FGF23 and Klotho Levels are Independently Associated with Diabetic Foot Syndrome in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Samples and Biochemical Markers
2.3. Immunohistochemistry
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
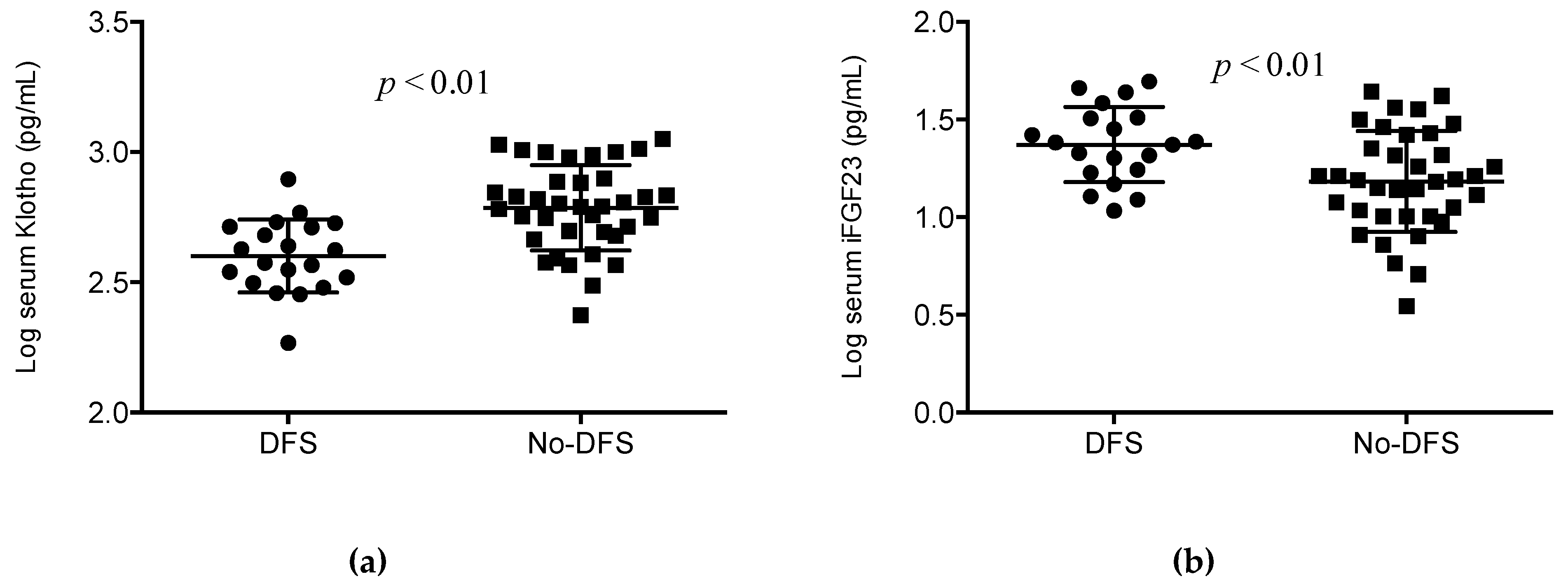
3. Results
3.1. Characteristics of the Patients and Biochemical Parameters
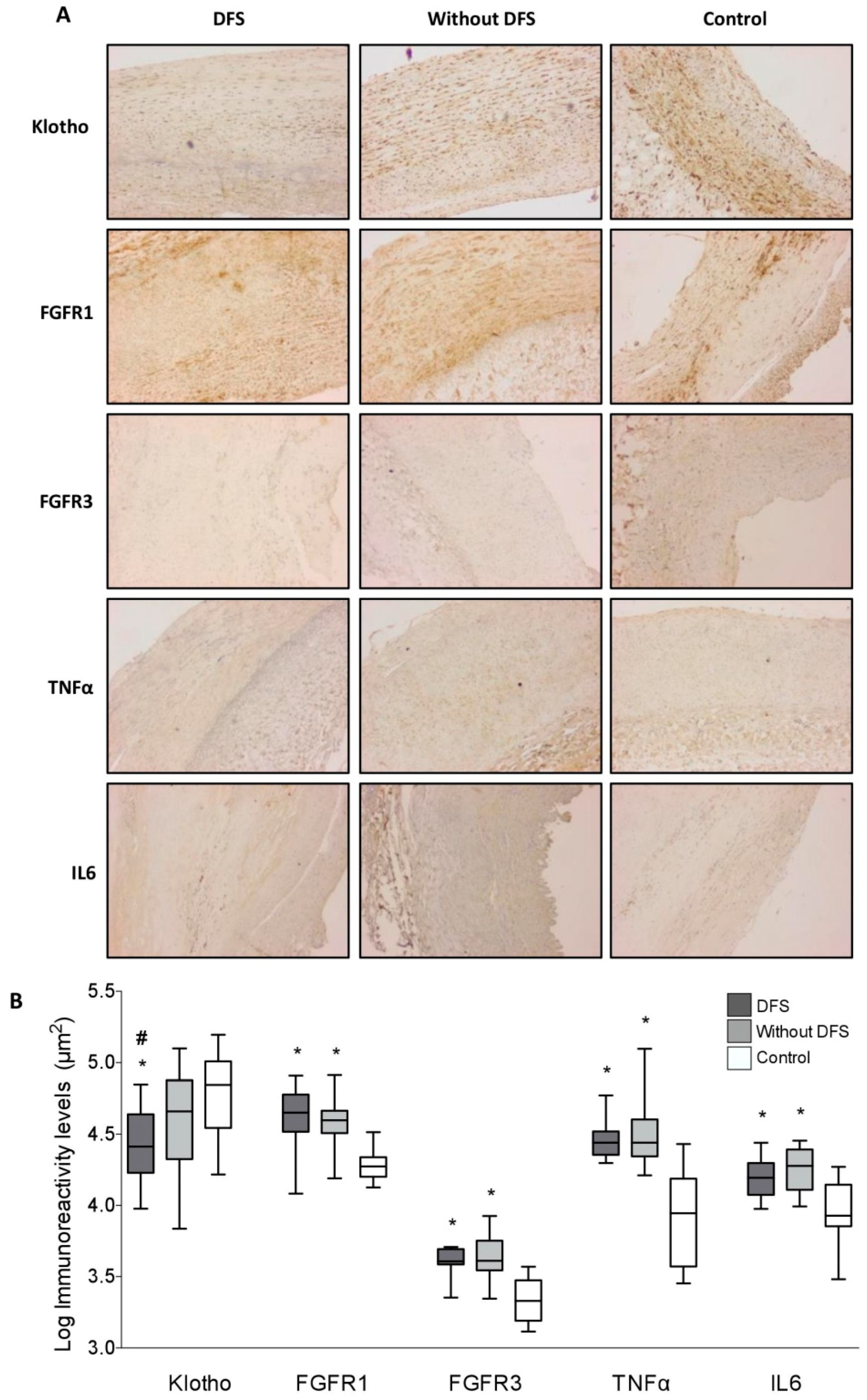
3.2. Immunohistochemical Analysis
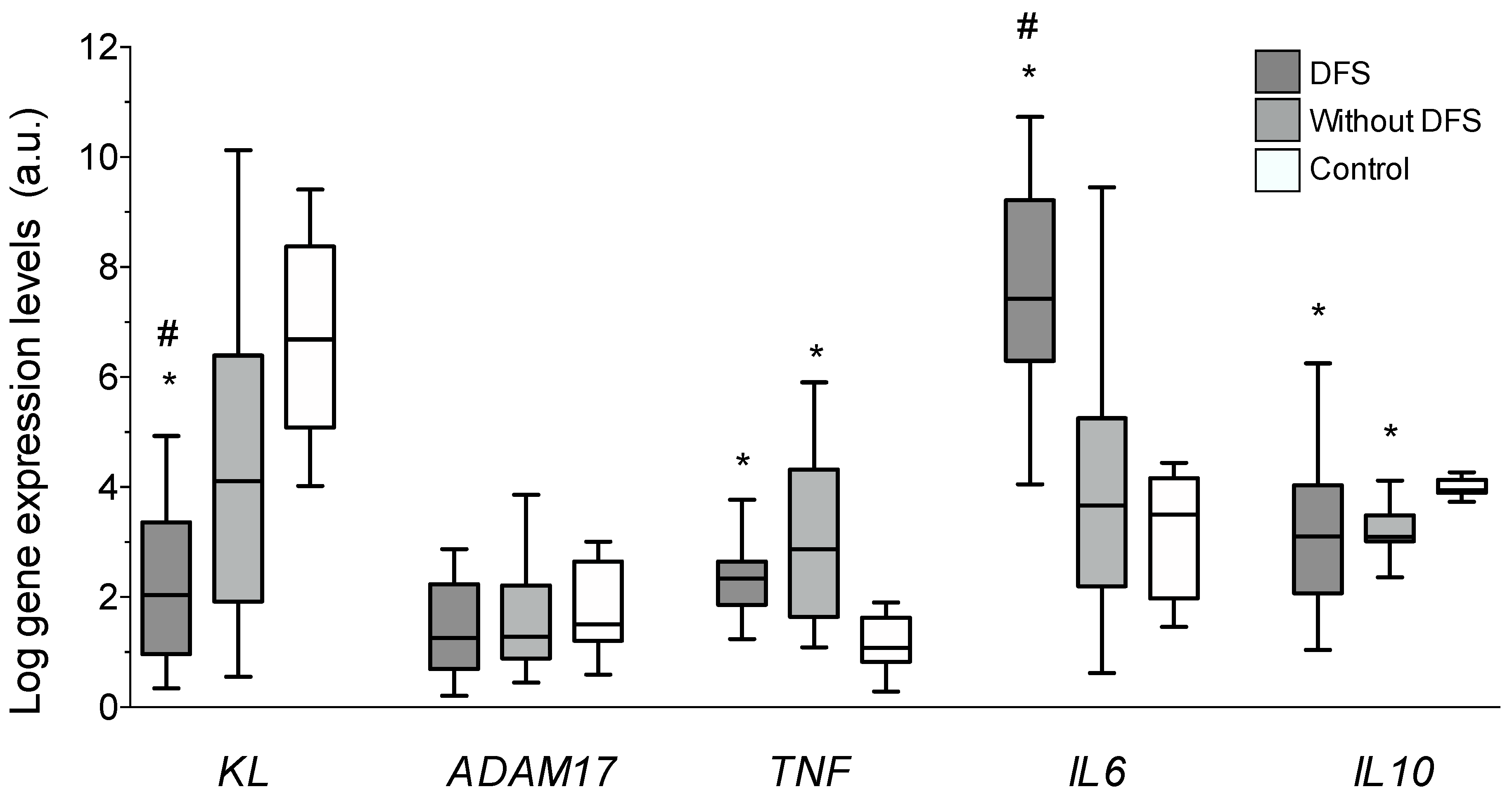
3.3. Vascular Gene Expression
3.4. Correlations and Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Bild, D.E.; Selby, J.V.; Sinnock, P.; Browner, W.S.; Braveman, P.; Showstack, J.A. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989, 12, 24–31. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Wrobel, J.; Robbins, J.M. Guest Editorial: Are diabetes-related wounds and amputations worse than cancer? Int. Wound J. 2007, 4, 286–287. [Google Scholar] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; Donate-Correa, J.; Cazaña-Pérez, V.; García-Pérez, J. Effect of phosphate binders on serum inflammatory profile, soluble CD14, and endotoxin levels in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros, M.; Herrera, H.; García, J. Mineral metabolism and inflammation in chronic kidney disease patients: A cross−sectional study. Clin. J. Am. Soc. Nephrol. 2009, 4, 1646–1654. [Google Scholar] [CrossRef]
- Yu, X.; Ibrahimi, O.A.; Goetz, R.; Zhang, F.; Davis, S.I.; Garringer, H.J.; Linhardt, R.J.; Ornitz, D.M.; Mohammadi, M.; White, K.E. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 2005, 146, 4647–4656. [Google Scholar] [CrossRef]
- Imura, A.; Iwano, A.; Tohyama, O.; Tsuji, Y.; Nozaki, K.; Hashimoto, N.; Fujimori, T.; Nabeshima, Y.-I. Secreted Klotho protein in sera and CSF: Implication for post−translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004, 565, 143–147. [Google Scholar] [CrossRef]
