner, C.E.; Aphram, G.; Mairy, Y.; Vanbelighen, C.; Etienne, P.Y. Complete myocardial revascularization using only bilateral internal thoracic arteries provides a low-risk and durable 10-year clinical outcome. Eur. J. Cardio-Thorac. Surg. 2016, 50, 735–741. [Google Scholar] [CrossRef]
- Kinoshita, T.; Asai, T.; Suzuki, T.; Kambara, A.; Matsubayashi, K. Off-Pump Bilateral Versus Single Skeletonized Internal Thoracic Artery Grafting in High-Risk Patients. Circulation 2011, 124, S130–S134. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Asai, T.; Suzuki, T. Off-pump bilateral skeletonized internal thoracic artery grafting in patients with chronic kidney disease. J. Thorac. Cardiovasc. Surg. 2015, 150, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, M.; Sergeant, P.; Meuris, B. Bilateral internal thoracic artery grafting increases long-term survival in elderly patients. Eur. J. Cardiothorac. Surg. 2015, 47, 703–709. [Google Scholar] [CrossRef][Green Version]
- Galbut, D.L.; Kurlansky, P.A.; Traad, E.A.; Dorman, M.J.; Zucker, M.; Ebra, G. Bilateral internal thoracic artery grafting improves long-term survival in patients with reduced ejection fraction: A propensity-matched study with 30-year follow-up. J. Thorac. Cardiovasc. Surg. 2012, 143, 844–853. [Google Scholar] [CrossRef]
- Puskas, J.D.; Sadiq, A.; Vassiliades, T.A.; Kilgo, P.D.; Lattouf, O.M. Bilateral Internal Thoracic Artery Grafting Is Associated with Significantly Improved Long-Term Survival, Even Among Diabetic Patients. Ann. Thorac. Surg. 2012, 94, 710–715. [Google Scholar] [CrossRef]
- Dalén, M.; Ivert, T.; Holzmann, M.J.; Sartipy, U. Bilateral versus Single Internal Mammary Coronary Artery Bypass Grafting in Sweden from 1997–2008. PLoS ONE 2014, 9, e86929. [Google Scholar] [CrossRef]
- Yang, M.; Wu, Y.; Wang, G.; Xiao, C.; Zhang, H.; Gao, C. Robotic Total Arterial Off-Pump Coronary Artery Bypass Grafting: Seven-Year Single-Center Experience and Long-Term Follow-Up of Graft Patency. Ann. Thorac. Surg. 2015, 100, 1367–1373. [Google Scholar] [CrossRef]
- Gong, W.; Cai, J.; Wang, Z.; Chen, A.; Ye, X.; Li, H.; Zhao, Q. Robot-assisted coronary artery bypass grafting improves short-term outcomes compared with minimally invasive direct coronary artery bypass grafting. J. Thorac. Dis. 2016, 8, 459–468. [Google Scholar] [CrossRef]
- Ishikawa, N.; Watanabe, G.; Tomita, S.; Yamaguchi, S.; Nishida, Y.; Iino, K. Robot-Assisted Minimally Invasive Direct Coronary Artery Bypass Grafting. ThoraCAB Circ. J. 2014, 78, 399–402. [Google Scholar] [CrossRef]
- Canale, L.S.; Bonatti, J. Mammary artery harvesting using the Da Vinci Si robotic system. Rev. Bras. Cir. Cardiovasc. 2014, 29, 107–109. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Cai, J.-F. Traditional coronary artery bypass graft versus totally endoscopic coronary artery bypass graft or robot-assisted coronary artery bypass graft-meta-analysis of 16 studies. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 790–797. [Google Scholar]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Tamura, M.K.; Feldman, H.I. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef]
- Marui, A.; Okabayashi, H.; Komiya, T.; Tanaka, S.; Furukawa, Y.; Kita, T.; Kimura, T.; Sakata, R.; CREDO-Kyoto Investigators. Impact of occult renal impairment on early and late outcomes following coronary artery bypass grafting. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 638–643. [Google Scholar] [CrossRef]
