Anatomical and Functional Changes of the Retina and the Choroid after Resolved Chronic CSCR
Abstract
1. Introduction
2. Experimental Section
2.1. Materials Study Population
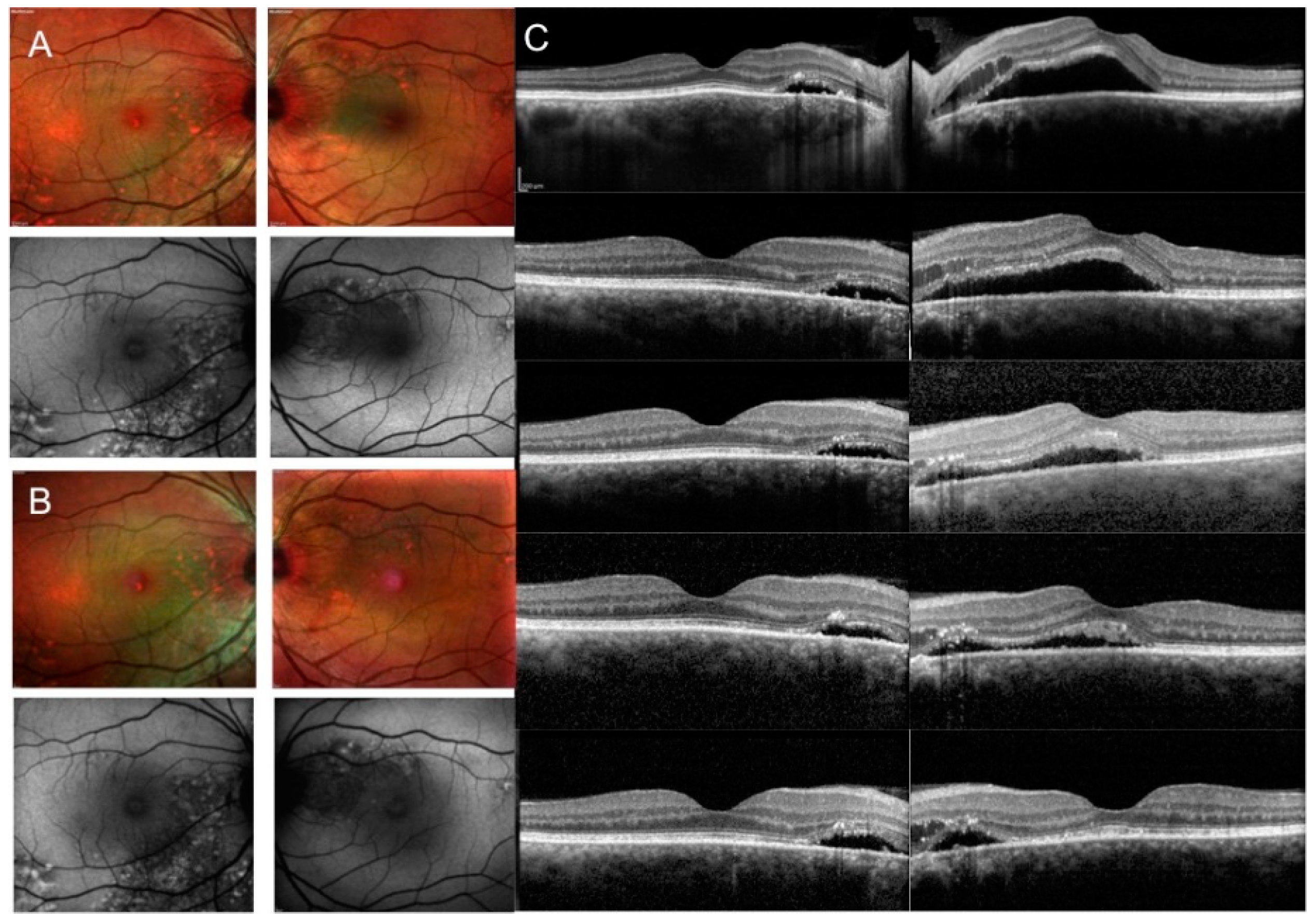
2.2. SD OCT Analysis
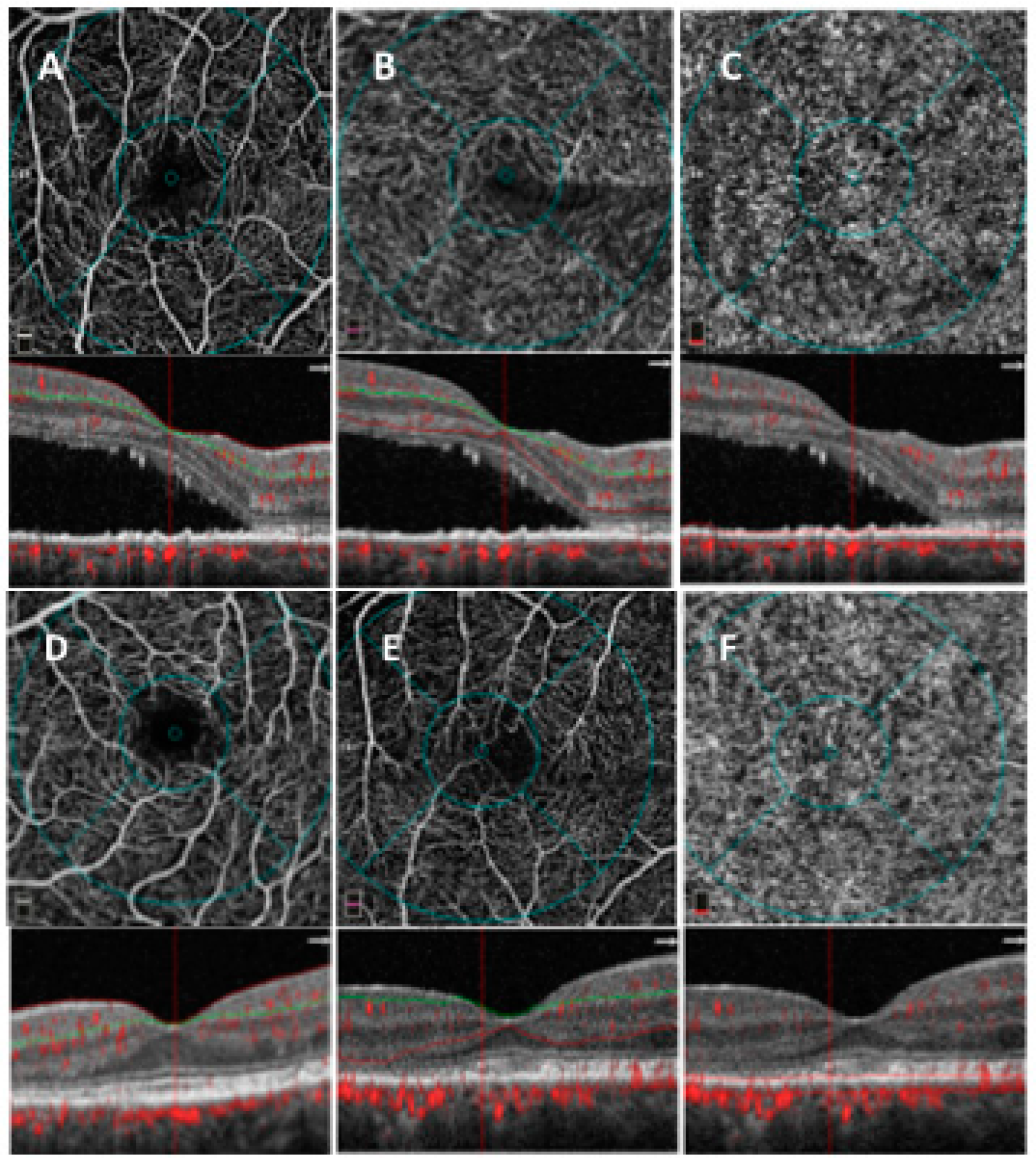
2.3. OCTA Analysis
2.3.1. SD-OCT Angiography with XR Avanti and Vascular Layer Segmentation
2.3.2. Quantitative Vessel Analysis
2.4. Main Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Iacono, P.; Battaglia Parodi, M.; Falcomatà, B.; Bandello, F. Central Serous Chorioretinopathy Treatments: A Mini Review. Ophthalmic Res. 2015, 55, 76–83. [Google Scholar] [CrossRef]
- Wang, M.; Munch, I.C.; Hasler, P.W.; Prünte, C.; Larsen, M. Central serous chorioretinopathy. Acta Ophthalmol. 2008, 86, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, LA. Central serous chorioretinopathy: A personal perspective. Am. J. Ophthalmol. 2010, 149, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.M.; Owens, S.L.; Smith, P.D.; Fine, S.L. Long-term follow-up of central serous chorioretinopathy. Br. J. Ophthalmol. 1984, 68, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef]
- Schwartz, R.; Habot-Wilner, Z.; Martinez, M.R.; Nutman, A.; Goldenberg, D.; Cohen, S. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol. 2017, 95, e610–e618. [Google Scholar] [CrossRef]
- Sacconi, R.; Baldin, G.; Carnevali, A.; Querques, L.; Rabiolo, A.; Marchini, G.; Bandello, F.; Querques, G. Response of central serous chorioretinopathy evaluated by multimodal retinal Imaging. Eye (Lond) 2018, 32, 734–742. [Google Scholar] [CrossRef]
- Cakir, B.; Fischer, F.; Ehlken, C.; Bühler, A.; Stahl, A.; Schlunck, G.; Böhringer, D.; Agostini, H.; Lange, C. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Dirani, A.; Gallice, M.; Nicholson, L.; Sivaprasad, S. Oral Mineralocorticoid-Receptor Antagonists: Real-Life Experience in Clinical Subtypes of Nonresolving Central Serous Chorioretinopathy With Chronic Epitheliopathy. Transl. Vis. Sci Technol. 2016, 5, 2. [Google Scholar] [CrossRef]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Senatore, A.; Di Antonio, L.; Di Nicola, M.; Carpineto, P.; Mastropasqua, L. Macular Features in Retinitis Pigmentosa: Correlations Among Ganglion Cell Complex Thickness, Capillary Density, and Macular Function. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6360–6366. [Google Scholar] [CrossRef]
