The Extended Use of Eculizumab in Pregnancy and Complement Activation–Associated Diseases Affecting Maternal, Fetal and Neonatal Kidneys—The Future Is Now?
Abstract
1. Introduction
2. Antiphospholipid Syndrome
3. Sickle-Cell Trait/Anemia/Disease
4. HELLP Syndrome
5. Paroxysmal Nocturnal Hemoglobinuria (PNH)
6. Atypical Hemolytic Uremic Syndrome (aHUS)
7. Preterm Birth and Its Influence on the Renal Development and Function in Offspring
8. Conclusions and Further Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Agust, P. Obstetric nephrology: Pregnancy and the kidney—Inextricably linked. Clin. J. Am. Soc. Nephrol. 2012, 7, 2071–2072. [Google Scholar] [CrossRef] [PubMed]
- Regal, J.F.; Gilbert, J.S.; Burwick, R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015, 67, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Figueiredo, N.; Borges, A. Acute kidney injury in pregnancy: A clinical challenge. J. Nephrol. 2012, 25, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Stritzke, A.; Thomas, S.; Amin, H.; Fusch, C.; Lodha, A. Renal consequences of preterm birth. Mol. Cell. Pediatr. 2017, 4, 2. [Google Scholar] [CrossRef]
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef]
- Ray, J.G.; Burows, R.F.; Ginsberg, J.S.; Burrows, E.A. Paroxysmal nocturnal hemoglobinuria and the risk of venous thrombosis: Review and recommendations for management of the pregnant and nonpregnant patient. Haemostasis 2000, 30, 103–117. [Google Scholar] [CrossRef]
- Dashe, J.S.; Ramin, S.M.; Cunningham, F.G. The long-term consequences of thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome) in pregnancy. Obstet. Gynecol. 1998, 91, 662–668. [Google Scholar]
- Girardi, G.; Berman, J.; Redecha, P.; Spruce, L.; Thurman, J.M.; Kraus, D.; Hollmann, T.J.; Casali, P.; Caroll, M.C.; Wetsel, R.A.; et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 2003, 112, 1644–1654. [Google Scholar] [CrossRef]
- Romay-Penabad, Z.; Carrera Marin, A.; Willis, R.; Weston-Davies, W.; Machin, S.; Cohen, H.; Brasier, A.; Gonzalez, E.B. Complement C5-inhibitor rEV576 (coversin) ameliorates in-vivo effects of antiphospholipid antibodies. Lupus 2014, 23, 1324–1326. [Google Scholar] [CrossRef]
- Gustavsen, A.; Skattum, L.; Bergseth, G.; Lorentzen, B.; Floisand, Y.; Bosnes, V.; Mollnes, T.; Barratt-Due, A. Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: A case report. Medicine 2017, 96, e6338. [Google Scholar] [CrossRef] [PubMed]
- Rovere-Querini, P.; Canti, V.; Erra, R.; Bianchi, E.; Slaviero, G.; D’Angelo, A.; Rosa, S.; Candiani, M.; Castiglioni, M. Eculizumab in a pregnant patient with laboratory onset of catastrophic antiphospholipid syndrome: A case report. Medicine 2018, 97, e12584. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Mainou, M.; Christodoulou, I.; Koravou, E.-E.; Paleta, A.; Touloumenidou, T.; Papalexandri, A.; Athanasiadou, A.; Apostolou, C.; Klonizakis, P.; et al. In vitro evidence of complement activation in patients with sickle cell disease. Haematologica 2017, 102, e481–e482. [Google Scholar] [CrossRef]
- Chonat, S.; Chandrakasan, S.; Kalinyak, K.A.; Ingala, D.; Gruppo, R.; Kalfa, T.A. Atypical haemolytic uraemic syndrome in a patient with sickle cell disease, successfully treated with eculizumab. Br. J. Haematol. 2016, 175, 744–747. [Google Scholar] [CrossRef]
- Kirui, L.C.; Scully, M.; Mcqueen, N.; Porter, J.; Eleftheriou, P. Use of Eculizumab for the Treatment of Hyperhaemolysis in Pregnancy in Sickle Cell Disease: A Case Report. Blood 2018, 132, 4922. [Google Scholar] [CrossRef]
