Ultrasound Tissue Characterization of Carotid Plaques Differs Between Patients with Type 1 Diabetes and Subjects without Diabetes
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Carotid Ultrasound Imaging
2.3. Statistical Analysis
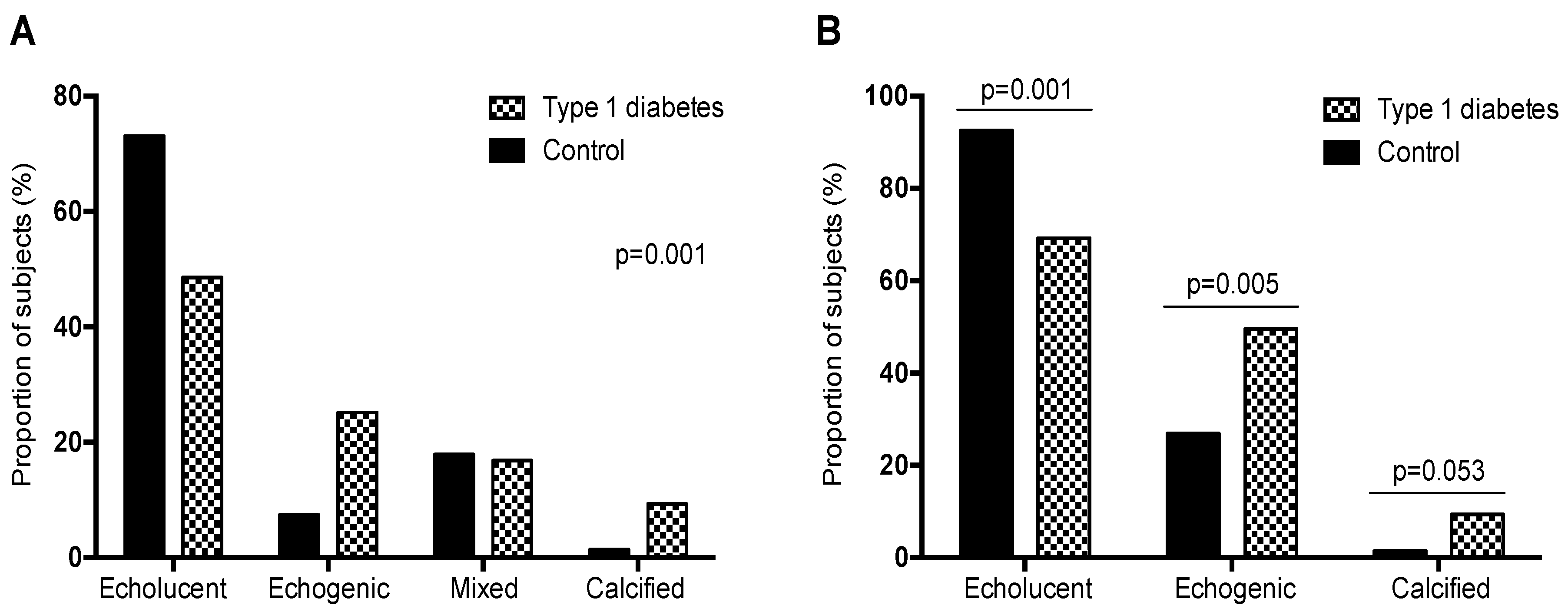
3. Results
3.1. Ultrasound Examination
3.2. Measurements of the Plaque Volume
3.3. Characteristics of Patients with Echogenic Plaques
3.4. Logistic Regression Model of Echogenic Plaques
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Non-Echogenic Plaque | Echogenic | p-Value Plaque |
|---|---|---|---|
| n = 103 | n = 71 | ||
| Age, years | 51.2 (9.22) | 56.7 (9.11) | <0.001 |
| Sex, women, n (%) | 52 (50.5%) | 29 (40.8%) | 0.272 |
| Race, non Caucasian, n (%) | 2 (1.94%) | 0 (0.00%) | 0.514 |
| Diabetes mellitus type 1, n (%) | 54 (52.4%) | 53 (74.6%) | 0.005 |
| Diabetes duration, years | 22.2 (11.8) | 27.2 (11.8) | 0.030 |
| Tobacco exposure, n (%) | 58 (56.9%) | 47 (66.2%) | 0.281 |
| Hypertension, n (%) | 33 (32.0%) | 40 (56.3%) | 0.002 |
| Dyslipidaemia, n (%) | 47 (45.6%) | 43 (60.6%) | 0.075 |
| BMI, kg/m2 | 27 [25;29.9] | 26.7 [24.2;29.8] | 0.424 |
| Waist, cm | 96.0 (12.0) | 94.5 (14.6) | 0.469 |
| Systolic blood pressure, mmHg | 132 (17.4) | 139 (15.5) | 0.004 |
| Diastolic blood pressure, mmHg | 76.3 (10.2) | 76.9 (11.3) | 0.730 |
| Heart rate, beat/min | 74.1 (12.8) | 75.6 (10.6) | 0.391 |
| Antiplatelet treatment, n (%) | 23 (29.1%) | 33 (57.9%) | 0.001 |
| Statin treatment, n (%) | 43 (41.7%) | 41 (57.7%) | 0.055 |
| Glucose, mg/dL | 99 [87.5; 140] | 127 [88; 211] | 0.034 |
| HbA1c, % | 6.7 [5.5; 7.6] | 7.40 [6.3; 7.9] | 0.006 |
| HbA1c, mmol/mol | 49.7 [36.6; 60] | 57 [45; 63] | 0.006 |
| Triglycerides, mg/dL | 74 [59; 106] | 81 [60.5; 126] | 0.313 |
| Total cholesterol, mg/dL | 192 [170; 218] | 178 [160; 202] | 0.016 |
| HDL, mg/dL | 58 [49.5; 70] | 56 [47.5; 68.5] | 0.296 |
| LDL, mg/dL | 112 [92.5; 141] | 107 [82.5; 120] | 0.036 |
| Creatinine, mg/dL | 0.8 [0.67; 0.9] | 0.79 [0.66; 0.92] | 0.756 |
| GFR, mL/min/1.73 m2 | 97.6 [87.4; 106] | 96 [86.8; 104] | 0.485 |
| Plaque, n (%) | <0.001 | ||
| One plaque | 67 (65%) | 18 (25.4%) | |
| Multiple plaques | 36 (35%) | 53 (74.6%) |

References
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Articles Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography carotid intima-media thickness task force endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [PubMed]
- Baber, U.; Mehran, R.; Sartori, A.; Schoos, M.M.; Sillesen, H.; Muntendam, P.; Garcia, M.J.; Gregson, J.; Pocock, S.; Falk, E.; et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults. J. Am. Coll. Cardiol. 2015, 65, 1065–1074. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis 2012, 220, 128–133. [Google Scholar] [CrossRef]