- Akimoto, T.; Yoshizawa, H.; Watanabe, Y.; Numata, A.; Yamazaki, T.; Takeshima, E.; Iwazu, K.; Komada, T.; Otani, N.; Morishita, Y.; et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012, 13, 155. [Google Scholar]
- Matsumura, Y.; Aizama, H.; Shiraki-lida, T.; Naqai, R.; Kuro-o, M.; Nabeshima, Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys. Res. Commun. 1998, 242, 626–630. [Google Scholar] [CrossRef]
- Kanbay, M.; Nicoleta, M.; Selcoki, Y.; Ikizek, M.; Aydin, M.; Eryonucu, B.; Duranay, M.; Akcay, A.; Armutcu, F.; Covic, A. Fibroblast growth factor 23 and fetuin A are independent predictors for the coronary artery disease extent in mild chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutierrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Mirza, M.A.; Larsson, A.; Lind, L.; Larsson, T.E. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009, 205, 385–390. [Google Scholar] [CrossRef]
- Lim, K.; Lu, T.S.; Molostvov, G.; Lee, C.; Lam, F.T.; Zehnder, D.; Hsiao, L.L. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to FGF-23. Circulation 2012, 125, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Renal and extrarenal actions of Klotho. Semin. Nephrol. 2013, 33, 118–129. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Mora-Fernández, C.; Martínez-Sanz, R.; Muros-De-Fuentes, M.; Perez, H.; Meneses-Pérez, B.; Cazaña-Pérez, V.; Navarro-Gonzalez, J.F. Expression of FGF23/KLOTHO system in human vascular tissue. Int. J. Cardiol. 2013, 165, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, E.; Donate-Correa, J.; López-Castillo, Á.; Delgado-Molinos, A.; Ferri, C.; Rodríguez-Ramos, S.; Cerro, P.; Pérez-Delgado, N.; Castro, V.; Hernández-Carballo, C.; et al. Soluble levels and endogenous vascular gene expression of KLOTHO are related to inflammation in human atherosclerotic disease. Clin. Sci. (Lond.) 2017, 131, 2601–2609. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Núñez, E.; Martínez-Sanz, R.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Pérez-Delgado, N.; Navarro-González, J.F. Influence of Klotho gene polymorphisms on vascular gene expression and its relationship to cardiovascular disease JF. J. Cell Mol. Med. 2016, 20, 128–133. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Donate-Correa, J.; Muros de Fuentes, M.; Pérez-Hernández, H.; Martínez-Sanz, R.; Mora-Fernández, C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart 2014, 100, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; Nabuurs-Franssen, M.H. The diabetic foot: Pathogenesis and clinical evaluation. Semin. Vasc. Med. 2002, 2, 221–228. [Google Scholar] [CrossRef]
- Mirza, M.A.; Hansen, T.; Johansson, L.; Ahlström, H.; Larsson, A.; Lind, L. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol. Dial. Transplant. 2009, 24, 3125–3131. [Google Scholar] [CrossRef]
- Kuro-O, M.; Matsumura, Y.; Aizawa, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; Iwasaki, H.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nakamura, T.; Ohtama, Y.; Suzuki, T.; Iida, A.; Shiraki-Iida, T.; Kuro-O, M.; Nabeshima, Y.-I.; Kurabayashi, M.; Nagai, R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem. Biophys. Res. Commun. 2000, 276, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Saito, Y.; Ohyama, Y.; Aizawa, H.; Suga, T.; Nakamura, T.; Kurabayashi, M.; Kuroo, M. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell. Mol. Life Sci. 2000, 57, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Keles, N.; Caliskan, M.; Dogan, B.; Keles, N.N.; Kalcik, M.; Aksu, F.; Kostek, O.; Aung, S.M.; Isbilen, B.; Oguz, A. Low serum level of Klotho is an early predictor of atherosclerosis. Tohoku J. Exp. Med. 2015, 237, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and cardiovascular disease. J. Am. Geriatr. Soc. 2011, 59, 1596–1601. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T. Clinical implication of alterations in serum Klotho levels in patients with type 2 diabetes mellitus and its associated complications. J. Diabetes Complicat. 2018, 32, 922–930. [Google Scholar] [CrossRef]
- Ruiz-Andrés, O.; Sánchez-Niño, M.D.; Moreno, J.A.; Ruiz-Ortega, M.; Ramos, A.M.; Sanz, A.B.; Ortiz, A. Downregulation of kidney protective factors by inflammation: Role of transcription factors and epigenetic mechanisms. Am. J. Physiol. Renal Physiol. 2016, 311, 1329–1340. [Google Scholar] [CrossRef]
| Variable | Overall | DFS | No. DFS | p–Value |
|---|---|---|---|---|
| n | 57 | 20 | 37 | |
| Age (years) | 69.8 ± 9.7 | 71.8 ± 10.2 | 68.7 ± 9.4 | NS |
| Male gender, n (%) | 46 (80) | 15 (75) | 31 (84) | NS |
| BMI (kg/m2) | 30.1 ± 3.8 | 30.8 ± 4.7 | 29.7 ± 3.3 | NS |
| Hypertension, n (%) | 51 (89.4) | 17 (85) | 34 (91.9) | NS |
| Alcoholism, n (%) | 22 (38.6) | 7 (35) | 15 (40.5) | NS |
| Smoking habits, n (%) | 36 (63.2) | 12 (60) | 24 (64.8) | NS |
| Total Cholesterol (mg/dL) | 159.2 ± 38.9 | 159 ± 45.8 | 159.3 ± 35.3 | NS |
| HDL (mg/dL) | 42.4 ± 10.2 | 40 ± 9.8 | 43.7 ± 10.4 | NS |
| LDL (mg/dL) | 84.8 ± 29.4 | 87.5 ± 33.4 | 83.3 ± 27.3 | NS |
| Triglycerides, mg/dL | 155 ± 80.2 | 156.6 ± 100 | 154.2 ± 68.4 | NS |
| HbA1c, % | 7.3 ± 1.4 | 7.3 ± 1.9 | 7.2 ± 1.3 | NS |
| Glucose | 132.9 ± 42.8 | 126.3 ± 40.1 | 136.6 ± 44.4 | NS |
| eGFR, mL/min/1.73 m2 | 77.5 ± 24.7 | 76.6 ± 24 | 77.8 ± 25.4 | NS |
| Albumin (g/L) | 3.8 ± 0.7 | 3.7 ± 0.8 | 3.8 ± 0.6 | NS |
| Calcium (mg/dl) | 9.1 ± 0.6 | 9 ± 0.6 | 9.1 ± 0.6 | NS |
| Phosphorous (mg/dl) | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | NS |
