Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty
Abstract
1. Introduction
2. Experimental Section
2.1. Ethics Statement
2.2. Patient Recruitment and Survey
2.3. Blood Sampling
2.4. Cytokine Array
2.5. Enzyme Linked Immunosorbent Assay (ELISA)
2.6. Statistics
2.7. Data Availability
3. Results
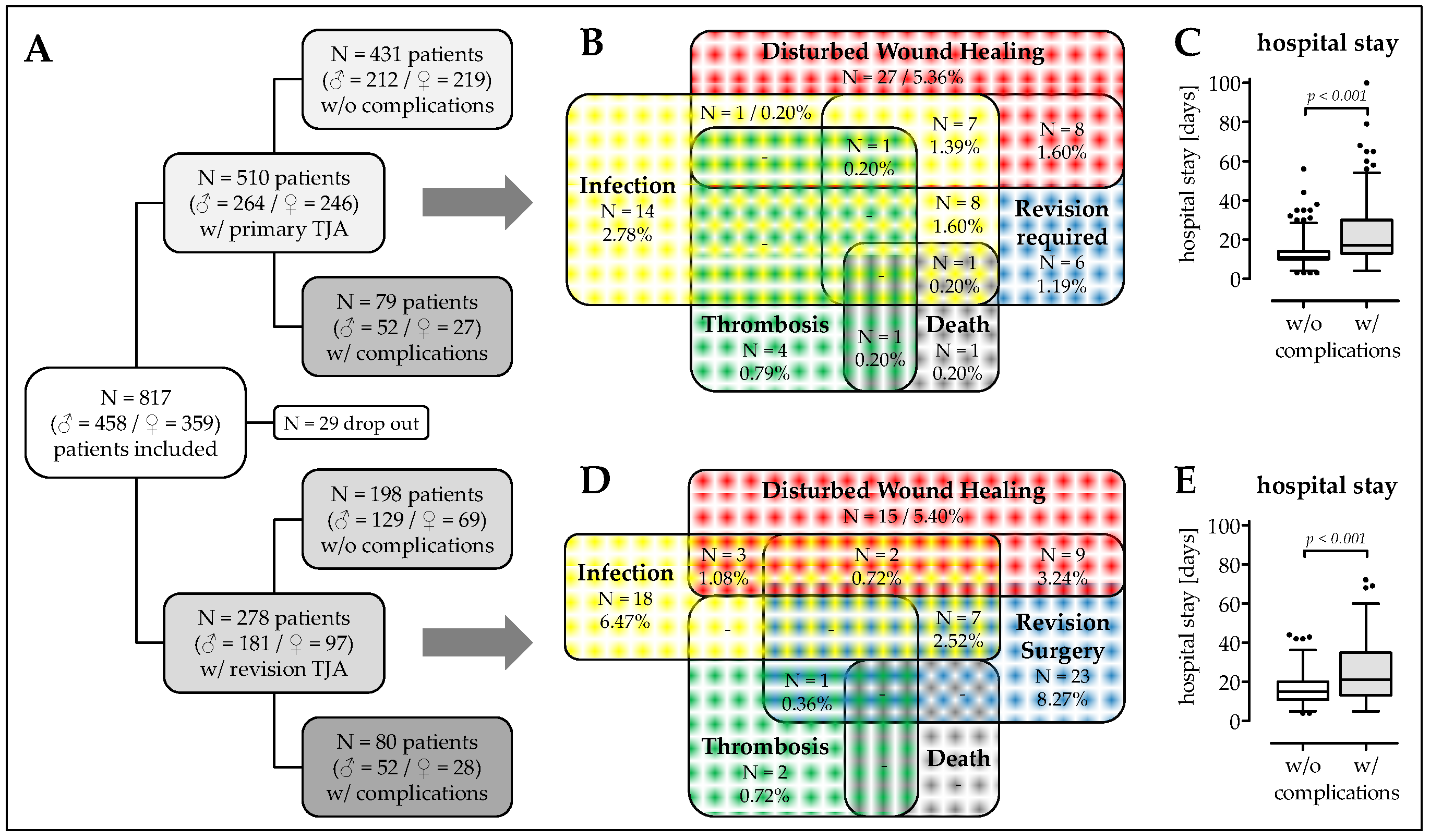
3.1. Patient Recruitment and Description of The Study Cohort
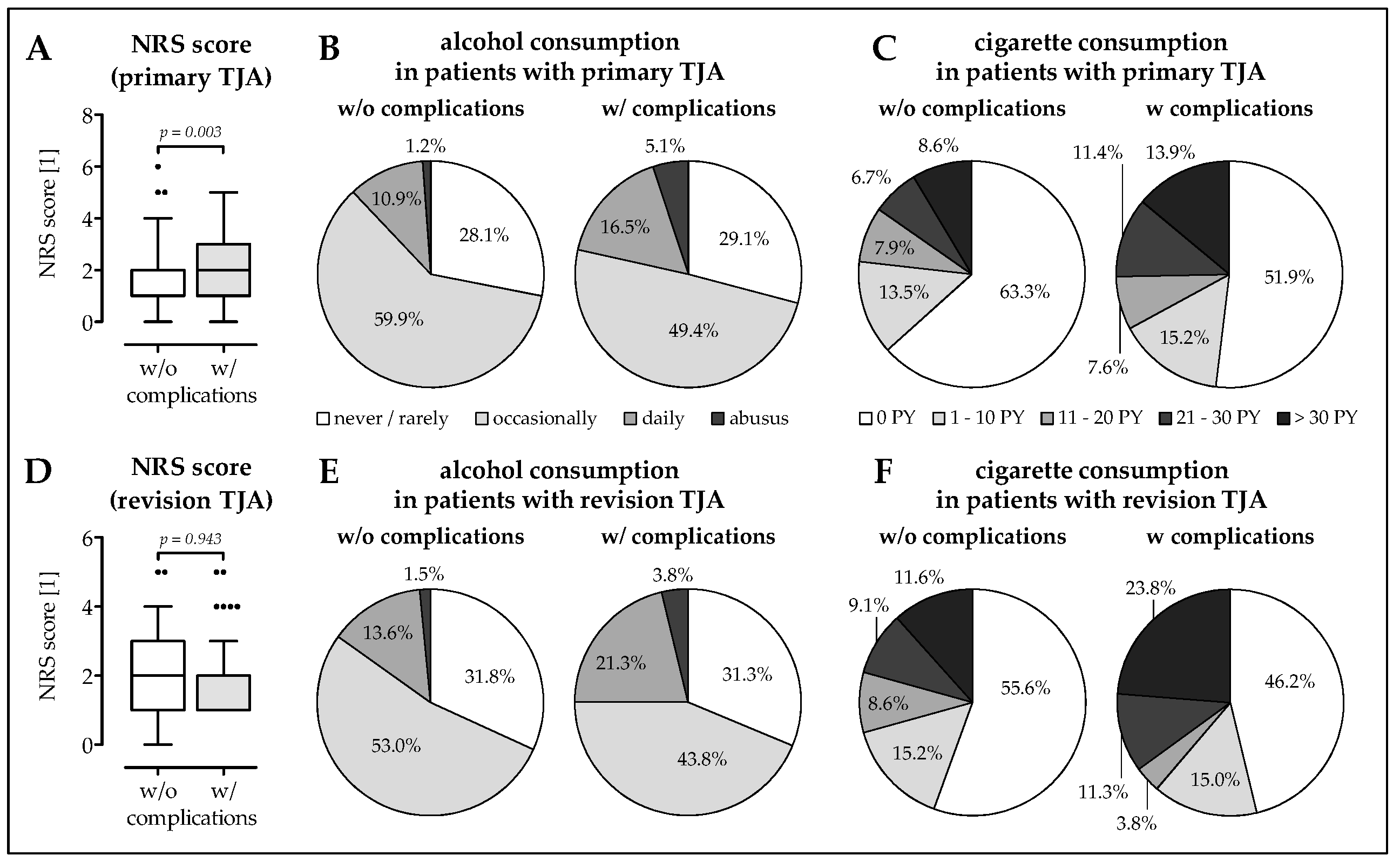
3.2. Higher Frequency of Malnourished Patients and Smokers in The Complication Group
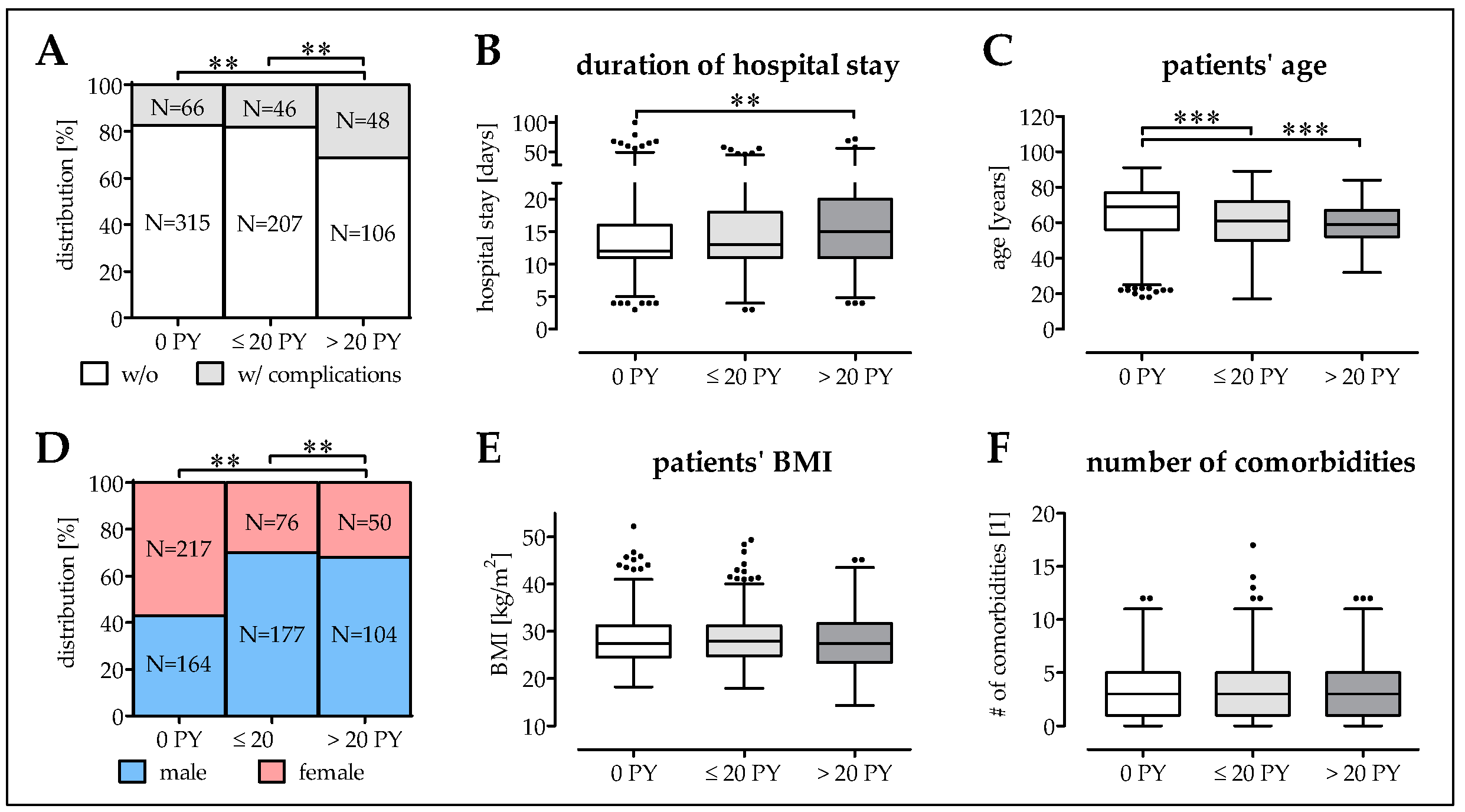
3.3. Smokers Have More Complications at a Younger Age
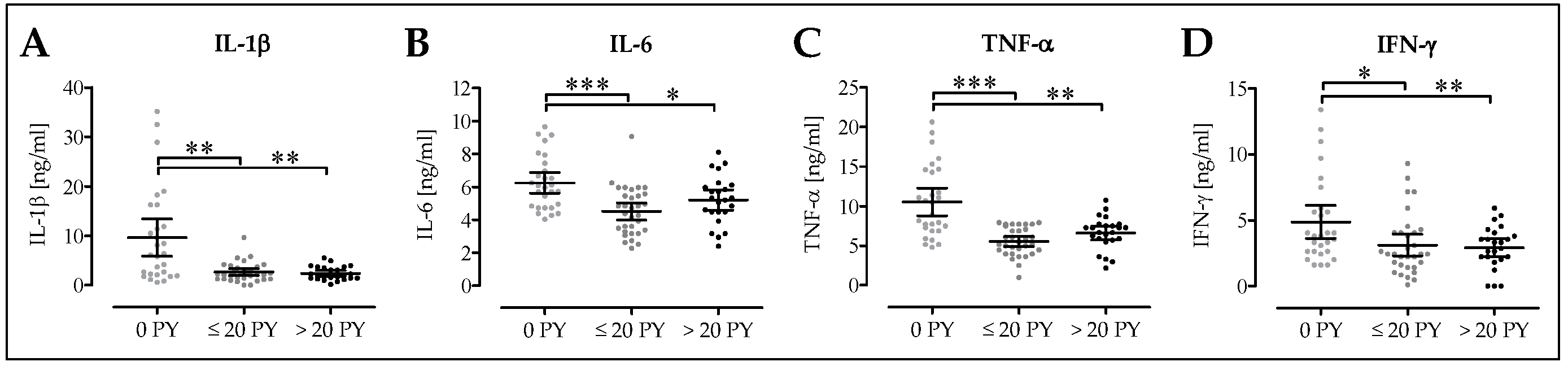
3.4. Cytokine Levels are Altered in Smokers’ Blood
3.5. Decreased Osteoblast Activity in Smokers Following Surgery
3.6. Pro-Inflammatory Cytokine Levels are Decreased in Smokers Following Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniell, H.W. Osteoporosis of the slender smoker. Vertebral compression fractures and loss of metacarpal cortex in relation to postmenopausal cigarette smoking and lack of obesity. Arch. Intern. Med. 1976, 136, 298–304. [Google Scholar] [CrossRef]
- Benson, B.W.; Shulman, J.D. Inclusion of tobacco exposure as a predictive factor for decreased bone mineral content. Nicotine Tob. Res. 2005, 7, 719–724. [Google Scholar] [CrossRef]
- Rudang, R.; Darelid, A.; Nilsson, M.; Nilsson, S.; Mellstrom, D.; Ohlsson, C.; Lorentzon, M. Smoking is associated with impaired bone mass development in young adult men: A 5-year longitudinal study. J. Bone Miner. Res. 2012, 27, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Sloan, A.; Hussain, I.; Maqsood, M.; Eremin, O.; El-Sheemy, M. The effects of smoking on fracture healing. Surgeon 2010, 8, 111–116. [Google Scholar] [CrossRef]
- Scolaro, J.A.; Schenker, M.L.; Yannascoli, S.; Baldwin, K.; Mehta, S.; Ahn, J. Cigarette smoking increases complications following fracture: A systematic review. J. Bone Joint Surg. Am. 2014, 96, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.E.; Carstensen, S.E.; Moore, S.; Dacus, A.R. Smoking increases postoperative complications after distal radius fracture fixation: A review of 417 patients from a level 1 trauma center. Hand (N Y) 2018. [Google Scholar] [CrossRef]
