Impact of Long Non-Coding RNA HOTAIR Genetic Variants on the Susceptibility and Clinicopathologic Characteristics of Patients with Urothelial Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations, Ethics, and Consent
2.2. Genomic DNA Extraction and Selection of HOTAIR SNPs
2.3. Genotyping of HOTAIR SNPs
2.4. HOTAIR Expression Profiles of Bladder Cancer Patients from Gene Expression Omnibus (GEO) and the Cancer Genome Atlas (TCGA) Data Sets
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Associations between HOTAIR Gene Polymorphisms and UCC Susceptibility in Different Genders
3.3. Relationships of Clinicopathological Characteristics with HOTAIR Genetic Polymorphisms in UCC Patients
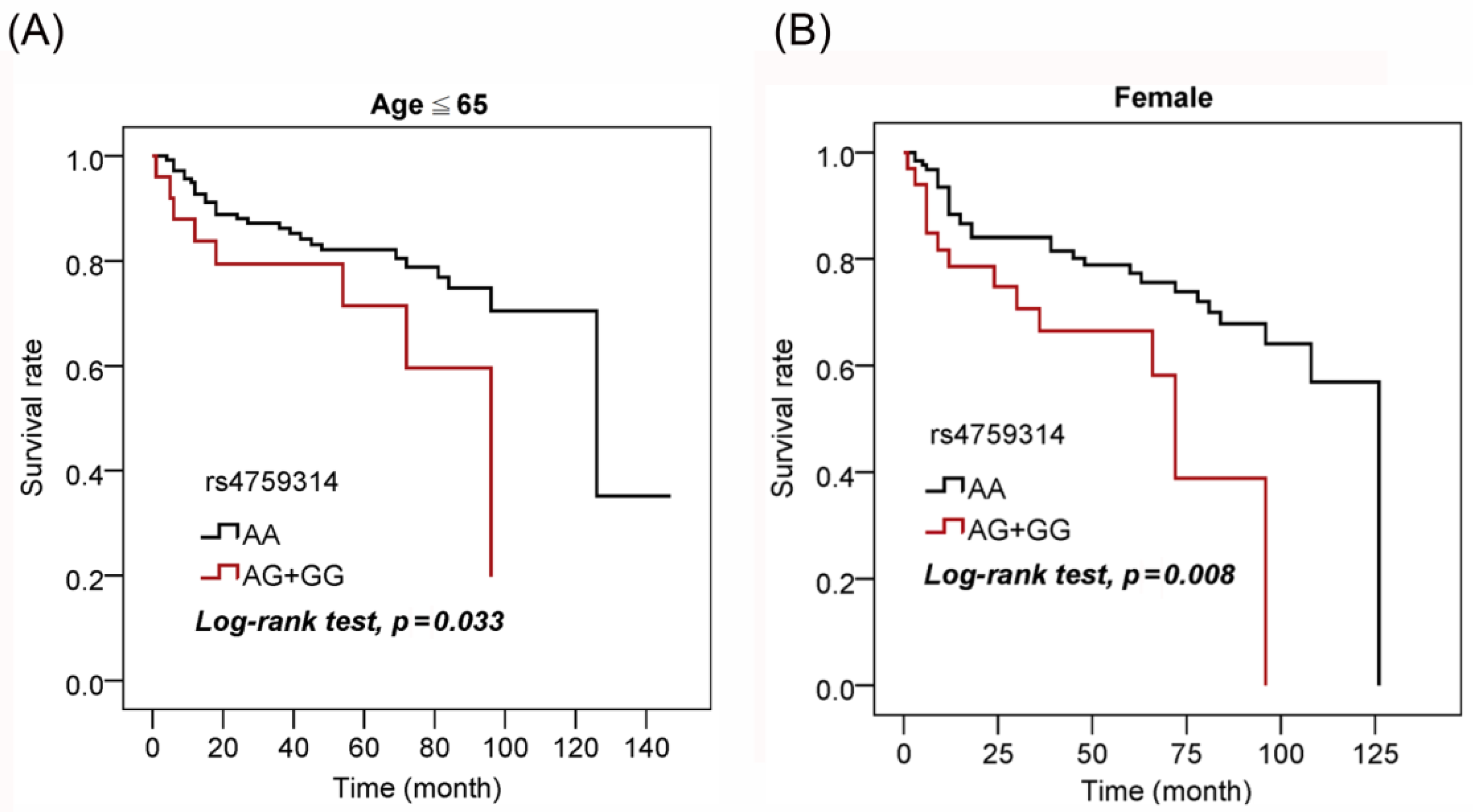
3.4. Associations between HOTAIR Gene Polymorphisms and the overall survival of UCC Patients
3.5. Clinical Relevance of HOTAIR Levels in Urothelial Bladder Cancer Patients Obtained from TCGA and GEO Databases
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix
| Variable | Controls (n = 566), n (%) | Patients (n = 272), n (%) | OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| rs920778 | ||||
| TT | 292 (51.6%) | 138 (50.7%) | 1.00 (reference) | 1.00 (reference) |
| TC | 240 (42.4%) | 115 (42.3%) | 1.01 (0.75–1.37) | 0.95 (0.68–1.32) |
| CC | 34 (6%) | 19 (7%) | 1.18 (0.65–2.15) | 1.41 (0.73–2.73) |
| TC + CC | 274 (48.4%) | 134 (49.3%) | 1.03 (0.77–1.38) | 1.00 (0.73–1.37) |
| rs1899663 | ||||
| GG | 359 (63.4%) | 170 (62.5%) | 1.00 (reference) | 1.00 (reference) |
| GT | 191 (33.7%) | 91 (33.5%) | 1.01 (0.74–1.37) | 0.94 (0.67–1.32) |
| TT | 16 (2.8%) | 11 (4%) | 1.45 (0.66–3.20) | 1.98 (0.81–4.84) |
| GT + TT | 207 (36.6%) | 102 (37.5%) | 1.04 (0.77–1.40) | 1.01 (0.72–1.40) |
| rs4759314 | ||||
| AA | 471 (83.2%) | 237 (87.1%) | 1.00 (reference) | 1.00 (reference) |
| AG | 93 (16.4%) | 35 (12.9%) | 0.75 (0.49–1.14) | 0.79 (0.50–1.24) |
| GG | 2 (0.4%) | 0 (0%) | - | - |
| AG + GG | 95 (16.8%) | 35 (12.9%) | 0.73 (0.48–1.11) | 0.77 (0.49–1.22) |
| rs12427129 | ||||
| CC | 451 (79.7%) | 222 (81.6%) | 1.00 (reference) | 1.00 (reference) |
| CT | 114 (20.1%) | 48 (17.6%) | 0.86 (0.59–1.24) | 0.88 (0.58–1.32) |
| TT | 1 (0.2%) | 2 (0.7%) | 4.06 (0.37–45.05) | 6.94 (0.28–169.70) |
| CT+TT | 115 (20.3%) | 50 (18.4%) | 0.88 (0.61–1.28) | 0.91 (0.61–1.36) |
| Variable | HOTAIR (rs1899663) | |||
| GG (%) (n = 265) | GT+TT (%) (n = 166) | OR (95% CI) | p-Value | |
| Stage | ||||
| Non-muscle invasive tumor | 141 (53.2%) | 94 (56.6%) | 1.00 (reference) | |
| Muscle invasive tumor | 124 (46.8%) | 72 (43.4%) | 0.87 (0.59–1.29) | 0.488 |
| Tumor T status | ||||
| Ta–Tcis | 62 (23.4%) | 28 (16.9%) | 1.00 (reference) | |
| T1–T4 | 203 (76.6%) | 138 (83.1%) | 1.51 (0.92–2.47) | 0.106 |
| Lymph node status | ||||
| N0 | 230 (86.8%) | 150 (90.4%) | 1.00 (reference) | |
| N1+N2+N3 | 35 (13.2%) | 16 (9.6%) | 0.70 (0.37–1.31) | 0.266 |
| Metastasis | ||||
| M0 | 255 (96.2%) | 162 (97.6%) | 1.00 (reference) | |
| M1 | 10 (3.8%) | 4 (2.4%) | 0.63 (0.19–2.04) | 0.441 |
| Histopathologic grading | ||||