- Sabau, L.; Terriou, L.; Provot, F.; Fourrier, F.; Roumier, C.; Caron, C.; Susen, S.; Ducloy-Bouthors, A.S. Are there any additional mechanisms for haemolysis in HELLP syndrome? Thromb. Res. 2016, 142, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Vaught, A.J.; Gavriilaki, E.; Hueppchen, N.; Blakemore, K.; Yuan, X.; Seifert, S.M.; York, S.; Brodsky, R.A. Direct evidence of complement activation in HELLP syndrome: A link to atypical hemolytic uremic syndrome. Exp. Hematol. 2016, 44, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Burwick, R.M.; Feinberg, B.B. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 2013, 34, 201–203. [Google Scholar] [CrossRef]
- Jeremic, K.; Stefanovic, A.; Dotlic, J.; Stojnic, J.; Kadija, S.; Vilendecic, Z.; Janjic, T.; Jeremic, J. Neonatal outcome in pregnant patients with antiphospholipid syndrome. J. Perinat. Med. 2015, 43, 761–768. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V.; Maruotti, G.M.; Ghi, T.; Rizzo, G.; Simonazzi, G.; Rizzo, N.; Facchinetti, F.; Dall’Asta, A.; Visentin, S.; et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: The PREGNANTS study. Am. J. Obstet. Gynecol. 2017, 216, 525. [Google Scholar] [CrossRef]
- Gabbay-Benziv, R.; Zafrir-Danieli, H.; Blickstein, D.; Shmueli, A.; Salman, L.; Hadar, E. Antiphospholipid syndrome characteristics and adverse pregnancy outcomes after 20 weeks of pregnancy. Int. J. Gynaecol. Obstet. 2018, 142, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Deng, X.L.; Liu, X.Y. [Pregnancy outcome in 54 patients with antiphospholipid syndrome: A retrospective clinical study]. Beijing Da Xue Xue Bao Yi Xue Ban. 2014, 46, 323–328. (In Chinese) [Google Scholar]
- Chou, A.-K.; Hsieh, S.-C.; Su, Y.-N.; Jeng, S.-F.; Chen, C.-Y.; Chou, H.-C.; Tsao, P.-N.; Hsieh, W.-S. Neonatal and Pregnancy Outcome in Primary Antiphospholipid Syndrome: A 10-year Experience in One Medical Center. Pediatr. Neonatol. 2009, 50, 143–146. [Google Scholar] [CrossRef]
- Muganyizi, P.S.; Kidanto, H. Sickle Cell Disease in Pregnancy: Trend and Pregnancy Outcomes at a Tertiary Hospital in Tanzania. PLoS ONE 2013, 8, e56541. [Google Scholar] [CrossRef]
- Elenga, N.; Adeline, A.; Balcaen, J.; Vaz, T.; Calvez, M.; Terraz, A.; Accrombessi, L.; Carles, G. Pregnancy in Sickle Cell Disease Is a Very High-Risk Situation: An Observational Study. Obstet. Gynecol. Int. 2016, 069054. [Google Scholar] [CrossRef] [PubMed]
- Alkhunaizi, A.M.; Al-Khatti, A.A.; Alkhunaizi, M.A. Prevalence of Microalbuminuria in Adult Patients with Sickle Cell Disease in Eastern Saudi Arabia. Int. J. Nephrol. 2018, 5015764. [Google Scholar] [CrossRef]
- Oteng-Ntim, E.; Meeks, D.; Seed, P.T.; Webster, L.; Howard, J.; Doyle, P.; Chappell, L.C. Adverse maternal and perinatal outcomes in pregnant women with sickle cell disease: Systematic review and meta-analysis. Blood 2015, 125, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Ozdogu, H. Pregnancy and sickle cell disease: A review of the current literature. Crit. Rev. Oncol. Hematol. 2016, 98, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.A.; Thomas, E.J.; Sissons, J.G. Complement activation in asymptomatic patients with sickle cell anaemia. Clin. Exp. Immunol. 1979, 36, 130–139. [Google Scholar]
- DeCeulaer, K.; Wilson, W.A.; Morgan, A.G.; Serjeant, G.R. Plasma haemoglobin and complement activation in sickle cell disease. J. Clin. Lab. Immunol. 1981, 6, 57–60. [Google Scholar]
- Mohamed, A.O.; Hashim, M.S.; Nilsson, U.R.; Venge, P. Increased in vivo activation of neutrophils and complement in sickle cell disease. Am. J. Trop. Med. Hyg. 1993, 49, 799–803. [Google Scholar] [CrossRef]
- Chapin, J.; Terry, H.S.; Kleinert, D.; Laurence, J. The role of complement activation in thrombosis and hemolytic anemias. Transfus. Apher. Sci. 2016, 54, 191–198. [Google Scholar] [CrossRef]
- Maga, T.K.; Nishimura, C.J.; Weaver, A.E.; Frees, K.L.; Smith, R.J. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum. Mutat. 2010, 31, E1445–E1460. [Google Scholar] [CrossRef]