- Pearson, R.G.; Clement, R.G.; Edwards, K.L.; Scammell, B.E. Do smokers have greater risk of delayed and non-union after fracture, osteotomy and arthrodesis? A systematic review with meta-analysis. BMJ Open 2016, 6, e010303. [Google Scholar] [CrossRef]
- Abate, M.; Vanni, D.; Pantalone, A.; Salini, V. Cigarette smoking and musculoskeletal disorders. Muscles Ligaments Tendons J. 2013, 3, 63–69. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; De Laet, C.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; McCloskey, E.V.; Mellstrom, D.; et al. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef]
- Behfarnia, P.; Saied-Moallemi, Z.; Javanmard, S.H.; Naseri, R. Serum, saliva, and gcf concentration of rankl and osteoprotegerin in smokers versus nonsmokers with chronic periodontitis. Adv. Biomed. Res. 2016, 5, 80. [Google Scholar]
- Bostrom, E.A.; Kindstedt, E.; Sulniute, R.; Palmqvist, P.; Majster, M.; Holm, C.K.; Zwicker, S.; Clark, R.; Onell, S.; Johansson, I.; et al. Increased eotaxin and mcp-1 levels in serum from individuals with periodontitis and in human gingival fibroblasts exposed to pro-inflammatory cytokines. PLoS One 2015, 10, e0134608. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N. The rankl-opg system in clinical periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ozcaka, O.; Nalbantsoy, A.; Kose, T.; Buduneli, N. Plasma osteoprotegerin levels are decreased in smoker chronic periodontitis patients. Aust. Dent. J. 2010, 55, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Buduneli, E.; Kutukculer, N. Interleukin-17, rankl, and osteoprotegerin levels in gingival crevicular fluid from smoking and non-smoking patients with chronic periodontitis during initial periodontal treatment. J. Periodontol. 2009, 80, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Biyikoglu, B.; Sherrabeh, S.; Lappin, D.F. Saliva concentrations of rankl and osteoprotegerin in smoker versus non-smoker chronic periodontitis patients. J. Clin. Periodontol. 2008, 35, 846–852. [Google Scholar] [CrossRef]