| Low grade | 32 (12.1%) | 21 (12.7%) | 1.00 (reference) | |
| High grade | 233 (87.9%) | 145 (87.3%) | 0.95 (0.53–1.71) | 0.860 |
| Variable | HOTAIR (rs4759314) | |||
| AA (%) (n=363) | AG+GG (%) (n=68) | OR (95% CI) | p-Value | |
| Stage | ||||
| Non-muscle invasive tumor | 199 (54.8%) | 36 (52.9%) | 1.00 (reference) | |
| Muscle invasive tumor | 164 (45.2%) | 32 (47.1%) | 1.08 (0.64–1.81) | 0.775 |
| Tumor T status | ||||
| Ta–Tcis | 75 (20.7%) | 15 (22.1%) | 1.00 (reference) | |
| T1–T4 | 288 (79.3%) | 53 (77.9%) | 0.92 (0.49–1.72) | 0.795 |
| Lymph node status | ||||
| N0 | 317 (87.3%) | 63 (92.6%) | 1.00 (reference) | |
| N1+N2+N3 | 46 (12.7%) | 5 (7.4%) | 0.55 (0.21–1.43) | 0.219 |
| Metastasis | ||||
| M0 | 351 (96.7%) | 66 (97.1%) | 1.00 (reference) | |
| M1 | 12 (3.3%) | 2 (2.9%) | 0.89 (0.19–4.05) | 0.876 |
| Histopathologic grading | ||||
| Low grade | 44 (12.1%) | 9 (13.2%) | 1.00 (reference) | |
| High grade | 319 (87.9%) | 59 (86.8%) | 0.9 (0.42–1.95) | 0.797 |
| Variable | HOTAIR (rs12427129) | |||
| CC (%) (n = 350) | CT+TT (%) (n = 81) | OR (95% CI) | p-Value | |
| Stage | ||||
| Non-muscle invasive tumor | 185 (52.9%) | 50 (61.7%) | 1.00 (reference) | |
| Muscle invasive tumor | 165 (47.1%) | 31 (38.3%) | 0.70 (0.42–1.14) | 0.150 |
| Tumor T status | ||||
| Ta–Tcis | 77 (22%) | 13 (16%) | 1.00 (reference) | |
| T1–T4 | 273 (78%) | 68 (84%) | 1.48 (0.77–2.81) | 0.237 |
| Lymph node status | ||||
| N0 | 307 (87.7%) | 73 (90.1%) | 1.00 (reference) | |
| N1+N2+N3 | 43 (12.3%) | 8 (9.9%) | 0.78 (0.35–1.74) | 0.546 |
| Metastasis | ||||
| M0 | 339 (96.9%) | 78 (96.3%) | 1.00 (reference) | |
| M1 | 11 (3.1%) | 3 (3.7%) | 1.19 (0.32–4.35) | 0.798 |
| Histopathologic grading | ||||
| Low grade | 46 (13.1%) | 7 (8.6%) | 1.00 (reference) | |
| High grade | 304 (86.9%) | 74 (91.4%) | 1.6 (0.69–3.69) | 0.270 |
| Variable | Smoker | Non-Smokers | Aged ≤65 Years | Aged >65 Years |
|---|---|---|---|---|
| HOTAIR (rs920778) | ||||
| Stage | p = 0.525 a | p = 0.424 | p = 0.869 | p = 0.238 |
| Tumor T status | p = 0.100 b | p = 0.840 | p = 0.202 | p = 0.677 |
| Lymph node status | p = 0.126 c | p = 0.128 | p = 0.073 | p = 0.210 |
| Metastasis | p = 0.127 d | p = 0.665 | p = 0.275 | p = 0.710 |
| Histopathologic grading | p = 0.263 e | p = 0.086 | p = 0.871 | p = 0.239 |
| HOTAIR (rs1899663) | ||||
| Stage | p = 0.178 | p = 0.944 | p = 0.847 | p = 0.468 |
| Tumor T status | p = 0.118 | p = 0.380 | p = 0.162 | p = 0.334 |
| Lymph node status | p = 0.317 | p = 0.544 | p = 0.589 | p = 0.310 |
| Metastasis | p = 0.134 | p = 0.354 | p = 0.604 | p = 0.574 |
| Histopathologic grading | p = 0.236 | p = 0.303 | p = 0.736 | p = 0.595 |
| HOTAIR (rs4759314) | ||||
| Stage | p = 0.819 | p = 0.812 | p = 0.161 | p = 0.472 |
| Tumor T status | p = 0.769 | p = 0.872 | p = 0.599 | p = 0.479 |
| Lymph node status | p = 0.608 | p = 0.098 | p = 0.969 | p = 0.127 |
| Metastasis | p = 0.353 | p = 0.999 | p = 0.953 | p = 0.888 |
| Histopathologic grading | p = 0.467 | p = 0.910 | p = 0.366 | p = 0.266 |
| HOTAIR (rs12427129) | ||||
| Stage | p = 0.074 | p = 0.611 | p = 0.752 | p = 0.087 |