- Naik, R.P.; Smith-Whitley, K.; Hassell, K.L.; Umeh, N.I.; de Montalembert, M.; Sahota, P.; Haywood, C., Jr.; Jenkins, J.; Lloyd-Puryear, M.A.; Joiner, C.H.; et al. Clinical Outcomes Associated With Sickle Cell Trait: A Systematic Review. Ann. Intern. Med. 2018, 169, 619–627. [Google Scholar] [CrossRef]
- Maitra, P.; Caughey, M.; Robinson, L.; Desai, P.C.; Jones, S.; Nouraie, M.; Gladwin, M.T.; Hinderliter, A.; Cai, J.; Ataga, K.I. Risk factors for mortality in adult patients with sickle cell disease: A meta-analysis of studies in North America and Europe. Haematologica 2017, 102, 626–636. [Google Scholar] [CrossRef]
- Uzan, J.; Carbonnel, M.; Piconne, O.; Asmar, R.; Ayoubi, J.M. Pre-eclampsia: Pathophysiology, diagnosis, and management. Vasc. Health Risk Manag. 2011, 7, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.M.; Murphy, J.R.; Byers, T.; Gibbs, R.S.; Neville, M.C.; Giclas, P.C.; Salmon, J.E.; Holers, V.M. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am. J. Obstet. Gynecol. 2008, 198, 385.e1–385.e9. [Google Scholar] [CrossRef]
- Qing, X.; Redecha, P.B.; Burmeister, M.A.; Tomlinson, S.; D’Agati, V.D.; Davisson, R.L.; Salmon, J.E. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011, 79, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kong, L.R.; Ge, Q.; Lu, Y.Y.; Hong, M.N.; Zhang, Y.; Ruan, C.C.; Gao, P.J. Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J. Cell. Mol. Med. 2018, 22, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Vaught, A.J.; Braunstein, E.M.; Jasem, J.; Yuan, X.; Makhlin, I.; Eloundou, S.; Baines, A.C.; Merrill, S.A.; Chaturvedi, S.; Blakemore, K.; et al. Germline mutations in the alternative pathway of complement predispose to HELLP syndrome. JCI Insight. 2018, 22, e99128. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Bresin, E.; Mele, C.; Remuzzi, G. Genetic atypical hemolytic-uremic syndrome. In GeneReviews(R); University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Gul, A.; Cebeci, A.; Aslan, H.; Polat, I.; Ozdemir, A.; Ceylan, Y. Perinatal outcomes in severe preeclampsia-eclampsia with and without HELLP syndrome. Gynecol. Obstet. Invest. 2005, 59, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Roelofsen, A.C.; van Pampus, M.G.; Aarnoudse, J.G. The HELLP-syndrome; maternal-fetal outcome and follow up of infants. J. Perinat. Med. 2003, 31, 201–208. [Google Scholar] [CrossRef]
- Erdemoğlu, M.; Kuyumcuoğlu, U.; Kale, A.; Akdeniz, N. Factors affecting maternal and perinatal outcomes in HELLP syndrome: Evaluation of 126 cases. Clin. Exp. Obstet. Gynecol. 2010, 37, 213–216. [Google Scholar]
- Kongwattanakul, K.; Saksiriwuttho, P.; Chaiyarach, S.; Thepsuthammarat, K. Incidence, characteristics, maternal complications, and perinatal outcomes associated with preeclampsia with severe features and HELLP syndrome. Int. J. Womens Health 2018, 10, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Sohn, Y.S.; Lim, J.H.; Kim, E.H.; Kwon, J.Y.; Park, Y.W.; Kim, Y.H. Neonatal Outcome after Preterm Delivery in HELLP Syndrome. Yonsei Med. J. 2006, 47, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hupuczi, P.; Rigó, B.; Sziller, I.; Szabó, G.; Szigeti, Z.; Papp, Z. Follow-up analysis of pregnancies complicated by HELLP syndrome. Fetal Diagn. Ther. 2006, 21, 519–522. [Google Scholar] [CrossRef]
- Ayansina, D.; Black, C.; Hall, S.J.; Marks, A.; Millar, C.; Prescott, G.J.; Wilde, K.; Bhattacharya, S. Long term effects of gestational hypertension and pre-eclampsia on kidney function: Record linkage study. Pregnancy Hypertens. 2016, 6, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Yong, P.J.; Espinosa, V.; Côté, A.M.; Chen, I.; von Dadelszen, P. Expectant management of severe preeclampsia remote from term: A structured systematic review. Hypertens. Pregnancy 2009, 28, 312–347. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.; Liu, S.; Bartholomew, S. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: Population based retrospective cohort study. Br. Med. J. 2014, 349, g4731. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.; Dahhou, M.; Joseph, K.S. Investigation of a rise in obstetric acute renal failure in the United States, 1999–2011. Obstet. Gynecol. 2016, 127, 899–906. [Google Scholar] [CrossRef]