- Lappin, D.F.; Sherrabeh, S.; Jenkins, W.M.; Macpherson, L.M. Effect of smoking on serum rankl and opg in sex, age and clinically matched supportive-therapy periodontitis patients. J. Clin. Periodontol. 2007, 34, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and cotinine inhibit catalase and glutathione reductase activity contributing to the impaired osteogenesis of scp-1 cells exposed to cigarette smoke. Oxid. Med. Cell Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, V.; Aspera-Werz, R.; Ehnert, S.; Strobel, J.; Tendulkar, G.; Heid, D.; Schreiner, A.; Arnscheidt, C.; Nussler, A.K. Resveratrol protects primary cilia integrity of human mesenchymal stem cells from cigarette smoke to improve osteogenic differentiation in vitro. Arch. Toxicol. 2018, 92, 1525–1538. [Google Scholar] [CrossRef]
- Ehnert, S.; Braun, K.F.; Buchholz, A.; Freude, T.; Egana, J.T.; Schenck, T.L.; Schyschka, L.; Neumaier, M.; Dobele, S.; Stockle, U.; et al. Diallyl-disulphide is the effective ingredient of garlic oil that protects primary human osteoblasts from damage due to cigarette smoke. Food Chem. 2012, 132, 724–729. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (mcp-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Morrison, N.A.; Day, C.J.; Nicholson, G.C. Dominant negative mcp-1 blocks human osteoclast differentiation. J. Cell Biochem. 2014, 115, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ninomiya, K.; Sonoda, K.H.; Miyauchi, Y.; Hoshi, H.; Iwasaki, R.; Miyamoto, H.; Yoshida, S.; Sato, Y.; Morioka, H.; et al. Mcp-1 expressed by osteoclasts stimulates osteoclastogenesis in an autocrine/paracrine manner. Biochem. Biophys. Res. Commun. 2009, 383, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Day, C.J.; Morrison, N.A. Mcp-1 is induced by receptor activator of nuclear factor-{kappa}b ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J. Biol. Chem. 2005, 280, 16163–16169. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.A.; Hashimi, S.M.; Bakr, M.M.; Forwood, M.R.; Morrison, N.A. Ccl2 and ccr2 are essential for the formation of osteoclasts and foreign body giant cells. J. Cell. Biochem. 