Metformin Affects Serum Lactate Levels in Predicting Mortality of Patients with Sepsis and Bacteremia
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
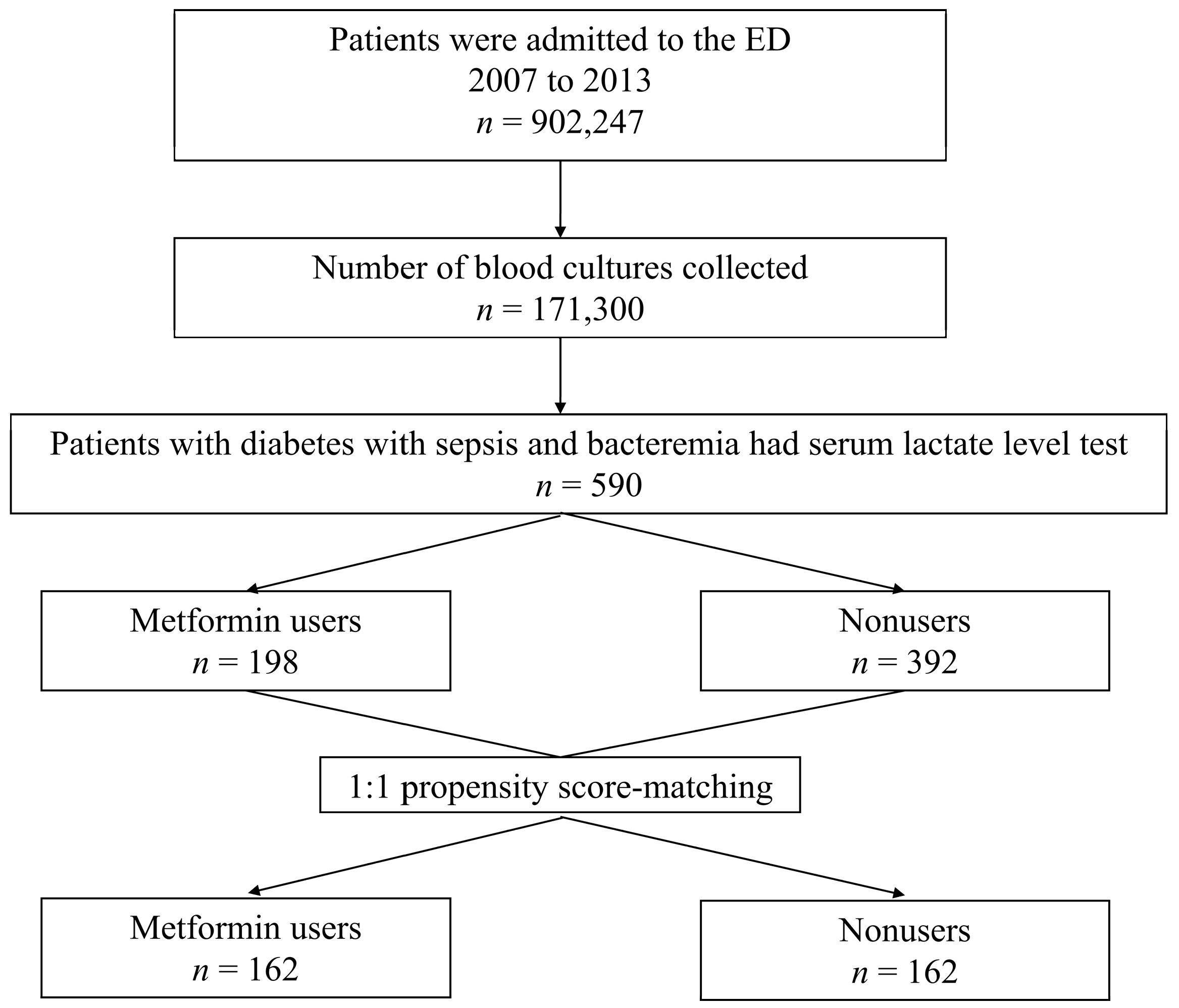
2.2. Study Setting and Population
2.3. Measurement and Data Collection
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Multivariate Logistic Regression Analyses for 28-Day Mortality
3.3. Subgroup Analysis by qSOFA Score
3.4. Receiver Operating Characteristic Curve Analysis for Lactate in Predicting 28-Day Mortality
3.5. Microbiology Results of Blood Culture
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Glickman, S.W.; Cairns, C.B.; Otero, R.M.; Woods, C.W.; Tsalik, E.L.; Langley, R.J.; van Velkinburgh, J.C.; Park, L.P.; Glickman, L.T.; Fowler, V.G., Jr.; et al. Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad. Emerg. Med. 2010, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Kuan, W.S.; Batech, M.; Shrikhande, P.; Mahadevan, M.; Li, C.H.; Ray, S.; Dengel, A.; Investigators, A. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: A multi-national evaluation. Crit. Care 2011, 15, R229. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; Dellinger, R.P.; Chansky, M.E.; Arnold, R.C.; Schorr, C.; Milcarek, B.; Hollenberg, S.M.; Parrillo, J.E. Serum lactate as a predictor of mortality in patients with infection. Intens. Care Med. 2007, 33, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Miltiades, A.N.; Gaieski, D.F.; Goyal, M.; Fuchs, B.D.; Shah, C.V.; Bellamy, S.L.; Christie, J.D. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit. Care Med. 2009, 37, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycaemia in type 2 diabetes, 2015: A patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015, 58, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Van Berlo-van de Laar, I.R.; Vermeij, C.G.; Doorenbos, C.J. Metformin associated lactic acidosis: Incidence and clinical correlation with metformin serum concentration measurements. J. Clin. Pharm. Ther. 2011, 36, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Green, J.P.; Berger, T.; Garg, N.; Suarez, A.; Hagar, Y.; Radeos, M.S.; Panacek, E.A. Impact of metformin use on the prognostic value of lactate in sepsis. Am. J. Emerg. Med. 2012, 30, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Doenyas-Barak, K.; Beberashvili, I.; Marcus, R.; Efrati, S. Lactic acidosis and severe septic shock in metformin users: A