| AP (mU/mL) | 78.8 ± 58.1 | 77.9 ± 43.3 | 79.2 ± 65.3 | NS |
| hsCRP (mg/dL) | 5.8 (2.4–11.9) | 6 (2.3–10.5) | 5.3 (2.4–13.6) | <0.05 |
| IL6, pg/mL | 11.2 (1.1–28.1) | 22 (5.1–28.6) | 6.42 (0.7–26.7) | <0.05 |
| IL10, pg/mL | 1.3 (0.5–6.7) | 1.1 (0.6–6.7) | 3.9 (0.5–6.8) | <0.01 |
| TNFα, pg/mL | 1.3 (0.8–1.3) | 1.4 (0.9–2.1) | 1.1 (0.7–1.7) | NS |
| KLOTHO, pg/mL | 532.8 (375–677) | 397.4 (318–515) | 617.3 (484–779) | <0.01 |
| iFGF23, pg/mL | 17.5 (12–27) | 23.8 (17–32) | 15.5 (10.1–24.5) | <0.01 |
| Log Serum KL | Log Vascular KL | |||
|---|---|---|---|---|
| Variable | r | p-Value | r | p-Value |
| BMI (kg/m2) | 0.129 | NS | −0.062 | NS |
| Total Cholesterol (mg/dL) | 0.055 | NS | −0.139 | NS |
| HDL (mg/dL) | 0.255 | <0.05 | −0.131 | NS |
| LDL (mg/dL) | 0.021 | NS | −0.152 | NS |
| Triglycerides, mg/dL | −0.058 | NS | 0.134 | NS |
| HbA1c, % | −0.034 | NS | 0.131 | NS |
| Hemoglobin | 0.344 | <0.05 | 0.104 | NS |
| Albumin (g/L) | −0.038 | NS | 0.065 | NS |
| Hematocrit | 0.341 | <0.05 | 0.109 | NS |
| Calcium (mg/dl) | 0.206 | NS | −0.51 | NS |
| Phosphorous (mg/dl) | 0.071 | NS | 0.17 | NS |
| AP (mU/mL) | −0.62 | NS | 0.03 | NS |
| Uric acid (mg/dL) | 0.096 | NS | −0.342 | <0.01 |
| eGFR, mL/min/1.73 m2 | 0.329 | <0.01 | 0.354 | <0.01 |
| hsCRP (mg/dL) | −0.196 | <0.05 | 0.076 | NS |
| Serum IL6 (pg/mL) | −0.204 | NS | −0.094 | NS |
| Serum IL10 (pg/mL) | 0.172 | NS | −0.099 | NS |
| Serum TNFα (pg/mL) | −0.159 | NS | −0.114 | NS |
| Serum Klotho (pg/mL) | 0.125 | NS | ||
| Serum iFGF23 (pg/mL) | 0.042 | NS | −0.064 | NS |
| VIR Klotho (µm2) | 0.288 | <0.05 | 0.097 | NS |
| VIR FGFR1 (µm2) | −0.15 | NS | 0.158 | NS |
| VIR FGFR3 (µm2) | 0.153 | NS | −0.106 | NS |
| VIR IL6 (µm2) | −0.124 | NS | −0.337 | <0.01 |
| VIR TNFα (µm2) | 0.076 | NS | −0.177 | NS |
| Log KL mRNA (a.u.) | 0.125 | NS | ||
| Log ADAM17 mRNA (a.u.) | 0.183 | NS | 0.369 | <0.01 |