- Catalan, M.; Herreras, Z.; Pinyol, M.; Sala-Vila, A.; Amor, A.J.; de Groot, E.; Gilabert, R.; Ros, E.; Ortega, E. Prevalence by sex of preclinical carotid atherosclerosis in newly diagnosed type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, M.; Castelblanco, E.; Valldeperas, X.; Betriu, À.; Traveset, A.; Granado-Casas, M.; Hernández, M.; Vázquez, F.; Martín, M.; Rubinat, E.; et al. Diabetic retinopathy is associated with the presence and burden of subclinical carotid atherosclerosis in type 1 diabetes. Cardiovas. Diabetol. 2018, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Grønholdt, M.-L.M. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arter. Thromb. Vasc. Biol. 1999, 19, 2–13. [Google Scholar] [CrossRef]
- Gray-Weale, A.C.; Graham, J.C.; Burnett, J.R.; Byrne, K.; Lusby, R.J. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. 1988, 29, 676–681. [Google Scholar]
- Mitchell, C.C.; Stein, J.H.; Cook, T.D.; Salamat, S.; Wang, X.; Varghese, T.; Jackson, D.C.; Sandoval Garcia, C.; Wilbrand, S.M.; Dempsey, R.J. Histopathological Validation of Grayscale Carotid Plaque Characteristics Related to Plaque Vulnerability. Ultrasound Med. Biol. 2017, 43, 129–137. [Google Scholar] [CrossRef]
- Mathiesen, E.B.; Bønaa, K.H.; Joakimsen, O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: The tromsø study. Circulation 2001, 103, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Grønholdt, M.-L.M.; Nordestgaard, B.G.; Schroeder, T.; Vorstrup, S.; Sillesen, H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation 2001, 104, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Morgan, T.; Herrington, D.M.; Xu, J.; Cox, A.J.; Freedman, B.I.; Carr, J.J.; Bowden, D.W. Coronary calcium score and prediction of all-cause mortality in diabetes: The diabetes heart study. Diabetes Care 2011, 34, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Hsu, F.-C.; Agarwal, S.; Freedman, B.I.; Herrington, D.M.; Carr, J.J.; Bowden, D.W. Prediction of mortality using a multi-bed vascular calcification score in the Diabetes Heart Study. Cardiovasc. Diabetol. 2014, 13, 160. [Google Scholar] [CrossRef]
- Allison, M.A.; Hsi, S.; Wassel, C.L.; Morgan, C.; Ix, J.H.; Wright, C.M.; Criqui, M.H. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arter. Thromb. Vasc. Biol. 2011, 32, 140–146. [Google Scholar] [CrossRef]
- Katakami, N.; Takahara, M.; Kaneto, H.; Sakamoto, K.; Yoshiuchi, K.; Irie, Y.; Kubo, F.; Katsura, T.; Yamasaki, Y.; Kosugi, K.; et al. Ultrasonic tissue characterization of carotid plaque improves the prediction of cardiovascular events in diabetic patients: A pilot study. Diabetes Care 2012, 35, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Katakami, N.; Kaneto, H.; Takahara, M.; Nishio, M.; Kasami, R.; Sakamoto, K.; Umayahara, Y.; Sumitsuji, S.; Ueda, Y.; et al. The utility of ultrasonic tissue characterization of carotid plaque in the prediction of cardiovascular events in diabetic patients. Atherosclerosis 2013, 230, 399–405. [Google Scholar] [CrossRef]
- Vigili de Kreutzenberg, S.; Fadini, G.P.; Guzzinati, S.; Mazzucato, M.; Volpi, A.; Coracina, A.; Avogaro, A. Carotid plaque calcification predicts future cardiovascular events in type 2 diabetes. Diabetes Care 2015, 38, 1937–1944. [Google Scholar] [CrossRef]