- Sibai, B.M.; Ramadan, M.K.; Usta, I.; Salama, M.; Mercer, B.M.; Friedman, S.A. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am. J. Obstet. Gynecol. 1993, 169, 1000–1006. [Google Scholar] [CrossRef]
- Weinstein, L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am. J. Obstet. Gynecol. 1982, 142, 159–167. [Google Scholar] [CrossRef]
- Fang, C.J.; Fremeaux-Bacchi, V.; Liszewski, M.K.; et al. Membrane cofactor protein mutations in atypical hemolytic uremic syndrome (aHUS), fatal Stx-HUS, C3 glomerulonephritis, and the HELLP syndrome. Blood 2008, 111, 624–632. [Google Scholar] [CrossRef]
- Burwick, R.M.; Fichorova, R.N.; Dawood, H.Y.; Yamamoto, H.S.; Feinberg, B.B. Urinary excretion of C5b-9 in severe preeclampsia: Tipping the balance of complement activation in pregnancy. Hypertension 2013, 62, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Burwick, R.M.; Burwick, N.R.; Feinberg, B.B. Eculizumab fails to inhibit generation of C5a in vivo. Blood 2014, 124, 3502–3503. [Google Scholar] [CrossRef] [PubMed]
- Geha, M.; Burwick, R.M.; Feinberg, B.B. Eculizumab, a Novel Treatment for Preeclampsia/HELLP Syndrome. In Proceedings of the American Academy of Pediatrics AAP Experience National Conference & Exhibition, Orlando, FL, USA, 26–29 October 2013. [Google Scholar]
- Vento, M.; Aguar, M.; Escobar, J.; Arduini, A.; Escrig, R.; Brugada, M.; Izquierdo, I.; Asensi, M.A.; Sastre, J.; Saenz, P.; et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: Influence of gender and timing. Antioxid. Redox. Signal. 2009, 11, 2945–2955. [Google Scholar] [CrossRef] [PubMed]
- Turitz, A.L.; Too, G.T.; Gyamfi-Bannerman, C. Proximity of magnesium exposure to delivery and neonatal outcomes. Am. J. Obstet. Gynecol. 2016, 215. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.E.; Heuser, C.; Triebwasser, M.; Liszewski, M.K.; Kavanagh, D.; Roumenina, L.; Branch, D.W.; Goodship, T.; Fremeaux-Bacchi, V.; Atkinson, J.P. Mutations in complement regulatory proteins predispose to preeclampsia: A genetic analysis of the PROMISSE cohort. PLoS Med. 2011, 8, e1001013. [Google Scholar] [CrossRef]
- Dmytrijuk, A.; Robie-Suh, K.; Cohen, M.H.; Rieves, D.; Weiss, K.; Pazdur, R. FDA report: Eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist 2008, 13, 993–1000. [Google Scholar] [CrossRef]
- Hill, A.; Kelly, R.J.; Hillmen, P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013, 121, 4985–4996. [Google Scholar] [CrossRef]
- Sahin, F.; Ozkan, M.C.; Mete, N.G.; Yilmaz, M.; Oruc, N.; Gurgun, A.; Kayikcioglu, M.; Guler, A.; Gokcay, F.; Bilgir, F.; et al. Multidisciplinary clinical management of paroxysmal nocturnal hemoglobinuria. Am. J. Blood Res. 2015, 5, 1–9. [Google Scholar]
- Hillmen, P.; Muus, P.; Röth, A.; Elebute, M.O.; Risitano, A.M.; Schrezenmeier, H.; Szer, J.; Browne, P.; Maciejewski, J.P.; Schubert, J.; et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2013, 162, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Peffault de Latour, R.; Schrezenmeier, H.; Bacigalupo, A.; Blaise, D.; de Souza, C.A.; Vigouroux, S.; Willemze, R.; Terriou, L.; Tichelli, A.; Mohty, M.; et al. Allogeneic stem cell transplantation in paroxysmal nocturnal hemoglobinuria. Haematologica 2012, 97, 1666–1673. [Google Scholar] [CrossRef]
- Muñoz-Linares, C.; Ojeda, E.; Forés, R.; Pastrana, M.; Cabero, M.; Morillo, D.; Bautista, G.; Baños, I.; Monteserín, C.; Bravo, P.; et al. Paroxysmal nocturnal hemoglobinuria: A single Spanish center’s experience over the last 40 year. Eur. J. Haematol. 2014, 93, 309–319. [Google Scholar] [CrossRef]