2016, 117, 382–389. [Google Scholar] [CrossRef]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stockle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial role of vitamin d in the musculoskeletal system. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin d endocrine system and osteoclasts. Bonekey Rep. 2014, 3, 495. [Google Scholar] [CrossRef]
- Wang, Y.C.; Hsieh, C.C.; Kuo, H.F.; Tsai, M.K.; Yang, S.N.; Kuo, C.H.; Lee, M.S.; Hung, C.H. Effect of vitamin d3 on monocyte chemoattractant protein 1 production in monocytes and macrophages. Acta Cardiol. Sin. 2014, 30, 144–150. [Google Scholar]
- Baldock, P.A.; Thomas, G.P.; Hodge, J.M.; Baker, S.U.; Dressel, U.; O’Loughlin, P.D.; Nicholson, G.C.; Briffa, K.H.; Eisman, J.A.; Gardiner, E.M. Vitamin d action and regulation of bone remodeling: Suppression of osteoclastogenesis by the mature osteoblast. J. Bone Miner. Res. 2006, 21, 1618–1626. [Google Scholar] [CrossRef]
- Knapik, J.J.; Bedno, S.A. Epidemiological evidence and possible mechanisms for the association between cigarette smoking and injuries (part 1). J. Spec. Oper. Med. 2018, 18, 108–112. [Google Scholar]
- Bon, J.; Zhang, Y.; Leader, J.K.; Fuhrman, C.; Perera, S.; Chandra, D.; Bertolet, M.; Diergaarde, B.; Greenspan, S.L.; Sciurba, F.C. Radiographic emphysema, circulating bone biomarkers, and progressive bone mineral density loss in smokers. Ann. Am. Thorac. Soc. 2018, 15, 615–621. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin d: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Javed, F.; Al-Kheraif, A.A.; Al Amri, M.D.; Alshehri, M.; Vohra, F.; Al-Askar, M.; Malmstrom, H.; Romanos, G.E. Periodontal status and whole salivary cytokine profile among smokers and never-smokers with and without prediabetes. J. Periodontol. 2015, 86, 890–898. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakanishi, K.; Yoneda, M.; Hirofuji, T.; Hanioka, T. Relationship between salivary stress biomarker levels and cigarette smoking in healthy young adults: An exploratory analysis. Tob. Induc. Dis. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Bogner, P.N.; Thatcher, T.H.; Sime, P.J.; Phipps, R.P.; Thanavala, Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J. Immunol. 2014, 192, 5226–5235. [Google Scholar] [CrossRef]