cohort study. Crit. Care 2016, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hwang, S.Y.; Jo, I.J.; Jeon, K.; Suh, G.Y.; Lee, T.R.; Yoon, H.; Cha, W.C.; Sim, M.S.; Carriere, K.C.; et al. Impact of Metformin Use on Lactate Kinetics in Patients with Severe Sepsis and Septic Shock. Shock 2017, 47, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Hwang, S.; Lee, Y.H.; Lee, S.H.; Lee, Y.M.; Kang, H.P.; Han, E.; Lee, W.; Lee, B.W.; Kang, E.S.; et al. Association between Metformin Use and Risk of Lactic Acidosis or Elevated Lactate Concentration in Type 2 Diabetes. Yonsei Med. J. 2017, 58, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lin, W.L.; Lin, C.C.; Hsieh, W.H.; Hsieh, C.H.; Wu, M.H.; Wu, J.Y.; Lee, C.C. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Yo, C.H.; Lee, M.T.; Gi, W.T.; Chang, S.S.; Tsai, K.C.; Chen, S.C.; Lee, C.C. Prognostic determinants of community-acquired bloodstream infection in type 2 diabetic patients in ED. Am. J. Emerg. Med. 2014, 32, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intens. Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Jones, G.C.; Macklin, J.P.; Alexander, W.D. Contraindications to the use of metformin. BMJ 2003, 326, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Lalau, J.D. Lactic acidosis induced by metformin: Incidence, management and prevention. Drug Saf. 2010, 33, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.C.; Kung, C.T.; Cheng, H.H.; Cheng, C.Y.; Tsai, T.C.; Hsiao, S.Y.; Su, C.M. Quick Sepsis-related Organ Failure Assessment predicts 72-h mortality in patients with suspected infection. Eur. J. Emerg. Med. 2018. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Patients (n = 590) | Propensity-Matched Patients (n = 324) | ||||
|---|---|---|---|---|---|---|
| Metformin Users (n = 198) | Nonusers (n = 392) | p-Value | Metformin Users (n = 162) | Nonusers (n = 162) | p-Value | |
| Age, years | 70 ± 12 | 69 ± 13 | 0.363 | 69 ± 12 | 69 ± 13 | 0.935 |
| Male sex, n (%) | 87 (44%) | 188 (48%) | 0.451 | 65 (40%) | 75 (46%) | 0.313 |
| Comorbidities | ||||||
| Liver cirrhosis | 7 (4%) | 44 (11%) | 0.002 a | 4 (3%) | 5 (3%) | 1.000 |
| Chronic renal insufficiency | 42 (21%) | 120 (31%) | 0.019 a | 37 (23%) | 40 (25%) | 0.794 |
| Congestive heart failure | 12 (6%) | 35 (9%) | 0.262 | 12 (7%) | 10 (6%) | 0.826 |
| Malignancy | 26 (13%) | 71 (18%) | 0.128 | 24 (15%) | 24 (15%) | 1.000 |
| Suspected infection focus | ||||||