| Tumor T status | p = 0.046 | p = 0.933 | p = 0.046 | p = 0.761 |
| Lymph node status | p = 0.523 | p = 0.749 | p = 0.556 | p = 0.605 |
| Metastasis | p = 0.448 | p = 0.210 | p = 0.585 | p = 0.380 |
| Histopathologic grading | p = 0.128 | p = 0.867 | p = 0.235 | p = 0.649 |
References
- Hung, C.F.; Yang, C.K.; Ou, Y.C. Urologic cancer in Taiwan. Jpn. J. Clin. Oncol. 2016, 46, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef] [PubMed]
- De Maturana, E.L.; Rava, M.; Anumudu, C.; Saez, O.; Alonso, D.; Malats, N. Bladder cancer genetic susceptibility. A systematic review. Bladder Cancer 2018, 4, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Zhou, H.; Fan, H.; Yuan, Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget 2017, 8, 110635–110649. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.A.; Luske, C.M.; Roppel, S.; Schaudinn, A.; Zimmer, C.; Pfluger, R.; Haubrock, M.; Rapp, J.; Gungor, C.; Bockhorn, M.; et al. Relevance of SP binding site polymorphism in WWOX for treatment outcome in pancreatic cancer. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, K.; Gong, P.; Qiao, F.; Wang, L.; Cui, H.; Sui, X.; Gao, J.; Fan, H. A novel functional TagSNP Rs7560488 in the DNMT3A1 promoter is associated with susceptibility to gastric cancer by modulating promoter activity. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, W. Explaining the disease phenotype of intergenic SNP through predicted long range regulation. Nucleic Acids Res. 2016, 44, 8641–8654. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Sharathchandra, A.; Ponnuswamy, A.; Grover, R.; Das, S. Effect of a natural mutation in the 5′ untranslated region on the translational control of p53 mRNA. Oncogene 2013, 32, 4148–4159. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.T.; Liu, Y.F.; Hsu, C.L.; Cheng, T.Y.; Yang, C.Y.; Chang, J.S.; Lee, W.J.; Hsiao, M.; Juan, H.F.; Chien, M.H.; et al. 3′UTR polymorphisms of carbonic anhydrase IX determine the miR-34a targeting efficiency and prognosis of hepatocellular carcinoma. Sci. Rep. 2017, 7, 4466. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Gui, J.; Hu, T.; Wyszynski, A.; Marsit, C.J.; Kelsey, K.T.; Schned, A.R.; Tanyos, S.A.; Pendleton, E.M.; Ekstrom, R.M.; et al. Genetic polymorphisms modify bladder cancer recurrence and survival in a USA population-based prognostic study. BJU Int. 2015, 115, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Fei, T.; Verhaak, R.G.; Su, Z.; Zhang, Y.; Brown, M.; Chen, Y.; Liu, X.S. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat. Struct. Mol. Biol. 2013, 20, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Calle, A.; Kawamura, Y.; Yamamoto, Y.; Takeshita, F.; Ochiya, T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018, 109, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Hashad, D.; Elbanna, A.; Ibrahim, A.; Khedr, G. Evaluation of the role of circulating long non-coding RNA H19 as a promising novel biomarker in plasma of patients with gastric cancer. J. Clin. Lab. Anal. 2016, 30, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, T.; Wang, Y.; Yu, J.; Liu, Y.; Lin, Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol. Ther. 2016, 17, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; He, Q.; Wang, J. Clinical values of long non-coding RNAs in bladder cancer: A systematic review. Front. Physiol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Berrondo, C.; Flax, J.; Kucherov, V.; Siebert, A.; Osinski, T.; Rosenberg, A.; Fucile, C.; Richheimer, S.; Beckham, C.J. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS ONE 2016, 11, e0147236. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.H.; Lu, S.W.; Huang, Y.Q.; Que, G.B.; Chen, J.H.; Chen, Y.P.; Zhang, H.B.; Liang, X.L.; Jiang, J.H. Upregulation of the long noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladder cancer. Tumour Biol. 2014, 35, 10249–10257. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, M.; Feber, A.; Duenas, M.; Segovia, C.; Rubio, C.; Fernandez, M.; Villacampa, F.; Duarte, J.; Lopez-Calderon, F.F.; Gomez-Rodriguez, M.J.; et al. Analysis of the polycomb-related LncRNAs HOTAIR and ANRIL in bladder cancer. Clin. Epigenet. 2015, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, M.; Salavaty, A. HOTAIR: An oncogenic long non-coding rna in different cancers. Cancer Biol. Med. 2015, 12, 1–9. [Google Scholar] [PubMed]
- Li, J.; Cui, Z.; Li, H.; Lv, X.; Gao, M.; Yang, Z.; Bi, Y.; Zhou, B.; Yin, Z. Long non-coding RNA Hotair polymorphism and susceptibility to cancer: An updated meta-analysis. Environ. Health Prev. Med. 2018, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Mu, X.; Tong, A.; Qian, Y.; Ling, C.; Yi, T.; Zhao, X. The association between HOTAIR polymorphisms and cancer susceptibility: An updated systemic review and meta-analysis. Onco Targets Ther. 2018, 11, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Tong, X.; Zhang, W.N.; Fu, W.N. Association between the HOTAIR polymorphisms and cancer risk: An updated meta-analysis. Oncotarget 2017, 8, 4460–4470. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Lin, C.W.; Chuang, C.Y.; Liu, Y.F.; Yeh, C.M.; Yang, S.F. Impact of HOTAIR gene polymorphism and environmental risk on oral cancer. J. Dent. Res. 2018, 97, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Li, Y.W.; Zhang, H.B.; Gong, H.; Yuan, Y.; Li, W.T.; Liu, H.Y.; Chen, J. HOTAIR LncRNA SNPs Rs920778 and Rs1899663 are associated with smoking, male gender, and squamous cell carcinoma in a chinese lung cancer population. Acta Pharmacol. Sin. 2018, 39, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Wang, J.; Huang, B.; Chen, A.; Li, G.; Li, X.; Wang, J. Association of HOTAIR polymorphisms Rs4759314 and Rs920778 with cancer susceptibility on the basis of ethnicity and cancer type. Oncotarget 2016, 7, 38775–38784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Lin, Z.; Ji, X.; Pi, L.; Lin, X.; Tian, N.; Liu, G.; Liu, Q.; Lin, Z.; et al. SNP-SNP and SNP-environment interactions of potentially functional HOTAIR SNPs modify the risk of hepatocellular carcinoma. Mol. Carcinog. 2018. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lu, X.; Zheng, L.; Liu, X.; Hu, M. Association of long non-coding RNA HOTAIR polymorphisms with cervical cancer risk in a chinese population. PLoS ONE 2016, 11, e0160039. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Liu, L.; Wei, J.; Ge, Y.; Zhang, J.; Chen, H.; Zhou, L.; Yuan, Q.; Zhou, C.; Yang, M. A functional LncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol. Carcinog. 2016, 55, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, L.; Fu, G.; Sun, F.; Shi, J.; Wei, J.; Lu, C.; Zhou, C.; Yuan, Q.; Yang, M. The identification of an ESCC susceptibility SNP Rs920778 that regulates the expression of LncRNA HOTAIR via a novel intronic enhancer. Carcinogenesis 2014, 35, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, W.; Jin, H.; Wang, Q.; Ge, Y.; Lu, J.; Ma, G.; Chu, H.; Tong, N.; Zhu, H.; et al. The association analysis of LncRNA HOTAIR genetic variants and gastric cancer risk in a chinese population. Oncotarget 2015, 6, 31255–31262. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Cheng, H.L.; Liu, Y.F.; Chou, M.C.; Yang, S.F.; Chou, Y.E. Functional genetic variant of WW domain-containing oxidoreductase (WWOX) gene is associated with hepatocellular carcinoma risk. PLoS ONE 2017, 12, e0176141. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Habuchi, T.; Takahashi, T.; Mitsumori, K.; Kamoto, T.; Kakehi, Y.; Kakinuma, H.; Sato, K.; Nakamura, A.; Ogawa, O.; et al. Cyclin D1 gene polymorphism is associated with an increased risk of urinary bladder cancer. Carcinogenesis 2002, 23, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, N.A.; Nassir, A.; Saada, H.; Dannoun, A.; Qoqandi, O.; Alsharif, A.; Tayeb, M.T. Combined genetic biomarkers confer susceptibility to risk of urothelial bladder carcinoma in a Saudi population. Dis. Mark. 2017, 2017, 1474560. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Castelao, J.E.; Yuan, J.M.; Stern, M.C.; Conti, D.V.; Cortessis, V.K.; Pike, M.C.; Gago-Dominguez, M. Cigarette smoking and subtypes of bladder cancer. Int. J. Cancer 2012, 130, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Skinner, E.C.; Lieskovsky, G.; Skinner, D.G. Radical cystectomy in the elderly patient. J. Urol. 1984, 131, 1065–1068. [Google Scholar] [CrossRef]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin. Cancer Res. 2014, 20, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Chen, Q.; Wang, Y.; Zhou, Z.; Huang, Y.; Qiu, F. Upregulated malat-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol. Biosyst. 2012, 8, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Pang, H.; Li, X.; Li, H.; Pan, J.; Chen, W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016, 107, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Omrani, M.D.; Ghafouri-Fard, S. Long non-coding RNA expression in bladder cancer. Biophys. Rev. 2018, 10, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Heubach, J.; Monsior, J.; Deenen, R.; Niegisch, G.; Szarvas, T.; Niedworok, C.; Schulz, W.A.; Hoffmann, M.J. The long noncoding RNA HOTAIR has tissue and cell type-dependent effects on HOX gene expression and phenotype of urothelial cancer cells. Mol. Cancer 