- Zhao, M.; C’el’erier, I.; Bousquet, E.; Jeanny, J.C.; Jonet, L.; Savoldelli, M.; Offret, O.; Curan, A.; Farman, N.; Jaisser, F.; et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J. Clin. Investig. 2012, 122, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Valamanesh, F.; Celerier, I.; Savoldelli, M.; Jonet, L.; Jeanny, J.C.; Jaisser, F.; Farman, N.; Behar-Cohen, F. The neuroretina is a novel mineralocorticoid target: Aldosterone up-regulates ion and water channels in Müller glial cells. FASEB J. 2010, 24, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, A. Potential involvement of mineralocorticoid receptor activation in the pathogenesis of central serous chorioretinopathy: Case report. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1369–1373. [Google Scholar]
- Herold, T.R.; Prause, K.; Wolf, A.; Mayer, W.J.; Ulbig, M.W. Spironolactone in the treatment of central serous chorioretinopathy—A case series. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1985–1991. [Google Scholar] [CrossRef]
- Bousquet, E.; Beydoun, T.; Rothschild, P.R.; Bergin, C.; Zhao, M.; Batista, R.; Brandely, M.L.; Couraud, B.; Farman, N.; Gaudric, A.; et al. Spironolactone for nonresolving central serous chorioretinopathy. A Randomized Controlled Crossover Study. Retina 2015, 35, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, E.; Beydoun, T.; Zhao, M.; Hassan, L.; Offret, O.; Behar-Cohen, F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: A pilot study. Retina 2013, 33, 2096–2102. [Google Scholar] [CrossRef]
- Singh, R.P.; Sears, J.E.; Bedi, R.; Schachat, A.P.; Ehlers, J.P.; Kaiser, P.K. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int. J. Ophthalmol. 2015, 8, 310–314. [Google Scholar]
- Salz, D.A.; Pitcher, J.D., III; Hsu, J.; Regillo, C.D.; Fineman, M.S.; Elliott, K.S.; Vander, J.F.; Fischer, D.H.; Spirn, M.J. Oral eplerenone for treatment of chronic central serous chorioretinopathy: A case series. Ophthalmic Surg. Lasers Imaging Retina 2015, 46, 439–444. [Google Scholar] [CrossRef]