| Respiratory tract | 64 (32%) | 131 (33%) | 0.853 | 50 (31%) | 48 (30%) | 0.904 |
| Urinary tract | 98 (50%) | 159 (41%) | 0.043 a | 75 (46%) | 77 (48%) | 0.911 |
| Skin and soft tissue | 14 (7%) | 36 (9%) | 0.436 | 11 (7%) | 12 (7%) | 1.000 |
| Intra–abdomen | 23 (12%) | 57 (15%) | 0.374 | 21 (13%) | 24 (15%) | 0.748 |
| Others | 35 (18%) | 88 (22%) | 0.198 | 34 (21%) | 27 (17%) | 0.394 |
| Septic shock | 46 (23%) | 95 (24%) | 0.838 | 31 (19%) | 33 (20%) | 0.889 |
| qSOFA score ≥2 | 54 (27%) | 105 (27%) | 0.922 | 40 (25%) | 37 (23%) | 0.794 |
| 28-day mortality | 35 (18%) | 84 (21%) | 0.328 | 29 (18%) | 27 (17%) | 0.883 |
| Serum lactate (mmol/L) | 4.7 ± 4.1 | 3.8 ± 3.4 | 0.009 a | 4.7 ± 4.3 | 3.9 ± 2.9 | 0.044 a |
| C-reactive protein (mg/L) | 154.2 ± 125.4 | 148.2 ± 113.3 | 0.579 | 155.1 ± 125.8 | 143.3 ± 112. | 0.397 |
| WBC (1000/mm3) | 13.6 ± 7.4 | 14.9 ± 12.8 | 0.214 | 13.6 ± 7.5 | 14.2 ± 8.0 | 0.491 |
| BUN (mg/dL) | 40.5 ± 33.1 | 41.5 ± 34.3 | 0.772 | 41.9 ± 35.1 | 38.4 ± 29.2 | 0.410 |
| Creatinine (mg/dL) | 1.8 ± 1.4 | 2.4 ± 2.3 | 0.002 a | 1.8 ± 1.5 | 2.1 ± 1.9 | 0.291 |
| Variable | All Patients (n = 590) | Propensity-Matched Patients (n = 324) | ||||||
|---|---|---|---|---|---|---|---|---|
| COR (95% CI) | p-Value | AOR (95% CI) | p-Value | COR (95% CI) | p-Value | AOR (95% CI) | p-Value | |
| Age (+1 year) | 1.02 (1.01–1.04) | 0.037 a | 1.02 (0.99–1.04) | 0.063 | 1.00 (0.97–1.03) | 0.990 | - | - |
| Male sex | 1.14 (0.70–1.86) | 0.595 | - | - | 0.93 (0.44–1.98) | 0.859 | - | - |
| Liver cirrhosis | 1.13 (0.52–2.46) | 0.754 | - | - | 3.64 (0.63–21.02) | 0.149 | - | - |
| Chronic renal insufficiency | 1.92 (1.16–3.19) | 0.011 a | 1.98 (1.21–3.23) | 0.007 a | 1.84 (0.84–4.06) | 0.129 | - | - |
| Congestive heart failure | 0.67 (0.29–1.56) | 0.353 | - | - | 0.44 (0.10–1.90) | 0.274 | - | - |
| Malignancy | 2.59 (1.49–4.54) | 0.001 a | 2.67 (1.54–4.62) | <0.001 a | 4.51 (1.96–10.41) | <0.001 a | 4.64 (2.13–10.08) | <0.001 a |
| Respiratory tract infection | 2.31 (1.16–4.59) | 0.018 a | 2.18 (1.37–3.46) | 0.001 a | 4.56 (1.54–13.50) | 0.006 a | 3.55 (1.82–6.90) | <0.001 a |
| Urinary tract infection | 0.42 (0.22–0.79) | 0.008 a | 0.39 (0.24–0.65) | <0.001 a | 0.50 (0.18–1.35) | 0.169 | 0.44 (0.21–0.93) | 0.032 a |
| Skin and soft tissue infection | 1.28 (0.51–3.25) | 0.603 | - | - | 0.79 (0.15–4.15) | 0.781 | - | - |