2015, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Fog, C.K.; Galli, G.G.; Lund, A.H. PRDM proteins: Important players in differentiation and disease. Bioessays 2012, 34, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Payer, B.; Rosenberg, M.; Yamaji, M.; Yabuta, Y.; Koyanagi-Aoi, M.; Hayashi, K.; Yamanaka, S.; Saitou, M.; Lee, J.T. Tsix RNA and the germline factor, PRDM14, link X reactivation and stem cell reprogramming. Mol. Cell 2013, 52, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Hemelt, M.; Yamamoto, H.; Cheng, K.K.; Zeegers, M.P. The effect of smoking on the male excess of bladder cancer: A meta-analysis and geographical analyses. Int. J. Cancer 2009, 124, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, F.; Xu, Y.; Wang, B.; Zhao, Y.; Xu, W.; Shi, L.; Lu, X.; Liu, Q. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2015, 282, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.E.; Lin, C.C.; Lee, I.T.; Hsu, C.K.; Kou, Y.R.; Yang, C.M. Cigarette smoke extract regulates cytosolic phospholipase A2 expression via NADPH oxidase/MAPKs/AP-1 and p300 in human tracheal smooth muscle cells. J. Cell. Biochem. 2011, 112, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Zhang, Q.; Gu, D.; Zhang, K.; Ge, Y.; Chu, H.; Du, M.; Xu, B.; Wang, M.; et al. Tagging snps in the hotair gene are associated with bladder cancer risk in a chinese population. Gene 2018, 664, 22–26. [Google Scholar] [CrossRef] [PubMed]

| Variable | Controls (n = 862) n (%) | Patients (n = 431) n (%) | p-Value |
|---|---|---|---|
| Age (years) | |||
| mean ± S.D. | 57.2 ± 10 | 68.6 ± 11.8 | p < 0.001 |
| ≤65 | 697 (80.9%) | 166 (38.5%) | p < 0.001 |
| >65 | 165 (19.1%) | 265 (61.5%) | |
| Gender | |||
| Male | 566 (65.7%) | 272 (63.1%) | 0.365 |
| Female | 296 (34.3%) | 159 (36.9%) | |
| Tobacco consumption | |||
| No | 562 (65.2%) | 300 (69.6%) | 0.113 |
| Yes | 300 (34.8%) | 131 (30.4%) | |
| Stage | |||
| Non-muscle invasive tumor | 235 (54.5%) | ||
| Muscle invasive tumor | 196 (45.5%) | ||
| Tumor T Status | |||
| Ta | 90 (16.5%) | ||
| Tcis | 19 (4.4%) | ||
| T1 | 145 (33.6%) | ||
| T2 | 52 (12.1%) | ||
| T3 | 107 (24.8%) | ||
| T4 | 37 (8.6%) | ||
| Lymph Node Status | |||
| N0 | 380 (88.2%) | ||
| N1 | 13 (3.0%) | ||
| N2 | 33 (7.6%) | ||
| N3 | 5 (1.2%) | ||
| Metastasis | |||
| M0 | 417 (96.8%) | ||
| M1 | 14 (3.2%) | ||
| Histopathologic Grading | |||
| Low grade | 53 (12.3%) | ||
| High grade | 378 (87.7%) |
| Variable | Controls (n = 862) n (%) | Patients (n = 431) n (%) | OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| rs920778 | ||||
| TT | 447 (51.9%) | 217 (50.3%) | 1.00 (reference) | 1.00 (reference) |
| TC | 360 (41.8%) | 176 (40.8%) | 1.01 (0.79–1.28) | 0.96 (0.73–1.27) |
| CC | 55 (6.4%) | 38 (8.8%) | 1.42 (0.91–2.22) | 1.60 (0.96–2.68) |
| TC + CC | 415 (48.1%) | 214 (49.7%) | 1.06 (0.84–1.34) | 1.04 (0.80–1.35) |
| rs1899663 | ||||
| GG | 550 (63.8%) | 265 (61.5%) | 1.00 (reference) | 1.00 (reference) |
| GT | 285 (33.1%) | 148 (34.3%) | 1.08 (0.84–1.38) | 0.95 (0.71–1.25) |
| TT | 27 (3.1%) | 18 (4.2%) | 1.38 (0.75–2.56) | 1.85 (0.92–3.73) |