- Cooper, W.A.; O’Brien, S.M.; Thourani, V.H.; Guyton, R.A.; Bridges, C.R.; Szczech, L.A.; Petersen, R.; Peterson, E.D. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the society of thoracic surgeons national adult cardiac database. Circulation 2006, 113, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Hillis, G.S.; Croal, B.L.; Buchan, K.G.; El-Shafei, H.; Gibson, G.; Jeffrey, R.R.; Millar, C.G.; Prescott, G.J.; Cuthbertson, B.H. Renal function and outcome from coronary artery bypass grafting: Impact on mortality after a 2.3-year follow-up. Circulation 2006, 113, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Sartipy, U.; Evans, M.; Holzmann, M.J. Acute Kidney Injury After Coronary Artery Bypass Grafting and Long-Term Risk of End-Stage Renal Disease. Circulation 2014, 130, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Cochran, R.P.; Leavitt, B.J.; Dacey, L.J.; Ross, C.S.; MacKenzie, T.A.; Kunzelman, K.S.; Kramer, R.S.; Hernandez, F., Jr.; Helm, R.E.; et al. Multivariable Prediction of Renal Insufficiency Developing After Cardiac Surgery. Circulation 2007, 116, 139–143. [Google Scholar] [CrossRef]
- Hayashida, N.; Teshima, H.; Chihara, S.; Tomoeda, H.; Takaseya, T.; Hiratsuka, R.; Shoujima, T.; Takagi, K.; Kawara, T.; Aoyagi, S. Does Off-Pump Coronary Artery Bypass Grafting Really Preserve Renal Function? Circ. J. 2002, 66, 921–925. [Google Scholar] [CrossRef][Green Version]
- Ji, Q.; Mei, Y.; Wang, X.; Feng, J.; Wusha, D.; Cai, J.; Zhou, Y. Effect of Elapsed Time from Coronary Angiography Until Off-Pump Coronary Artery Bypass Surgery on Postoperative Renal Function. Circ. J. 2012, 76, 2356–2362. [Google Scholar] [CrossRef]
- Wiedemann, D.; Bonaros, N.; Schachner, T.; Weidinger, F.; Lehr, E.J.; Vesely, M.; Bonatti, J. Surgical problems and complex procedures: Issues for operative time in robotic totally endoscopic coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2012, 143, 639–647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolotin, G.; Scott, W.W., Jr.; Austin, T.C.; Charland, P.J.; Kypson, A.P.; Nifong, L.W.; Salleng, K.; Chitwood, W.R., Jr. Robotic skeletonizing of the internal thoracic artery: Is it safe? Ann. Thorac. Surg. 2004, 77, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Ragosta, M.; Singh, K.P. Robotic-Assisted Percutaneous Coronary Intervention: Rationale, Implementation, Case Selection and Limitations of Current Technology. J. Clin. Med. 2018, 7, 23. [Google Scholar] [CrossRef] [PubMed]
| Da Vinci Robotic Surgery (n = 22) | Sternotomy Surgery (n = 22) | p Value | |
|---|---|---|---|
| Male | 20 (90.9) | 21 (95.5) | 1.00 |
| Age (years) | 61.2 ± 12.0 | 62.9 ± 10.5 | 0.63 |
| Body mass index (kg/m2) | 26.9 ± 4.0 | 27.3 ± 3.2 | 0.70 |
| Risk factors for CAD | |||
| Diabetes mellitus | 11 (50.0) | 10 (45.5) | 1.00 |
| Hypertension | 17 (77.3) | 18 (81.8) | 1.00 |
| Dyslipidemia | 13 (59.1) | 12 (54.5) | 1.00 |
| Hyperuricemia | 1 (4.5) | 0 (0) | 1.00 |
| Drug allergy | 5 (22.7) | 1 (4.5) | 0.19 |
| Alcohol drinking | 6 (23.7) | 1 (4.5) | 0.95 |
| Cigarette smoking | 10 (45.5) | 11 (50.0) | 1.00 |
| Family history of CVD | 8 (36.4) | 2 (9.1) | 0.03 |
| CAD condition | |||
| LM | 10 (45.5) | 13 (59.1) | 0.55 |
| TVD | 17 (77.3) | 20 (90.9) | 0.41 |
| DVD | 5 (22.7) | 2 (9.1) | 0.41 |
| Grafting conduit | |||
| BIMA | 22 (100.0) | 22 (100.0) | 1.00 |
| Radial artery | 19 (86.4) | 19 (86.4) | 1.00 |
| GSV | 3 (13.6) | 3 (13.6) | 1.00 |
| Anastomosis number | 3.0 ± 0.6 | 3.4 ± 0.7 | 0.34 |