- Leisser, C.; Hirnschall, N.; Hackl, C.; Plasenzotti, P.; Findl, O. Eplerenone in patients with chronic recurring central serous chorioretinopathy. Eur. J. Ophthalmol. 2016, 26, 479–484. [Google Scholar] [CrossRef]
- Eandi, C.M.; Piccolino, F.C.; Alovisi, C.; Tridico, F.; Giacomello, D.; Grignolo, F.M. Correlation between fundus autofluorescence and central visual function in chronic central serous chorioretinopathy. Am. J. Ophthalmol. 2015, 159, 652–658. [Google Scholar] [CrossRef]
- Ghadiali, Q.; Jung, J.J.; Yu, S.; Patel, S.N.; Yannuzzi, L.A. Central serous chorioretinopathy treated with mineralocorticoid antagonists: A one-year pilot study. Retina 2015, 36, 611–618. [Google Scholar] [CrossRef]
- Goktas, A. Correlation of subretinal fluid volume with choroidal thickness and macular volume in acute central serous chorioretinopathy. Eye (Lond) 2014, 28, 1431–1436. [Google Scholar] [CrossRef]
- Maruko, I.; Iida, T.; Sugano, Y.; Ojima, A.; Sekiryu, T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina 2011, 31, 1603–1608. [Google Scholar] [CrossRef]
- Imamura, Y.; Fujiwara, F.; Margolis, R.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 2009, 29, 1469–1473. [Google Scholar] [CrossRef]
- Rabiolo, A.; Zucchiatti, I.; Marchese, A.; Baldin, G.; Sacconi, R.; Montorio, D.; Cicinelli, M.V.; Querques, L.; Bandello, F.; Querques, G.; et al. Multimodal retinal imaging in central serous chorioretinopathy treated with oral eplerenone or photodynamic therapy. Eye (Lond) 2018, 32, 55–56. [Google Scholar] [CrossRef]
| Ocular Characteristics | Group 1 (n = 21) | Group 2 (n = 21) | Healthy Controls (n = 21) | Kruskall–Wallis p-Value | Post-hoc p-Value Group 1 vs. Group 2 |
|---|---|---|---|---|---|
| Baseline visual acuity (logMAR), mean ± SD | 0.30 ± 0.11 ** | −0.01 ± 0.04 | 0.04 ± 0.08 | <0.001 | <0.001 |
| Foveal SRF (µm), mean ± SD | 217.14 ± 86.97 | - | - | ||
| CMT (µm), mean ± SD | 428.71 ± 80.05 ** | 237.71 ± 37.56 | 248.86 ± 17.25 | <0.001 | <0.001 |
| SFCT (µm), mean ± SD | 452.85 ± 132.74 ** | 410.28 ± 124.19 ** | 252.00 ± 53.39 | <0.001 | 0.387 |