- Hillmen, P.; Young, N.S.; Schubert, J.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef]
- Brodsky, R.A.; Young, N.S.; Antonioli, E.; Risitano, A.M.; Schrezenmeier, H.; Schubert, J.; Gaya, A.; Coyle, L.; de Castro, C.; Fu, C.L.; et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 2008, 111, 1840–1847. [Google Scholar] [CrossRef]
- Villegas, A.; Núñez, R.; Gaya, A.; Cuevas-Ruiz, M.V.; Bosch, J.M.; Carral, A.; Arrizabalaga, B.; Gómez-Roncero, M.I.; Mora, A.; Bravo, P.; et al. Presence of acute and chronic renal failure in patients with paroxysmal nocturnal hemoglobinuria: Results of a retrospective analysis from the Spanish PNH Registry. Ann. Hematol. 2017, 96, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Kanakura, Y.; Ohyashiki, K.; Shichishima, T.; Okamoto, S.; Ando, K.; Ninomiya, H.; Kawaguchi, T.; Nakao, S.; Nakakuma, H.; Nishimura, J.; et al. Long-term efficacy and safety of eculizumab in Japanese patients with PNH: AEGIS trial. Int. J. Hematol. 2013, 98, 406–416. [Google Scholar] [CrossRef]
- Al-Ani, F.; Chin-Yee, I.; Lazo-Langner, A. Eculizumab in the management of paroxysmal nocturnal hemoglobinuria: Patient selection and special considerations. Ther. Clin. Risk. Manag. 2016, 12, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Höchsmann, B.; Szer, J.; Kulasekararaj, A.; de Guibert, S.; Röth, A.; Weitz, I.C.; Armstrong, E.; Risitano, A.M.; Patriquin, C.J.; et al. Eculizumab in Pregnant Patients with Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2015, 373, 1032–1039. [Google Scholar] [CrossRef]
- Hallstensen, R.F.; Bergseth, G.; Foss, S.; Jæger, S.; Gedde-Dahl, T.; Holt, J.; Christiansen, D.; Lau, C.; Brekke, O.L.; Armstrong, E.; et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 2015, 220, 452–459. [Google Scholar] [CrossRef]
- Huerta, A.; Arjona, E.; Portoles, J.; Lopez-Sanchez, P.; Rabasco, C.; Espinosa, M.; Cavero, T.; Blasco, M.; Cao, M.; Manrique, J.; et al. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int. 2018, 93, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Nayer, A.; Haas, C.S. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: Case reports and a review of the evidence for treatment with eculizumab. J. Nephrol. 2017, 30, 347–362. [Google Scholar] [CrossRef]
- Fakhouri, F.; Roumenina, L.; Provot, F.; Sallée, M.; Caillard, S.; Couzi, L.; Essig, M.; Ribes, D.; Dragon-Durey, M.A.; Bridoux, F.; et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J. Am. Soc. Nephrol. 2010, 21, 859–867. [Google Scholar] [CrossRef]
- Bruel, A.; Kavanagh, D.; Noris, M.; Delmas, Y.; Wong, E.K.S.; Bresin, E.; Provôt, F.; Brocklebank, V.; Mele, C.; Remuzzi, G.; et al. Hemolytic Uremic Syndrome in Pregnancy and Postpartum. Clin. J. Am. Soc. Nephrol. 2017, 12, 1237–1247. [Google Scholar] [CrossRef]
- Gaggl, M.; Aigner, C.; Csuka, D.; Szilágyi, Á.; Prohászka, Z.; Kain, R.; Haninger, N.; Knechtelsdorfer, M.; Sunder-Plassmann, R.; Sunder-Plassmann, G.; Schmidt, A. Maternal and Fetal Outcomes of Pregnancies in Women with Atypical Hemolytic Uremic Syndrome. J. Am. Soc. Nephrol. 2018, 29, 1020–1029. [Google Scholar] [CrossRef]
- Rathbone, J.; Kaltenthaler, E.; Richards, A.; Tappenden, P.; Bessey, A.; Cantrell, A. A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ Open 2013, 3, e003573. [Google Scholar] [CrossRef]
- Cofiell, R.; Kukreja, A.; Bedard, K.; Yan, Y.; Mickle, A.P.; Ogawa, M.; Bedrosian, C.L.; Faas, S.J. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 2015, 125, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Andries, G.; Karass, M.; Yandrapalli, S.; Linder, K.; Liu, D.; Nelson, J.; Pawar, R.; Chugh, S. Atypical hemolytic uremic syndrome in first trimester pregnancy successfully treated with eculizumab. Exp. Hematol. Oncol. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Yazici, H.; Ozluk, Y.; Kilicaslan, I.; Turkmen, A. Pregnant woman with atypical hemolytic uremic syndrome delivered a healthy newborn under eculizumab treatment. Case Rep. Nephrol. Dial. 2016, 6, 143–148. [Google Scholar] [CrossRef]