| On pump | 10 (45.5) | 16 (72.7) | 0.12 |
| On pump time (min) | 123.9 ± 96.2 | 131.4 ± 59.8 | 0.81 |
| Operation room time (h) | 12.7 ± 1.7 | 8.5 ± 1.5 | <0.01 |
| Biomarkers at admission | |||
| Hemoglobin (g/dL) | 13.7 ± 1.6 | 12.7 ± 2.4 | 0.11 |
| Creatinine (mg/dL) | 1.2 ± 0.3 | 2.0 ± 1.7 | 0.04 |
| eGFR (mL/min/1.73 m2) | 65.4 ± 13.6 | 57.9 ± 31.5 | 0.32 |
| CKD stage | 0.11 | ||
| I/II | 15 (68.2) | 10 (45.5) | |
| III | 7 (31.8) | 7 (31.8) | |
| IV | 0 (0) | 3 (13.6) | |
| V | 0 (0) | 2 (9.1) |
| Da Vinci Robotic Surgery (n = 22) | Sternotomy Surgery (n = 22) | p Value | |
|---|---|---|---|
| Hospital stay (days) | 21.0 ± 8.8 | 24.4 ± 14.0 | 0.34 |
| ICU stay (days) | 4.8 ± 3.5 | 5.0 ± 3.3 | 0.90 |
| Ventilator weaning (days) | 2.2 ± 1.8 | 2.3 ± 2.3 | 0.94 |
| Renal events | |||
| CKD stages IV/V | 1 (4.5) | 10 (22.7) | <0.01 |
| Creatinine change >0.5 mg/dL | 5 (22.7) | 7 (31.8) | 0.50 |
| Doubling creatinine (mg/dL) | 1 (4.8) | 3 (13.6) | 0.61 |
| eGFR (mL/min/1.73 m2) * | 58.5 ± 22.1 | 45.5 ± 31.6 | 0.12 |
| Hemodialysis | 0 (0) | 1 (4.5) | 1.00 |
| The lowest hemoglobin (g/dL) | 11.9 ± 1.5 | 12.1 ± 1.5 | 0.57 |
| Blood transfusion (U) | |||
| FFP | 5.3 ± 3.8 | 3.8 ± 4.3 | 0.28 |
| PRBC | 2.1 ± 2.0 | 1.4 ± 2.2 | 0.30 |
| Adverse events | 4 (18.2) | 5 (27.8) | 0.70 |
| Death | 0 (0) | 0 (0) | 1.00 |
| Myocardial infarction | 0 (0) | 0 (0) | 1.00 |
| Stroke | 0 (0) | 0 (0) | 1.00 |
| Wound infection | 1 (4.5) | 1 (5.6) | 1.00 |
| Pneumonia | 1 (4.5) | 0 (0) | 1.00 |
| Pleural effusion | 1 (4.5) | 2 (11.1) | 1.00 |
| Post-operation IABP | 2 (9.1) | 3 (13.6) | 1.00 |
| Da Vinci Robotic Surgery (n = 22) | Sternotomy Surgery (n = 22) | p Value | |
|---|---|---|---|
| Finished six months follow-up | 19 (86.5) | 21 (95.5) | |
| Primary endpoints | 5 (22.7) | 12 (54.5) | 0.03 |
| Cardiovascular events | 1 (4.5) | 0 (0) | 1.00 |
| Mortality | 0 (0) | 0 (0) | 1.00 |
| Myocardial infarction | 1 (4.5) | 0 (0) | 1.00 |
| Stoke | 0 (0) | 0 (0) | 1.00 |
| Renal events | 1 (4.5) | 7 (31.8) | 0.02 |
| CKD stages IV/V | 0 (0) | 7 (31.8) | 0.02 |
| Creatinine doubling | 0 (0) | 1 (4.5) | 1.00 |
| Creatinine change >0.5 mg/dL | 1 (4.5) | 5 (22.7) | 0.18 |
| Hemodialysis | 0 (0) | 1 (4.5) | 1.00 |
| eGFR (mL/min/1.73 m2) | 72.1 ± 19.0 | 56.9 ± 34.0 | 0.08 |
| Re-hospitalization * | 4 (18.2) | 6 (27.3) | 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-J.; Chen, H.-H.; Cheng, P.-W.; Lu, W.-H.; Tseng, C.-J.; Lai, C.-C. Outcome of Robot-Assisted Bilateral Internal Mammary Artery Grafting via Left Pleura in Coronary Bypass Surgery. J. Clin. Med. 2019, 8, 502. https://doi.org/10.3390/jcm8040502
Wu C-J, Chen H-H, Cheng P-W, Lu W-H, Tseng C-J, Lai C-C. Outcome of Robot-Assisted Bilateral Internal Mammary Artery Grafting via Left Pleura in Coronary Bypass Surgery. Journal of Clinical Medicine. 2019; 8(4):502. https://doi.org/10.3390/jcm8040502
Chicago/Turabian StyleWu, Chieh-Jen, Hsin-Hung Chen, Pei-Wen Cheng, Wen-Hsien Lu, Ching-Jiunn Tseng, and Chi-Cheng Lai. 2019. "Outcome of Robot-Assisted Bilateral Internal Mammary Artery Grafting via Left Pleura in Coronary Bypass Surgery" Journal of Clinical Medicine 8, no. 4: 502. https://doi.org/10.3390/jcm8040502
APA StyleWu, C.-J., Chen, H.-H., Cheng, P.-W., Lu, W.-H., Tseng, C.-J., & Lai, C.-C. (2019). Outcome of Robot-Assisted Bilateral Internal Mammary Artery Grafting via Left Pleura in Coronary Bypass Surgery. Journal of Clinical Medicine, 8(4), 502. https://doi.org/10.3390/jcm8040502