- Alonso, N.; Traveset, A.; Rubinat, E.; Ortega, E.; Alcubierre, N.; Sanahuja, J.; Hernández, M.; Betriu, A.; Jurjo, C.; Fernández, E.; et al. Type 2 diabetes-associated carotid plaque burden is increased in patients with retinopathy compared to those without retinopathy. Cardiovasc. Diabetol. 2015, 14, 33–39. [Google Scholar] [CrossRef]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez, R.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011). Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Ludwig, M.; Zielinski, T.; Schremmer, D.; Stumpe, K.O. Reproducibility of 3-dimensional ultrasound readings of volume of carotid atherosclerotic plaque. Cardiovas. Ultrasound 2008, 26, 42. [Google Scholar] [CrossRef]
- European Carotid Plaque Study Group. Carotid Artery Plaque Composition—Relationship to Clinical Presentation and Ultrasound B-mode Imaging. Eur. J. Vasc. Endovasc. Surg. 1995, 10, 23–30. [Google Scholar] [CrossRef]
- Mayor, I.; Momjian, S.; Lalive, P.; Sztajzel, R. Carotid plaque: Comparison between visual and grey-scale median analysis. Ultrasound Med. Biol. 2003, 29, 961–966. [Google Scholar] [CrossRef]
- Larsen, J.R.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Brekke, M.; Arnesen, H.; Hanssen, K.F.; Nissen, S.E.; Dahl-Jorgensen, K. Intracoronary ultrasound examinations reveal significantly more advanced coronary atherosclerosis in people with type 1 diabetes than in age- and sex-matched non-diabetic controls. Diab. Vasc. Dis. Res. 2007, 4, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Costacou, T.; Edmundowicz, D.; Prince, C.; Conway, B.; Orchard, T.J. Progression of coronary artery calcium in type 1 diabetes mellitus. Am. J. Cardiol. 2007, 100, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Szklo, M.; Kronmal, R.A.; Burke, G.L.; Shea, S.; Zavodni, A.E.; O’Leary, D.H. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: The multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000087. [Google Scholar] [CrossRef] [PubMed]
- Djaberi, R.; Schuijf, J.D.; Boersma, E.; Kroft, L.J.; Pereira, A.M.; Romijn, J.A.; Scholte, A.J.; Jukema, J.W.; Bax, J.J. Differences in atherosclerotic plaque burden and morphology between type 1 and 2 diabetes as assessed by multislice computed tomography. Diabetes Care 2009, 32, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Zavodni, A.E.H.; Wasserman, B.A.; McClelland, R.L.; Gomes, A.S.; Folsom, A.R.; Polak, J.F.; Lima, J.A.; Bluemke, D.A. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2014, 271, 381–389. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Singh, R.; Zhou, X.; Ramas, R.; Sacco, R.L.; Rundek, T. Presence of calcified carotid plaque predicts vascular events: The Northern Manhattan Study. Atherosclerosis 2007, 195, e197–e201. [Google Scholar] [CrossRef]
