A Peri-Implant Disease Risk Score for Patients with Dental Implants: Validation and the Influence of the Interval between Maintenance Appointments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Study Population, Ethics, Inclusion and Exclusion Criteria
2.2. Sample Size
2.3. Outcome Measures, Variables
2.4. Statistical Analyses
3. Results
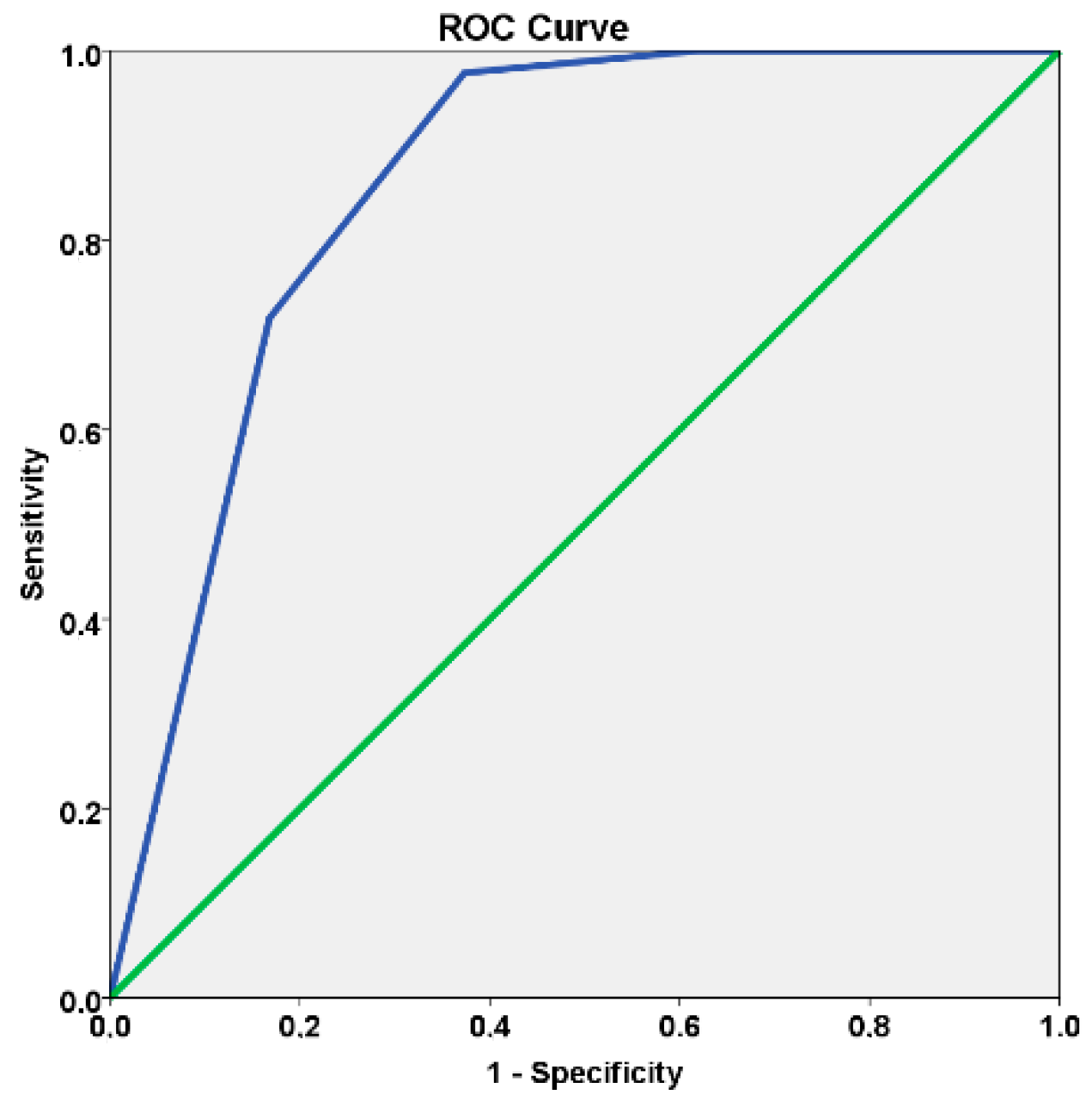
3.1. Primary Outcome Measure: Model Discrimination Capacity
3.2. Secondary Outcome Measure: Effect and Impact of Having > Six Months Intervals between Maintenance Visits
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T. Consensus report of session IV. In Proceedings of the First European Workshop on Periodontology, 1st ed.; Lang, N.P., Ed.; Quintessence: London, UK, 1984; pp. 365–369. [Google Scholar]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89, S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P.; Salvado, F. Attributable fractions, modifiable risk factors and risk stratification using a risk score for peri-implant pathology. J. Prosthodont. Res. 2017, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Mrdovic, I.; Savic, L.; Krljanac, G.; Asanin, M.; Lasica, R.; Djuricic, N.; Brdar, N.; Marinkovic, J.; Kocev, N.; Perunicic, J. Simple risk algorithm to predict serious bleeding in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. J. Circ. 2013, 77, 1719–1727. [Google Scholar] [CrossRef]
- Kobayashi, D.; Takahashi, O.; Ueda, T.; Deshpande, G.A.; Arioka, H.; Fukui, T. Risk factors for adverse reactions from contrast agents for computed tomography. BMC Med. Inform. Decis. Mak. 2013, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino Sr, R.B. Presentation of multivariate data for clinical use: The Framingham study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef] [PubMed]
- Cho-Yan Lee, J.; Mattheos, N.; Charles Nixon, K.; Ivanovski, S. Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin. Oral Implants Res. 2012, 23, 325–333. [Google Scholar] [CrossRef]
- Bidra, A.S.; Daubert, D.; Garcia, L.T.; Kosinski, T.F.; Nenn, C.A.; Olsen, J.A. Clinical practice guidelines for recall and maintenance of patients with tooth-borne and implant-borne dental retorations. J. Prosthodont. 2016, 25, S32–S40. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Tervonen, T. The Efficacy of supportive Peri-Implant Therapies in Preventing Peri-Implantitis and Implant Loss: A Systematic Review of the Literature. J. Oral Maxillofac. Res. 2016, 7, e12. [Google Scholar] [CrossRef]
- Monje, A.; Aranda, L.; Diaz, K.T.; Alarcón, M.A.; Bagramian, H.L.; Catena, A. Impact of maintenance therapy for the prevention of peri-implant diseases: A systematic review and meta-analysis. J. Dent. Res. 2016, 95, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.S. Implant maintenance treatment and peri-implant health. Evid. Based Dent. 2017, 18, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Bidra, A.S.; Daubert, D.; Garcia, L.T.; Gaunthier, M.F.; Kosinski, T.F.; Nenn, C.A. A systematic review of recall regimen of patients with dental restorations. Part 2: Implant-borne retorations. J. Prosthodont. 2016, 25, S16–S31. [Google Scholar] [CrossRef] [PubMed]
- Jemt, T.; Olsson, M.; Franke Stenport, V. Incidence of first implant failure: A retrospective study of 27 years of implant operations at one specialist clinic. Clin. Implant Dent. Relat. Res. 2015, 17, e501–e510. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P. Risk factors of peri-implant pathology. Eur. J. Oral Sci. 2015, 123, 131–139. [Google Scholar] [CrossRef]