| Intra-abdomen infection | 0.76 (0.31–1.87) | 0.546 | - | - | 0.80 (0.19–3.41) | 0.759 | - | - |
| Other infection | 1.12 (0.46–2.73) | 0.800 | - | - | 1.74 (0.45–6.70) | 0.422 | - | - |
| Septic shock | 3.22 (1.96–5.29) | <0.001 a | 3.21 (1.97–5.24) | <0.001 a | 3.48 (1.58–7.64) | 0.002 a | 3.70 (1.80–7.61) | <0.001 a |
| qSOFA score ≥2 | 1.62 (0.98–2.67) | 0.058 | 1.69 (1.04–2.78) | 0.035 a | 1.56 (0.70–3.44) | 0.275 | - | - |
| Metformin use | 0.79 (0.47–1.32) | 0.371 | - | - | 1.01 (0.50–2.02) | 0.987 | - | - |
| Serum lactate (+1 mmol/L) | 1.09 (1.03–1.16) | 0.003 a | 1.09 (1.03–1.15) | 0.003 a | 1.07 (0.99–1.16) | 0.093 | 1.09 (1.01–1.18) | 0.023 a |
| Outcome | Group | AUC | 95% CI | p | Cut–Off Point | Sensitivity | Specificity | Youden Index |
|---|---|---|---|---|---|---|---|---|
| 28-day mortality | All patients | 0.63 | 0.57–0.69 | <0.001 a | 2.0 | 0.81 | 0.31 | 0.12 |
| 3.6 b | 0.58 | 0.64 | 0.22 | |||||
| 4.0 | 0.51 | 0.69 | 0.21 | |||||
| Nonusers | 0.63 | 0.56–0.70 | <0.001 a | 2.0 | 0.79 | 0.34 | 0.14 | |
| 3.6 b | 0.54 | 0.69 | 0.22 | |||||
| 4.0 | 0.48 | 0.73 | 0.21 | |||||
| Metformin users | 0.66 | 0.55–0.77 | 0.003 a | 2.0 | 0.83 | 0.26 | 0.09 | |
| 4.0 | 0.60 | 0.63 | 0.23 | |||||
| 5.9 b | 0.49 | 0.85 | 0.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.-C.; Kung, C.-T.; Cheng, H.-H.; Cheng, C.-Y.; Tsai, T.-C.; Hsiao, S.-Y.; Wu, C.-H.; Su, C.-M. Metformin Affects Serum Lactate Levels in Predicting Mortality of Patients with Sepsis and Bacteremia. J. Clin. Med. 2019, 8, 318. https://doi.org/10.3390/jcm8030318
Chen F-C, Kung C-T, Cheng H-H, Cheng C-Y, Tsai T-C, Hsiao S-Y, Wu C-H, Su C-M. Metformin Affects Serum Lactate Levels in Predicting Mortality of Patients with Sepsis and Bacteremia. Journal of Clinical Medicine. 2019; 8(3):318. https://doi.org/10.3390/jcm8030318
Chicago/Turabian StyleChen, Fu-Cheng, Chia-Te Kung, Hsien-Hung Cheng, Chi-Yung Cheng, Tsung-Cheng Tsai, Sheng-Yuan Hsiao, Chien-Hung Wu, and Chih-Min Su. 2019. "Metformin Affects Serum Lactate Levels in Predicting Mortality of Patients with Sepsis and Bacteremia" Journal of Clinical Medicine 8, no. 3: 318. https://doi.org/10.3390/jcm8030318
APA StyleChen, F.-C., Kung, C.-T., Cheng, H.-H., Cheng, C.-Y., Tsai, T.-C., Hsiao, S.-Y., Wu, C.-H., & Su, C.-M. (2019). Metformin Affects Serum Lactate Levels in Predicting Mortality of Patients with Sepsis and Bacteremia. Journal of Clinical Medicine, 8(3), 318. https://doi.org/10.3390/jcm8030318