| Log IL6 mRNA (a.u.) | −0.81 | NS | 0.133 | NS |
| Log IL10 mRNA (a.u.) | −0.069 | NS | 0.223 | NS |
| Log TNF mRNA (a.u.) | 0.069 | NS | 0.183 | NS |
| Serum Klotho Concentrations | ||
| Serum hsCRP | r = −0.30 | p < 0.05 |
| Serum IL6 | r = −0.23 | p = 0.10 |
| Serum IL10 | r = 0.20 | p = 0.17 |
| Serum TNFα | r = −0.12 | p = 0.39 |
| Vascular mRNA KL Expression Levels | ||
| Expression ADAM17 | r = 0.41 | p = 0.001 |
| Expression IL6 | r = −0.31 | p < 0.05 |
| Expression IL10 | r = 0.19 | p = 0.20 |
| Expression TNF | r = 0.03 | p = 0.81 |
| Vascular Klotho Immunoreactivity Levels | ||
| Immunoreactivity IL6 | r = −0.29 | p < 0.05 |
| Immunoreactivity TNFα | r = −0.06 | p = 0.64 |
| Model 1 | ||
| Independent Variable | Odds Ratio (95% CI) | p-Value |
| Age | 1.03 (0.97 to 1.10) | 0.28 |
| Gender | 0.59 (0.13 to 2.78) | 0.51 |
| Uric acid | 0.96 (0.64 to 1.45) | 0.87 |
| eGFR | 1.05 (0.97 to 1.03) | 0.72 |
| hsCRP | 0.99 (0.93 to 1.06) | 0.95 |
| Model 2 (Model 1 + Serum Klotho, FGF23, IL6 and IL10) | ||
| Independent Variable | Odds Ratio (95% CI) | p-Value |
| Age | 1.00 (0.91 to 1.10) | 0.82 |
| Gender | 0.75 (0.08 to 7.04) | 0.80 |
| Uric acid | 1.09 (0.57 to 2.08) | 0.78 |
| eGFR | 1.01 (0.97 to 1.05) | 0.49 |
| hsCRP | 0.97 (0.89 to 1.07) | 0.64 |
| Serum Klotho | 0.99 (0.98 to 0.99) | <0.01 |
| Serum FGF23 | 1.10 (1.05 to 1.21) | <0.05 |
| Serum IL6 | 1.00 (0.98 to 1.01) | 0.88 |
| Serum IL10 | 1.06 (0.85 to 1.32) | 0.60 |
| Model 3 (Model 2 + Soluble Klotho) | ||
| Independent Variable | Odds Ratio (95% CI) | p-Value |
| Age | 0.91 (0.78 to 1.06) | 0.23 |
| Gender | 4.98 (0.17 to 14.63) | 0.35 |
| Uric acid | 0.64 (0.27 to 1.50) | 0.31 |
| eGFR | 1.04 (0.98 to 1.12) | 0.15 |
| hsCRP | 1.16 (0.93 to 1.33) | 0.23 |
| Serum Klotho | 0.99 (0.98 to 0.99) | <0.05 |
| Serum FGF23 | 1.22 (1.02 to 1.47) | <0.05 |