- Hunt, K.J.; Evans, G.W.; Folsom, A.R.; Sharrett, A.R.; Chambless, L.E.; Tegeler, C.H.; Heiss, G. Acoustic shadowing on B-mode ultrasound of the carotid artery predicts ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) study. Stroke 2001, 32, 1120–1126. [Google Scholar] [CrossRef]
- Wilson, P.W.; Kauppila, L.I.; O’Donnell, C.J.; Kiel, D.P.; Hannan, M.; Polak, J.M.; Cupples, L.A. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001, 103, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Nonin, S.; Iwata, S.; Sugioka, K.; Fujita, S.; Norioka, N.; Ito, A.; Nakagawa, M.; Yoshiyama, M. Plaque surface irregularity and calcification length within carotid plaque predict secondary events in patients with coronary artery disease. Atherosclerosis 2017, 256, 29–34. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [PubMed]
- Lin, R.; Chen, S.; Liu, G.; Xue, Y.; Zhao, X. Association between carotid atherosclerotic plaque calcification and intraplaque hemorrhage A Magnetic Resonance Imaging Study. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1228–1233. [Google Scholar] [CrossRef]
- Beckman, J.A.; Ganz, J.; Creager, M.A.; Ganz, P.; Kinlay, S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, J.; Stroh, C.I.; Tenenbaum, A.; Hod, H.; Boyko, V.; Fisman, E.Z.; Motro, M. Comparison of coronary calcium in stable angina pectoris and in first acute myocardial infarction utilizing double helical computerized tomography. Am. J. Cardiol. 1998, 81, 271–275. [Google Scholar] [CrossRef]
- Ehara, S. Spotty Calcification typifies the culprit plaque in patients with acute myocardial infarction: An Intravascular Ultrasound Study. Circulation 2004, 110, 3424–3429. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, N.; Kelly-Arnold, A.; Vengrenyuk, Y.; Laudier, D.; Fallon, J.T.; Virmani, R.; Cardoso, L.; Weinbaum, S. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: Potential implications for plaque rupture. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H619–H628. [Google Scholar] [CrossRef]
- Lee, S.E.; Chang, H.J.; Sung, J.M.; Park, H.B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM (Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging) Study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef]
- Puri, R.; Libby, P.; Nissen, S.E.; Wolski, K.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.; Raichlen, J.S.; Uno, K.; et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: Insights from SATURN. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 380–388. [Google Scholar] [CrossRef]
| Non-Diabetic Group (n = 67) | Type 1 Diabetes (n = 107) | p-Value | |
|---|---|---|---|
| Age, years | 52.6 (9.13) | 54 (9.8) | 0.351 |
| Sex, women, n (%) | 28 (41.8%) | 53 (49.5%) | 0.401 |
| Race, non-Caucasian, n (%) | 2 (3%) | 0 (0%) | 0.147 |
| Tobacco exposure, n (%) | 35 (53%) | 70 (65.4%) | 0.144 |
| Antiplatelet treatment, n (%) | 3 (10.3%) | 53 (49.5%) | <0.001 |
| Dyslipidaemia, n (%) | 20 (29.9%) | 78 (72.9%) | <0.001 |
| Hypertension, n (%) | 13 (19.4%) | 60 (56.1%) | <0.001 |
| Systolic blood pressure, mmHg | 131 (14.2) | 137 (18) | 0.010 |