- Ardissino, G.; Ossola, M.W.; Baffero, G.M.; Rigotti, A.; Cugno, M. Eculizumab for atypical hemolytic uremic syndrome in pregnancy. Obstet. Gynecol. 2013, 122, 487–489. [Google Scholar] [CrossRef]
- Sarno, L.; Tufano, A.; Maruotti, G.M.; Martinelli, P.; Balletta, M.M.; Russo, D. Eculizumab in pregnancy: A narrative overview. J. Nephrol. 2019, 32, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.; Devillard, N.; Frémeaux-Bacchi, V.; Hummel, A.; Salomon, L.; Contin-Bordes, C.; Gomer, H.; Legendre, C.; Delmas, Y. Atypical haemolytic uraemic syndrome and pregnancy: Outcome with ongoing eculizumab. Nephrol. Dial. Transpl. 2016, 31, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Lisonkova, S.; Hutcheon, J.A.; Joseph, K.S. Temporal trends in neonatal outcomes following iatrogenic preterm delivery. BMC Pregnancy Childbirth 2011, 11, 39. [Google Scholar] [CrossRef]
- Chen, A.; Feresu, S.A.; Barsoom, M.J. Heterogeneity of preterm birth subtypes in relation to neonatal death. Obstet. Gynecol. 2009, 114, 516–522. [Google Scholar] [CrossRef]
- Stevens, W.; Shih, T.; Incerti, D.; Ton, T.G.N.; Lee, H.C.; Peneva, D.; Macones, G.A.; Sibai, B.M.; Jena, A.B. Short-term costs of preeclampsia to the United States health care system. Am. J. Obstet. Gynecol. 2017, 217, 237–248. [Google Scholar] [CrossRef]
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet. Gynecol. 2016, 215, 103. [Google Scholar] [CrossRef] [PubMed]
- Korvenranta, E.; Linna, M.; Rautava, L.; Andersson, S.; Gissler, M.; Hallman, M.; Häkkinen, U.; Leipälä, J.; Peltola, M.; Tammela, O.; et al. Performance, Effectiveness, and Cost of Treatment Episodes (PERFECT) Preterm Infant Study Group. Hospital costs and quality of life during 4 years after very preterm birth. Arch. Pediatr. Adolesc. Med. 2010, 164, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Stelloh, C.; Allen, K.P.; Mattson, D.L.; Lerch-Gaggl, A.; Reddy, S.; El-Meanawy, A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl. Res. 2012, 159, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.C.; Brodsky, R.A. Complementopathies. Blood Rev. 2017, 31, 213–223. [Google Scholar] [CrossRef]
- Hill, A.; DeZern, A.E.; Kinoshita, T.; Brodsky, R.A. Paroxysmal nocturnal haemoglobinuria. Nat. Rev. Dis. Primers 2017, 3, 17028. [Google Scholar] [CrossRef]
- Brodsky, R.A. Complement in hemolytic anemia. Blood 2015, 126, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, R.A. Paroxysmal nocturnal hemoglobinuria. Blood 2014, 124, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Barratt-Due, A.; Mollnes, T.E. Complement in clinical medicine: Clinical trials, case reports and therapy monitoring. Mol. Immunol. 2017, 89, 10–21. [Google Scholar] [CrossRef] [PubMed]
- van den Brand, J.A.; Verhave, J.C.; Adang, E.M.; Wetzels, J.F. Cost-effectiveness of eculizumab treatment after kidney transplantation in patients with atypical haemolytic uraemic syndrome. Nephrol. Dial. Transpl. 2017, 32, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.; Cheung, M.C.; Evans, G.A. Opportunity cost of funding drugs for rare diseases: The cost-effectiveness of eculizumab in paroxysmal nocturnal hemoglobinuria. Med. Decis. Mak. 2014, 34, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Europan Medicines Agency. Soliris, Summary of Product Characteristics, Annex I. Available online: https://www.ema.europa.eu/en/documents/product-information/soliris-epar-product-information_en.pdf (accessed on 16 February 2019).