- Goncalves, R.B.; Coletta, R.D.; Silverio, K.G.; Benevides, L.; Casati, M.Z.; da Silva, J.S.; Nociti, F.H., Jr. Impact of smoking on inflammation: Overview of molecular mechanisms. Inflamm. Res. 2011, 60, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cowan, M.J.; Hasday, J.D.; Vogel, S.N.; Medvedev, A.E. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of il-1r-associated kinase, p38, and nf-kappab in alveolar macrophages stimulated with tlr2 and tlr4 agonists. J. Immunol. 2007, 179, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Ihle, C.; Freude, T.; Bahrs, C.; Zehendner, E.; Braunsberger, J.; Biesalski, H.K.; Lambert, C.; Stockle, U.; Wintermeyer, E.; Grunwald, J.; et al. Malnutrition - an underestimated factor in the inpatient treatment of traumatology and orthopedic patients: A prospective evaluation of 1055 patients. Injury 2017, 48, 628–636. [Google Scholar] [CrossRef]
- Ihle, C.; Bahrs, C.; Freude, T.; Bickel, M.; Spielhaupter, I.; Wintermeyer, E.; Stollhof, L.; Grunwald, L.; Ziegler, P.; Pscherer, S.; et al. Malnutrition in elderly trauma patients - comparison of two assessment tools. Z. Orthop. Unfall. 2017, 155, 184–193. [Google Scholar]
- Hitchman, S.C.; Fong, G.T. Gender empowerment and female-to-male smoking prevalence ratios. Bull. World Health Organ. 2011, 89, 195–202. [Google Scholar] [CrossRef]
- Kapasa, E.R.; Giannoudis, P.V.; Jia, X.; Hatton, P.V.; Yang, X.B. The effect of rankl/opg balance on reducing implant complications. J. Funct. Biomater. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, M.; Koepp, R.; Cortis, J.; Komrakova, M.; Schieker, M.; Hempel, U.; Siggelkow, H. Il-6, il-1beta, and tnf-alpha only in combination influence the osteoporotic phenotype in crohn’s patients via bone formation and bone resorption. Adv. Clin. Exp. Med. 2018, 27, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The impact of vitamin d levels on inflammatory status: A systematic review of immune cell studies. PLoS One 2015, 10, e0141770. [Google Scholar] [CrossRef]