| Serum IL6 | 0.99 (0.97 to 1.01) | 0.53 |
| Serum IL10 | 0.97 (0.77 to 1.23) | 0.83 |
| VIR Klotho | 1.00 (0.99 to 1.00) | 0.06 |
| KL gene expression | 0.66 (0.43 to 0.99) | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Hernández-Carballo, C.; Tagua, V.G.; Delgado-Molinos, A.; López-Castillo, Á.; Rodríguez-Ramos, S.; Cerro-López, P.; López-Tarruella, V.C.; et al. FGF23 and Klotho Levels are Independently Associated with Diabetic Foot Syndrome in Type 2 Diabetes Mellitus. J. Clin. Med. 2019, 8, 448. https://doi.org/10.3390/jcm8040448
Donate-Correa J, Martín-Núñez E, Ferri C, Hernández-Carballo C, Tagua VG, Delgado-Molinos A, López-Castillo Á, Rodríguez-Ramos S, Cerro-López P, López-Tarruella VC, et al. FGF23 and Klotho Levels are Independently Associated with Diabetic Foot Syndrome in Type 2 Diabetes Mellitus. Journal of Clinical Medicine. 2019; 8(4):448. https://doi.org/10.3390/jcm8040448
Chicago/Turabian StyleDonate-Correa, Javier, Ernesto Martín-Núñez, Carla Ferri, Carolina Hernández-Carballo, Víctor G. Tagua, Alejandro Delgado-Molinos, Ángel López-Castillo, Sergio Rodríguez-Ramos, Purificación Cerro-López, Victoria Castro López-Tarruella, and et al. 2019. "FGF23 and Klotho Levels are Independently Associated with Diabetic Foot Syndrome in Type 2 Diabetes Mellitus" Journal of Clinical Medicine 8, no. 4: 448. https://doi.org/10.3390/jcm8040448
APA StyleDonate-Correa, J., Martín-Núñez, E., Ferri, C., Hernández-Carballo, C., Tagua, V. G., Delgado-Molinos, A., López-Castillo, Á., Rodríguez-Ramos, S., Cerro-López, P., López-Tarruella, V. C., Arévalo-González, M. A., Pérez-Delgado, N., Mora-Fernández, C., & Navarro-González, J. F. (2019). FGF23 and Klotho Levels are Independently Associated with Diabetic Foot Syndrome in Type 2 Diabetes Mellitus. Journal of Clinical Medicine, 8(4), 448. https://doi.org/10.3390/jcm8040448