| Foveal SRF, n (%) | 21 (100.0) | - | - | ||
| Only extrafoveal SRF, n (%) | - | 9 (42.8) | - | ||
| Subtle RPE changes/widespread RPE atrophy, n | 6/15 | 12/6 | - | 0.017 b | |
| PED, n (%) | 3 (14.3) | 6 (28.6) | - | 0.259 a | |
| Intraretinal fluid, n (%) | 1 (4.8) | - | - | ||
| SCPD (%), mean ± SD | 46.78 ± 4.29 ** | 45.04 ± 4.52 * | 51.08 ± 3.00 | <0.001 | 0.869 |
| DCPD (%), mean ± SD | 46.34 ± 5.36 ** | 46.81 ± 4.66 ** | 58.68 ± 3.03 | <0.001 | 0.989 |
| CCD (%), mean ± SD | 54.72 ± 4.93 ** | 61.50 ± 5.58 ** | 66.70 ± 1.19 | <0.001 | <0.001 |
| Baseline | 7 days | 30 days | 60 days | 90 days | 120 days | p-Value a | p-Value b | p-Value c | |
|---|---|---|---|---|---|---|---|---|---|
| BCVA, logMAR | <0.001 | <0.001 | <0.001 | ||||||
| Group 2 | −0.01 ± 0.04 | −0.01 ± 0.03 | −0.01 ± 0.03 | 0.02 ± 0.07 | 0.02 ± 0.07 | 0.02 ± 0.07 | |||
| Group 1 | 0.30 ± 0.11 | 0.30 ± 0.11 | 0.13 ± 0.11 ** | 0.11 ± 0.10 ** | 0.10 ± 0.08 ** | 0.09 ± 0.09 ** | |||
| CMT, µm | <0.001 | <0.001 | <0.001 | ||||||
| Group 2 | 237.71 ± 37.56 | 223.28 ± 16.71 | 231.57 ± 27.48 | 223.85 ± 19.99 | 224.14 ± 19.60 | 224.14 ± 19.60 | |||
| Group 1 | 428.71 ± 80.05 | 427.86 ± 110.53 | 289.57 ± 80.26 ** | 267.71 ± 89.07 ** | 251.71 ± 63.46 ** | 213.57 ± 32.80 ** | |||
| SRF, µm | 0.037 d | - | |||||||
| Group 2 | |||||||||
| Group 1 | 217.14 ± 86.97 | 219.42 ± 112.98 | 153.57 ± 120.49 ** | 158.43 ± 119.53 ** | 134.71 ± 119.24 ** | 0 ** | |||
| SFCT, µm | <0.001 | 0.939 | 0.452 | ||||||
| Group 2 | 410.28 ± 124.19 | 392.28 ± 135.22 | 394.42 ± 134.85 | 374.14 ± 108.13 | 374.28 ± 108.32 | 373.71 ± 107.62 | |||
| Group 1 | 452.85 ± 132.74 | 427.71 ± 125.85 | 386.85 ± 130.69 ** | 354.85 ± 97.88 ** | 358.57 ± 93.47 ** | 358.28 ± 93.33 ** | |||
| SCPD, µm | 0.354 | 0.853 | 0.368 | ||||||
| Group 2 | 45.04 ± 4.52 | 45.90 ± 5.75 | 48.06 ± 2.63 | 48.02 ± 4.06 | 47.48 ± 3.24 | 47.94 ± 3.58 | |||
| Group 1 | 46.78 ± 4.29 | 46.76 ± 5.91 | 47.05 ± 4.55 | 47.05 ± 4.55 | 47.64 ± 4.48 | 47.68 ± 4.52 | |||
| DCPD, µm | 0.212 | 0.832 | 0.565 | ||||||
| Group 2 | 46.81 ± 4.66 | 47.59 ± 5.66 | 47.40 ± 3.58 | 48.07 ± 3.16 | 48.41 ± 3.39 | 48.58 ± 2.85 | |||