| GT + TT | 312 (36.2%) | 166 (38.5%) | 1.10 (0.87–1.40) | 1.01 (0.77–1.32) |
| rs4759314 | ||||
| AA | 727 (84.3%) | 363 (84.2%) | 1.00 (reference) | 1.00 (reference) |
| AG | 128 (14.8%) | 67 (15.5%) | 1.05 (0.76–1.45) | 1.07 (0.74–1.54) |
| GG | 7 (0.8%) | 1 (0.2%) | 0.29 (0.04–2.33) | 0.59 (0.06–5.58) |
| AG + GG | 135 (15.7%) | 68 (15.8%) | 1.01 (0.73–1.39) | 1.05 (0.73–1.51) |
| rs12427129 | ||||
| CC | 691 (80.2%) | 350 (81.2%) | 1.00 (reference) | 1.00 (reference) |
| CT | 167 (19.4%) | 76 (17.6%) | 0.90 (0.67–1.21) | 1.00 (0.71–1.41) |
| TT | 4 (0.5%) | 5 (1.2%) | 2.47 (0.66–9.25) | 2.23 (0.44–11.23) |
| CT + TT | 171 (19.8%) | 81 (18.8%) | 0.94 (0.70–1.26) | 1.03 (0.74–1.44) |
| Variable | Controls (n = 296), n (%) | Patients (n = 159), n (%) | OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| rs920778 | ||||
| TT | 155 (52.4%) | 79 (49.7%) | 1.000 (reference) | 1.00 (reference) |
| TC | 120 (40.5%) | 61 (38.4%) | 1.00 (0.66–1.50) | 1.03 (0.62–1.71) |
| CC | 21 (7.1%) | 19 (11.9%) | 1.78 (0.90–3.49) | 1.96 (0.84–4.61) |
| TC + CC | 141 (47.6%) | 80 (50.3%) | 1.11 (0.76–1.64) | 1.16 (0.72–1.88) |
| rs1899663 | ||||
| GG | 191 (64.5%) | 95 (59.7%) | 1.00 (reference) | 1.00 (reference) |
| GT | 94 (31.8%) | 57 (35.8%) | 1.22 (0.81–1.84) | 0.92 (0.55–1.54) |
| TT | 11 (3.7%) | 7 (4.4%) | 1.28 (0.48–3.41) | 1.79 (0.58–5.51) |
| GT + TT | 105 (35.5%) | 64 (40.3%) | 1.23 (0.82–1.82) | 1.00 (0.61–1.63) |
| rs4759314 | ||||
| AA | 256 (86.5%) | 126 (79.2%) | 1.00 (reference) | 1.00 (reference) |
| AG | 35 (11.8%) | 32 (20.1%) | 1.86 (1.10–3.14) p = 0.021 | 1.98 (1.03–3.82) p = 0.042 |
| GG | 5 (1.7%) | 1 (0.6%) | 0.41 (0.05–3.52) | 1.12 (0.09–13.66) |
| AG + GG | 40 (13.5%) | 33 (20.8%) | 1.68 (1.01–2.79) p = 0.046 | 1.92 (1.01–3.64) p = 0.047 |
| rs12427129 | ||||
| CC | 240 (81.1%) | 128 (80.5%) | 1.00 (reference) | 1.00 (reference) |
| CT | 53 (17.9%) | 28 (17.6%) | 0.99 (0.60–1.64) | 1.50 (0.80–2.80) |
| TT | 3 (1%) | 3 (1.9%) | 1.88 (0.37–9.42) | 1.40 (0.19–10.56) |
| CT+TT | 56 (18.9%) | 31 (19.5%) | 1.04 (0.64–1.69) | 1.49 (0.81–2.73) |
| Variable | HOTAIR (rs920778) | |||
|---|---|---|---|---|
| TT (%) (n = 217) | TC+CC (%) (n = 214) | OR (95% CI) | p-Value | |
| Stage | ||||
| Non-muscle invasive tumor (pTa–pT1) | 113 (52.1%) | 122 (57%) | 1.00 (reference) | |
| Muscle invasive tumor (pT2–pT4) | 104 (47.9%) | 92 (43%) | 0.82 (0.56–1.20) | 0.304 |
| Tumor T status | ||||
| Ta–Tcis | 50 (23%) | 40 (18.7%) | 1.00 (reference) | |
| T1–T4 | 167 (77%) | 174 (81.3%) | 1.30 (0.82–.077) | 0.267 |
| Lymph node status | ||||
| N0 | 184 (84.8%) | 196 (91.6%) | 1.00 (reference) | |
| N1+N2+N3 | 33 (15.2%) | 18 (8.4%) | 0.51 (0.28–0.94) | 0.031 |
| Metastasis | ||||
| M0 | 208 (95.9%) | 209 (97.7%) | 1.00 (reference) | |
| M1 | 9 (4.1%) | 5 (2.3%) | 0.55 (0.18–1.68) | 0.295 |
| Histopathologic grading | ||||
| Low grade | 24 (11.1%) | 29 (13.6%) | 1.00 (reference) | |
| High grade | 193 (88.9%) | 185 (86.4%) | 0.79 (0.45–1.41) | 0.432 |
| Variable | HOTAIR (rs12427129) | |||
|---|---|---|---|---|
| CC (%) (n = 103) | CT + TT (%), (n = 28) | OR (95% CI) | p-Value | |