- Komiyama, M.; Takanabe, R.; Ono, K.; Shimada, S.; Wada, H.; Yamakage, H.; Satoh-Asahara, N.; Morimoto, T.; Shimatsu, A.; Takahashi, Y.; et al. Association between monocyte chemoattractant protein-1 and blood pressure in smokers. J. Int. Med. Res. 2018, 46, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Coccini, T.; Roda, E.; Signorini, C.; Balbi, B.; Brunetti, G.; Ceriana, P. Blood mcp-1 levels are increased in chronic obstructive pulmonary disease patients with prevalent emphysema. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Koenigstein, A.; Joseph, J.; Sun, L.; Kalyanaraman, B.; Zaidi, M.; Avadhani, N.G. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann. N. Y. Acad. Sci. 2010, 1192, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in rankl-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Kim, K.H.; Burkhart, K.; Chen, P.; Frevert, C.W.; Randolph-Habecker, J.; Hackman, R.C.; Soloway, P.D.; Madtes, D.K. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am. J. Respir. Cell Mol. Biol. 2005, 33, 271–279. [Google Scholar] [CrossRef]
- Lieu, S.; Hansen, E.; Dedini, R.; Behonick, D.; Werb, Z.; Miclau, T.; Marcucio, R.; Colnot, C. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis. Model. Mech. 2011, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of timps in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Glantz, S.A. Heated tobacco products: The example of iqos. Tob. Control. 2018, 27, s1–s6. [Google Scholar] [CrossRef]
- Nasell, H.; Adami, J.; Samnegard, E.; Tonnesen, H.; Ponzer, S. Effect of smoking cessation intervention on results of acute fracture surgery: A randomized controlled trial. J. Bone Joint Surg. Am. 2010, 92, 1335–1342. [Google Scholar] [CrossRef]
- Lindstrom, D.; Sadr Azodi, O.; Wladis, A.; Tonnesen, H.; Linder, S.; Nasell, H.; Ponzer, S.; Adami, J. Effects of a perioperative smoking cessation intervention on postoperative complications: A randomized trial. Ann. Surg. 2008, 248, 739–745. [Google Scholar] [CrossRef]