| Group 1 | 46.34 ± 5.36 | 48.86 ± 5.51 | 45.37 ± 4.31 | 46.57 ± 4.46 | 46.60 ± 4.61 | 47.44 ± 3.53 | |||
| CCD, µm | 0.452 | 0.417 | 0.358 | ||||||
| Group 2 | 61.50 ± 5.58 | 60.92 ± 6.18 | 61.05 ± 6.31 | 61.35 ± 5.81 | 61.88 ± 5.21 | 62.84 ± 5.13 | |||
| Group 1 | 54.72 ± 4.93 | 55.87 ± 4.96 | 59.57 ± 5.91 | 60.84 ± 6.08 | 61.02 ± 4.54 | 61.28 ± 4.81 |
| Variable | 60 days n = 15 | 120 days n = 6 | Mann–Whitney U test p-Value |
|---|---|---|---|
| BCVA, logMAR | −0.05 (−0.20–0) | −0.10 (−0.28–0) | 0.566 |
| CMT, µm | −81.00 (−184.00–1.75) | −125.00 (−252.00–4.50) | 0.901 |
| SFCT, µm | −46.00 (−74.7–3.25) | −102.00 (−212.00–56.00) | 0.055 |
| SCPD, µm | 0.40 (−0.50–3.20) | 2.00 (−0.18–3.80) | 0.521 |
| DCPD, µm | 1.60 (0.60–2.90) | 1.40 (0.88–3.80) | 0.765 |
| CCD, µm | 4.00 (0.35–10.00) | 1.90 (0.80–4.70) | 0.547 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toto, L.; D’Aloisio, R.; Mastropasqua, R.; Di Antonio, L.; Di Nicola, M.; Di Martino, G.; Evangelista, F.; Erroi, E.; Doronzo, E.; Mariotti, C. Anatomical and Functional Changes of the Retina and the Choroid after Resolved Chronic CSCR. J. Clin. Med. 2019, 8, 474. https://doi.org/10.3390/jcm8040474
Toto L, D’Aloisio R, Mastropasqua R, Di Antonio L, Di Nicola M, Di Martino G, Evangelista F, Erroi E, Doronzo E, Mariotti C. Anatomical and Functional Changes of the Retina and the Choroid after Resolved Chronic CSCR. Journal of Clinical Medicine. 2019; 8(4):474. https://doi.org/10.3390/jcm8040474
Chicago/Turabian StyleToto, Lisa, Rossella D’Aloisio, Rodolfo Mastropasqua, Luca Di Antonio, Marta Di Nicola, Giuseppe Di Martino, Federica Evangelista, Emanuele Erroi, Emanuele Doronzo, and Cesare Mariotti. 2019. "Anatomical and Functional Changes of the Retina and the Choroid after Resolved Chronic CSCR" Journal of Clinical Medicine 8, no. 4: 474. https://doi.org/10.3390/jcm8040474
APA StyleToto, L., D’Aloisio, R., Mastropasqua, R., Di Antonio, L., Di Nicola, M., Di Martino, G., Evangelista, F., Erroi, E., Doronzo, E., & Mariotti, C. (2019). Anatomical and Functional Changes of the Retina and the Choroid after Resolved Chronic CSCR. Journal of Clinical Medicine, 8(4), 474. https://doi.org/10.3390/jcm8040474