| Stage | ||||
| Non-muscle invasive tumor (pTa–pT1) | 50 (48.5%) | 19 (67.9%) | 1.00 (reference) | |
| Muscle invasive tumor (pT2–pT4) | 53 (51.5%) | 9 (32.1%) | 0.45 (0.19–1.08) | 0.074 |
| Tumor T status | ||||
| Ta–Tcis | 27 (26.2%) | 2 (7.1%) | 1.00 (reference) | |
| T1–T4 | 76 (73.8%) | 26 (92.9%) | 4.62 (1.03–20.78) | 0.046 |
| Lymph node status | ||||
| N0 | 87 (84.5%) | 25 (89.3%) | 1.00 (reference) | |
| N1+N2+N3 | 16 (15.5%) | 3 (10.7%) | 0.65 (0.18–2.42) | 0.523 |
| Metastasis | ||||
| M0 | 95 (92.2%) | 27 (96.4%) | 1.00 (reference) | |
| M1 | 8 (7.8%) | 1 (3.6%) | 0.44 (0.05–3.67) | 0.448 |
| Histopathologic grading | ||||
| Low grade | 16 (15.5%) | 1 (3.6%) | 1.00 (reference) | |
| High grade | 87 (84.5%) | 27 (96.4%) | 4.97 (0.63–39.19) | 0.128 |
| Variable | HOTAIR (rs12427129) | |||
|---|---|---|---|---|
| CC (%), (n = 128) | CT + TT (%) (n = 38) | OR (95% CI) | p-Value | |
| Stage | ||||
| Non-muscle invasive tumor (pTa–pT1) | 67 (52.3%) | 21 (55.3%) | 1.00 (reference) | |
| Muscle invasive tumor (pT2–pT4) | 61 (47.7%) | 17 (44.7%) | 0.89 (0.43–1.84) | 0.752 |
| Tumor T status | ||||
| Ta–Tcis | 30 (23.4%) | 3 (7.9%) | 1.00 (reference) | |
| T1–T4 | 98 (76.6%) | 35 (92.1%) | 3.57 (1.03–12.44) | 0.046 |
| Lymph node status | ||||
| N0 | 106 (82.8%) | 33 (86.8%) | 1.00 (reference) | |
| N1 + N2 + N3 | 22 (17.2%) | 5 (13.2%) | 0.73 (0.26–2.08) | 0.556 |
| Metastasis | ||||
| M0 | 122 (95.3%) | 37 (97.4%) | 1.00 (reference) | |
| M1 | 6 (4.7%) | 1 (2.6%) | 0.55 (0.06–4.71) | 0.585 |
| Histopathologic grading | ||||
| Low grade | 20 (15.6%) | 3 (7.9%) | 1.00 (reference) | |
| High grade | 108 (84.4%) | 35 (92.1%) | 2.16 (0.61–7.71) | 0.235 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, M.-C.; Wen, Y.-C.; Wang, S.-S.; Lin, Y.-W.; Chow, J.-M.; Yang, S.-F.; Chien, M.-H. Impact of Long Non-Coding RNA HOTAIR Genetic Variants on the Susceptibility and Clinicopathologic Characteristics of Patients with Urothelial Cell Carcinoma. J. Clin. Med. 2019, 8, 282. https://doi.org/10.3390/jcm8030282
Tung M-C, Wen Y-C, Wang S-S, Lin Y-W, Chow J-M, Yang S-F, Chien M-H. Impact of Long Non-Coding RNA HOTAIR Genetic Variants on the Susceptibility and Clinicopathologic Characteristics of Patients with Urothelial Cell Carcinoma. Journal of Clinical Medicine. 2019; 8(3):282. https://doi.org/10.3390/jcm8030282
Chicago/Turabian StyleTung, Min-Che, Yu-Ching Wen, Shian-Shiang Wang, Yung-Wei Lin, Jyh-Ming Chow, Shun-Fa Yang, and Ming-Hsien Chien. 2019. "Impact of Long Non-Coding RNA HOTAIR Genetic Variants on the Susceptibility and Clinicopathologic Characteristics of Patients with Urothelial Cell Carcinoma" Journal of Clinical Medicine 8, no. 3: 282. https://doi.org/10.3390/jcm8030282
APA StyleTung, M.-C., Wen, Y.-C., Wang, S.-S., Lin, Y.-W., Chow, J.-M., Yang, S.-F., & Chien, M.-H. (2019). Impact of Long Non-Coding RNA HOTAIR Genetic Variants on the Susceptibility and Clinicopathologic Characteristics of Patients with Urothelial Cell Carcinoma. Journal of Clinical Medicine, 8(3), 282. https://doi.org/10.3390/jcm8030282