- Benamu, E.; Montoya, J.G. Infections associated with the use of eculizumab: Recommendations for prevention and prophylaxis. Curr. Opin. Infect. Dis. 2016, 29, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; King, M.; Jim, B.; Acharya, A. Recurrent case of pregnancy-induced atypical haemolytic uremic syndrome (P-aHUS). BMJ Case Rep. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G. Complement activation, a threat to pregnancy. Semin Immunopathol. 2018, 40, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Le Quintrec, M. Targeting the complement cascade: Novel treatments coming down the pike. Kidney Int. 2016, 90, 746–752. [Google Scholar] [CrossRef] [PubMed]
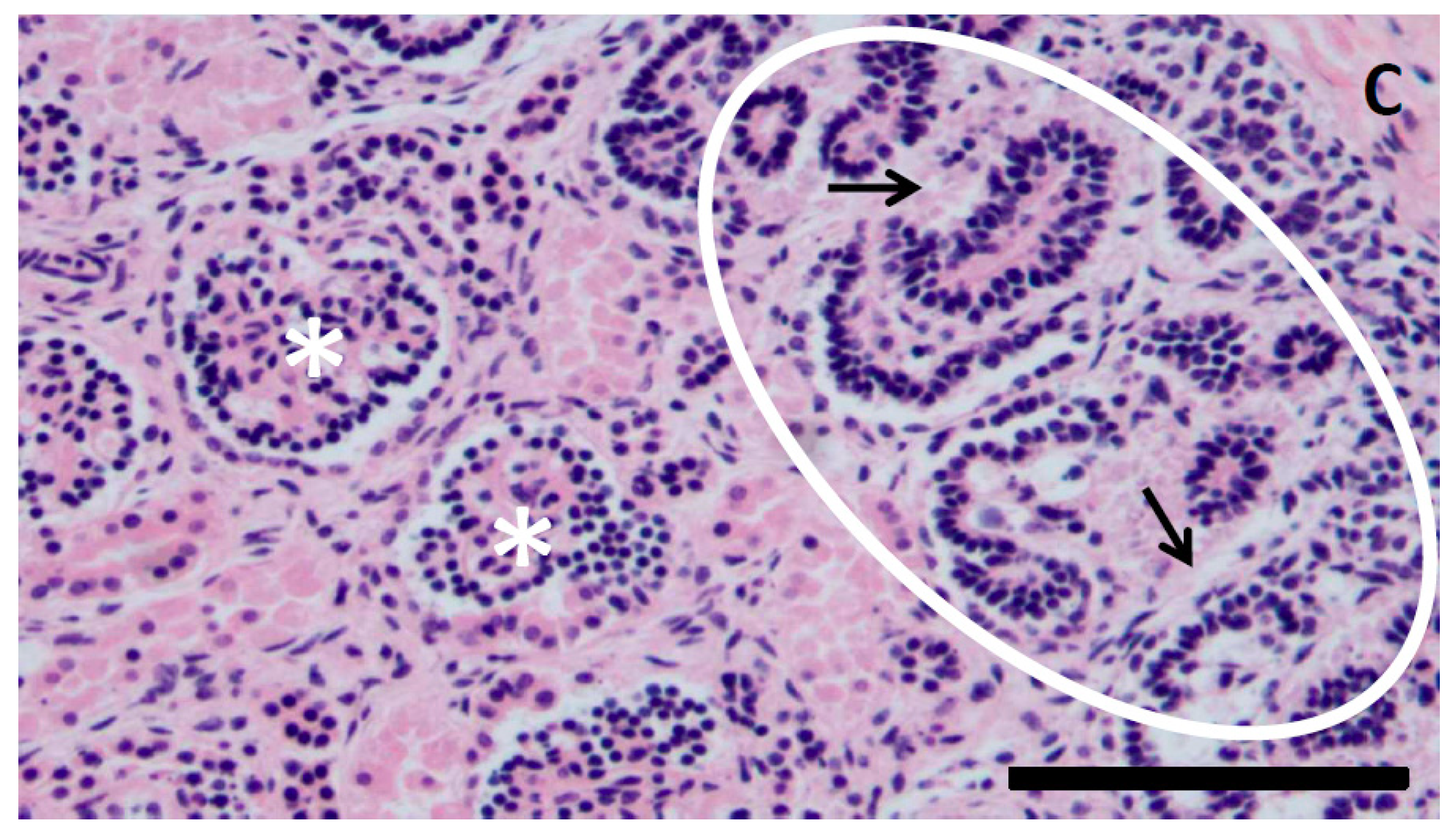
| Disease | Subject(s) | Treatment | Key Points | Ref. |
|---|---|---|---|---|
| Antiphospholipid syndrome | Pregnant mice | Human IgG containing aPL antibodies | Antibodies or peptides that block C5a-C5a receptor interactions prevent pregnancy complications. | [8] |
| Non-pregnant mice | IgG-APS/rEV576 (treatment group) and IgG-APS/phosphate buffer (controls) | Mice treated with IgG-APS/rEV576 [(coversin), a recombinant protein inhibitor of complement factor 5 activation] had significantly smaller thrombi than those treated with IgG-APS/phosphate buffer. This confirmed involvement of complement activation in antiphospholipid antibody-mediated thrombogenesis and suggested that this effect might be ameliorated by complement inhibition. | [9] | |
| Pregnant woman (case report) | Eculizumab | Triple positive aPL. Multiple previous arterial thromboses and ongoing ischemia during pregnancy. Significant risk of catastrophic APS. Eculizumab was administered twice before cesarean section which was performed at 32+4 gestational weeks with the birth of a heathy child. Complement activity surprisingly increased to normal levels within a week after both doses of eculizumab despite the evidence of complete inhibition of complement activity after each infusion. Pregnancy may influence pharmacodynamics and pharmacokinetics of eculizumab. Individual approach suggested. | [10] | |
| Pregnant woman (case report) | Eculizumab | Triple positive aPL. Abruptly developed microangiopathic hemolytic anemia, renal insufficiency, and thrombocytopenia at 30+6 weeks despite treatment with ASA, LMWH and hydroxycholoquine. Eculizumab was administered and pregnancy was safely continued for 9 days. The second eculizumab infusion was administered a week after the first treatment with rapid normalization of platelet count, renal function and hemoglobin level. | [11] | |
| Sickle-cell disease | Patients with thrombotic microangiopathies | Eculizumab | Increased complement activation was demonstrated in a portion of patients, especially older patients and those with higher HbS levels. In vitro study on the efficacy of complement inhibition by eculizumab in the modified Ham test. Mixing eculizumab-containing serum with complement-activated sera abolished complement-mediated cell killing in a dose-dependent relationship that was consistent across the three patients tested. | [12] |