- Ring, J.; Shoaib, A.; Shariff, R. Smoking cessation advice in limb reconstruction: An opportunity not to be missed. Injury 2017, 48, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Villebro, N.M.; Pedersen, T.; Moller, A.M.; Tonnesen, H. Long-term effects of a preoperative smoking cessation programme. Clin. Respir. J. 2008, 2, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, K.; Gucht, D.V.; Baeyens, F. Iqos(tm) vs. E-cigarette vs. Tobacco cigarette: A direct comparison of short-term effects after overnight-abstinence. Int. J. Environ. Res. Public Health 2018, 15, 2902. [Google Scholar] [CrossRef] [PubMed]


| Target | Function | ELISA Kit | Dilution Factor | |
|---|---|---|---|---|
| Order No. | Company | |||
| 25(OH)D3 | 25OH vitamin D3 | AC-57DF1 | IDS | - |
| BAP | Osteoblast activity | AC-20F1 | IDS | - |
| TRAP5b | Osteoclast activity | SB-TR201A | IDS | - |
| CTX-I | Bone resorption | AC-57SF1 | IDS | 3 |
| CICP | Collagen synthesis | 8003 | TecoMedical | 12.5 |
| MCP-1 | Stress marker | 900-K31 | Peprotech | 20 |
| sRANKL | Favors osteoclastogenesis | 900-K142 | Peprotech | 20 |
| TIMP-1 | Inhibitor for MMPs | 900-M438 | Peprotech | 20 |
| IL-1β | Inflammatory marker | 900-K95 | Peprotech | 10 |
| IL-6 | Inflammatory marker | 900-K16 | Peprotech | 10 |
| TNF-α | Inflammatory marker | 900-K25 | Peprotech | 10 |
| IFN-γ | Inflammatory marker | 900-K27 | Peprotech | 10 |
| TIMP-2 | Inhibitor for MMPs | ELH-TIMP2 | BioCat | 100 |
| OPG | Inhibitor for sRANKL | ELH-OPG | BioCat | 20 |
| OPN | Anchor for osteoclasts | ELH-OPN | BioCat | 20 |
| w/o Complications | w/ Complications | All Patients | p-Value | |||
|---|---|---|---|---|---|---|
| Primary Athroplasties | Age (a) | 63.1 ± 14.9 (61.7–64.5) | 60.8 ± 14.3 (57.5–64.0) | 62.7 ± 14.8 (61.5–64.0) | 0.106 | |
| Gender distribution | Male | 49.2% (N = 212) | 65.8% (N = 52) | 65.4% (N = 264) | 0.022 | |
| Female | 50.8% (N = 219) | 34.2% (N = 27) | 34.6% (N = 246) | |||
| BMI (kg/m2) | 28.1 ± 5.1 (27.7–28.6) | 29.0 ± 5.8 (27.7–30.3) | 28.3 ± 5.2 (27.8–28.7) | 0.263 | ||