| Diastolic blood pressure, mmHg | 79.6 (8) | 74.7 (11.6) | 0.001 |
| Statin treatment, n (%) | 8 (11.9%) | 76 (71%) | <0.001 |
| Heart rate, beats/min | 71 (11.1) | 76.8 (11.9) | 0.002 |
| Body mass index, kg/m2 | 27.8 [25; 30.3] | 26.4 [24.3; 29.6] | 0.124 |
| Waist, cm | 99.6 (12.8) | 92.9 (12.7) | 0.001 |
| HbA1c, % | 5.5 [5.3; 5.7] | 7.6 [7.15; 8.15] | <0.001 |
| HbA1c, mmol/mol | 36.6 [34; 39] | 60 [54; 65.5] | <0.001 |
| Total cholesterol, mg/dL | 203 [185; 228] | 173 [155; 198] | <0.001 |
| HDL cholesterol, mg/dL | 54 [46; 63] | 59 [52; 70] | 0.005 |
| LDL cholesterol, mg/dL | 124 [112; 148] | 97.4 [80.6; 113] | <0.001 |
| Triglycerides, mg/dL | 99 [65; 142] | 71 [57; 90] | 0.001 |
| Creatinine, mg/dL | 0.81 [0.7; 0.9] | 0.79 [0.7; 0.9] | 0.166 |
| GFR, mL/min/1.73 m2 | 97.9 [85.6; 105] | 96.1 [88.3; 105] | 0.899 |
| Diabetes duration, years | - | 24.7 (12) | - |
| Plaque | 0.581 | ||
| One plaque | 35 (52.2%) | 50 (46.7%) | |
| Multiple plaques (≥2) | 32 (47.8%) | 57 (53.3%) |
| Presence of any Echogenic Plaque vs. No Echogenic Plaque a | |||
|---|---|---|---|
| Odds Ratio | 95% CI | p | |
| Age | 1.062 | 1.023–1.103 | 0.002 |
| Sex, women | 0.539 | 0.272–1.068 | 0.076 |
| BMI | 0.949 | 0.872–1.032 | 0.221 |
| Diabetes | 2.276 | 1.104–4.690 | 0.026 |
| sBP | 1.019 | 0.998–1.040 | 0.075 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelblanco, E.; Betriu, À.; Hernández, M.; Granado-Casas, M.; Ortega, E.; Soldevila, B.; Ramírez-Morros, A.; Franch-Nadal, J.; Puig-Domingo, M.; Fernández, E.; et al. Ultrasound Tissue Characterization of Carotid Plaques Differs Between Patients with Type 1 Diabetes and Subjects without Diabetes. J. Clin. Med. 2019, 8, 424. https://doi.org/10.3390/jcm8040424
Castelblanco E, Betriu À, Hernández M, Granado-Casas M, Ortega E, Soldevila B, Ramírez-Morros A, Franch-Nadal J, Puig-Domingo M, Fernández E, et al. Ultrasound Tissue Characterization of Carotid Plaques Differs Between Patients with Type 1 Diabetes and Subjects without Diabetes. Journal of Clinical Medicine. 2019; 8(4):424. https://doi.org/10.3390/jcm8040424
Chicago/Turabian StyleCastelblanco, Esmeralda, Àngels Betriu, Marta Hernández, Minerva Granado-Casas, Emilio Ortega, Berta Soldevila, Anna Ramírez-Morros, Josep Franch-Nadal, Manel Puig-Domingo, Elvira Fernández, and et al. 2019. "Ultrasound Tissue Characterization of Carotid Plaques Differs Between Patients with Type 1 Diabetes and Subjects without Diabetes" Journal of Clinical Medicine 8, no. 4: 424. https://doi.org/10.3390/jcm8040424
APA StyleCastelblanco, E., Betriu, À., Hernández, M., Granado-Casas, M., Ortega, E., Soldevila, B., Ramírez-Morros, A., Franch-Nadal, J., Puig-Domingo, M., Fernández, E., Avogaro, A., Alonso, N., & Mauricio, D. (2019). Ultrasound Tissue Characterization of Carotid Plaques Differs Between Patients with Type 1 Diabetes and Subjects without Diabetes. Journal of Clinical Medicine, 8(4), 424. https://doi.org/10.3390/jcm8040424