| SCD patient who developed aHUS (case report) | Eculizumab | Respiratory and renal insufficiency unresponsible to plasmapheresis successfully treated with eculizumab. A clear evidence of complement activation was demonstrated by increased levels of sC5b-9 levels retrospectively analyzed from stored plasma prior to plasmapheresis. After eculizumab treatment and improvement of clinical symptoms and laboratory baseline, sC5b-9 levels normalized. This case demonstrated that patients with SCD may develop complement-mediated thrombotic microangiopathy i.e., aHUS, particularly when an underlying genetic defect in complement regulation is present. | [13] | |
| Pregnant woman (case report) | Eculizumab | A pregnant woman with HbSS who presented with hyperhemolysis at 25 gestational weeks and worsening anemia despite methylprednisolone and immunoglobulin treatment. Eculizumab treatment resulted in resolution of the hemolysis, with safe delivery at 34 weeks of gestation. | [14] | |
| HELLP syndrome | Sixteen pregnant women with HELLP | N/A | Incresed complement activation demonstration in sera. | [15] |
| Pregnant women with HELLP | N/A | Increased complement activation was observed in participants with classic or atypical HELLP compared with those with normal pregnancies and nonpregnant controls. Mixing HELLP serum with eculizumab-containing serum resulted in a significant decrease in cell killing compared with HELLP serum alone. Data of this study demonstrated striking similarities between HELLP and aHUS. | [16] | |
| Pregnant women with HELLP (case report) | Eculizumab | Prolongation of pregnancy from 26+3 to 28+6 (17 days) was achieved. In this case, reduction of soluble C5b-9 in plasma and urine correlated with clinical improvement, and resolution of hemolysis, thrombocytopenia and liver inflammation along with a prolonged pregnancy. | [17] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanovic, V. The Extended Use of Eculizumab in Pregnancy and Complement Activation–Associated Diseases Affecting Maternal, Fetal and Neonatal Kidneys—The Future Is Now? J. Clin. Med. 2019, 8, 407. https://doi.org/10.3390/jcm8030407
Stefanovic V. The Extended Use of Eculizumab in Pregnancy and Complement Activation–Associated Diseases Affecting Maternal, Fetal and Neonatal Kidneys—The Future Is Now? Journal of Clinical Medicine. 2019; 8(3):407. https://doi.org/10.3390/jcm8030407
Chicago/Turabian StyleStefanovic, Vedran. 2019. "The Extended Use of Eculizumab in Pregnancy and Complement Activation–Associated Diseases Affecting Maternal, Fetal and Neonatal Kidneys—The Future Is Now?" Journal of Clinical Medicine 8, no. 3: 407. https://doi.org/10.3390/jcm8030407
APA StyleStefanovic, V. (2019). The Extended Use of Eculizumab in Pregnancy and Complement Activation–Associated Diseases Affecting Maternal, Fetal and Neonatal Kidneys—The Future Is Now? Journal of Clinical Medicine, 8(3), 407. https://doi.org/10.3390/jcm8030407