| Number of comorbidities | 3.47 ± 2.65 (3.22–3.73) | 3.86 ± 3.49 (3.08–4.64) | 3.54 ± 2.80 (3.29–3.78) | 0.821 | ||
| Number of drugs | 3.50 ± 3.25 (3.19–3.81) | 4.19 ± 4.66 (3.15–5.23) | 3.61 ± 3.51 (3.30–3.92) | 0.844 | ||
| Frequency of malaise (%) | 9.3% (N = 40) | 17.7% (N = 14) | 10.6% (N = 54) | 0.097 | ||
| Revision Athroplasties | Age (a) | 60.3 ± 16.7 (58.0–62.7) | 59.1 ± 15.2 (55.8–62.5) | 60.0 ± 16.3 (58.1–61.9) | 0.437 | |
| Gender distribution | Male | 65.2% (N = 129) | 65.0% (N = 52) | 65.1% (N = 181) | 1.000 | |
| Female | 34.8% (N = 69) | 35.0% (N = 28) | 34.9% (N = 97) | |||
| BMI (kg/m2) | 28.2 ± 5.8 (27.4–29.1) | 29.0 ± 6.8 (27.5–30.5) | 28.5 ± 6.1 (27.7–29.2) | 0.809 | ||
| Number of comorbidities | 3.34 ± 2.87 (3.94–3.74) | 3.63 ± 3.12 (2.93–4.32) | 3.42 ± 2.94 (3.07–3.77) | 0.574 | ||
| Number of drugs | 3.55 ± 3.39 (3.08–4.03) | 3.56 ± 3.62 (2.75–4.37) | 3.56 ± 3.45 (3.14–3.97) | 0.795 | ||
| Frequency of malaise (%) | 8.6% (N = 17) | 31.3% (N = 25) | 15.1% (N = 42) | <0.001 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehnert, S.; Aspera-Werz, R.H.; Ihle, C.; Trost, M.; Zirn, B.; Flesch, I.; Schröter, S.; Relja, B.; Nussler, A.K. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. J. Clin. Med. 2019, 8, 406. https://doi.org/10.3390/jcm8030406
Ehnert S, Aspera-Werz RH, Ihle C, Trost M, Zirn B, Flesch I, Schröter S, Relja B, Nussler AK. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. Journal of Clinical Medicine. 2019; 8(3):406. https://doi.org/10.3390/jcm8030406
Chicago/Turabian StyleEhnert, Sabrina, Romina H. Aspera-Werz, Christoph Ihle, Markus Trost, Barbara Zirn, Ingo Flesch, Steffen Schröter, Borna Relja, and Andreas K. Nussler. 2019. "Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty" Journal of Clinical Medicine 8, no. 3: 406. https://doi.org/10.3390/jcm8030406
APA StyleEhnert, S., Aspera-Werz, R. H., Ihle, C., Trost, M., Zirn, B., Flesch, I., Schröter, S., Relja, B., & Nussler, A. K. (2019). Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. Journal of Clinical Medicine, 8(3), 406. https://doi.org/10.3390/jcm8030406