- Mombelli, A.; Van Oosten, M.A.; Schurch, E., Jr.; Lang, N.P. The microbiota associated with successful or failing titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Cole, P.; MacMahon, B. Attributable risk percent in case-control studies. Br. J. Prev. Soc. Med. 1971, 25, 242–244. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Zarb, G.; Koka, S.; Albrektsson, T. Hyperbole, clinical dissonance, and scratching the surface: Complication or disease? Int. J. Prosthodont. 2013, 26, 311. [Google Scholar]
- Becker, S.T.; Beck-Broichsitter, B.E.; Graetz, C.; Dörfer, C.E.; Wiltfang, J.; Häsler, R. Peri-implantitis versus periodontitis: Functional differences indicated by transcriptome profiling. Clin. Implant Dent. Relat. Res. 2014, 16, 401–411. [Google Scholar] [CrossRef]
- Carcuac, O.; Berglundh, T. Composition of human peri-implantitis and periodontal lesions. J. Dent. Res. 2014, 93, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, S292–S304. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Buser, D.; Chen, S.T.; Cochran, D.; DeBruyn, H.; Jemt, T.; Koka, S.; Nevins, M.; Sennerby, L.; Simion, M. Statements from the Estepona Consensus Meeting on Peri-implantitis, 2–4 February 2012. Clin. Implant Dent. Relat. Res. 2012, 14, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.K.; Kotsakis, G.A.; Gerdes, S.; Walter, M.H. Cross-sectional study on the prevalence and risk indicators of peri-implant diseases. Eur. J. Oral Implantol. 2018, 8, 75–88. [Google Scholar]
- De Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P.; Salvado, F. Peri-implant pathology: The prevalence(s) of the condition, the risk model(s) of the condition. Muskuloskeletal. Reg. 2016, 2, e1271. [Google Scholar] [CrossRef]
- Qian, J.; Wennerberg, A.; Albrektsson, T. Reasons for marginal bone loss around oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 792–807. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign body reaction to biomaterials: On mechanisms for buildup and breakdown of osseointegration. Clin. Implant Dent. Relat. Res. 2014. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, M.A. Causation and causal inference in Epidemiology. Am. J. Public Health 2005, 95, 144S–150S. [Google Scholar] [CrossRef]
- Marrone, A.; Lasserre, J.; Bercy, P.; Brecx, M. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin. Oral Implants Res. 2013, 24, 934–940. [Google Scholar] [CrossRef]
- Gurgel, B.C.V.; Montenegro, S.C.L.; Dantas, P.M.C.; Pascoal, A.L.B.; Lima, K.C.; Calderon, P.D.S. Frequency of peri-implant diseases and associated factors. Clin. Oral Implants Res. 2017, 28, 1211–1217. [Google Scholar] [CrossRef]
- Dalago, H.R.; Schuldt Filho, G.; Renvert, S.; Bianchini, M.A. Risk indicators for peri-implantitis. A cross-sectional study with 916 implants. Clin. Oral Implants Res. 2017, 28, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Moy, P.K.; Medina, D.; Shetty, V.; Aghaloo, T.L. Dental implant failure rates and associated risk factors. Int. J. Oral Maxillofac. Implants 2005, 20, 569–577. [Google Scholar] [PubMed]
- Chuang, S.K.; Wei, L.J.; Douglass, C.W.; Dodson, T.B. Risk factors for dental implant failure: A strategy for the analysis of clustered failure-time observations. J. Dent. Res. 2002, 81, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Brägger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 625–642. [Google Scholar] [CrossRef] [PubMed]
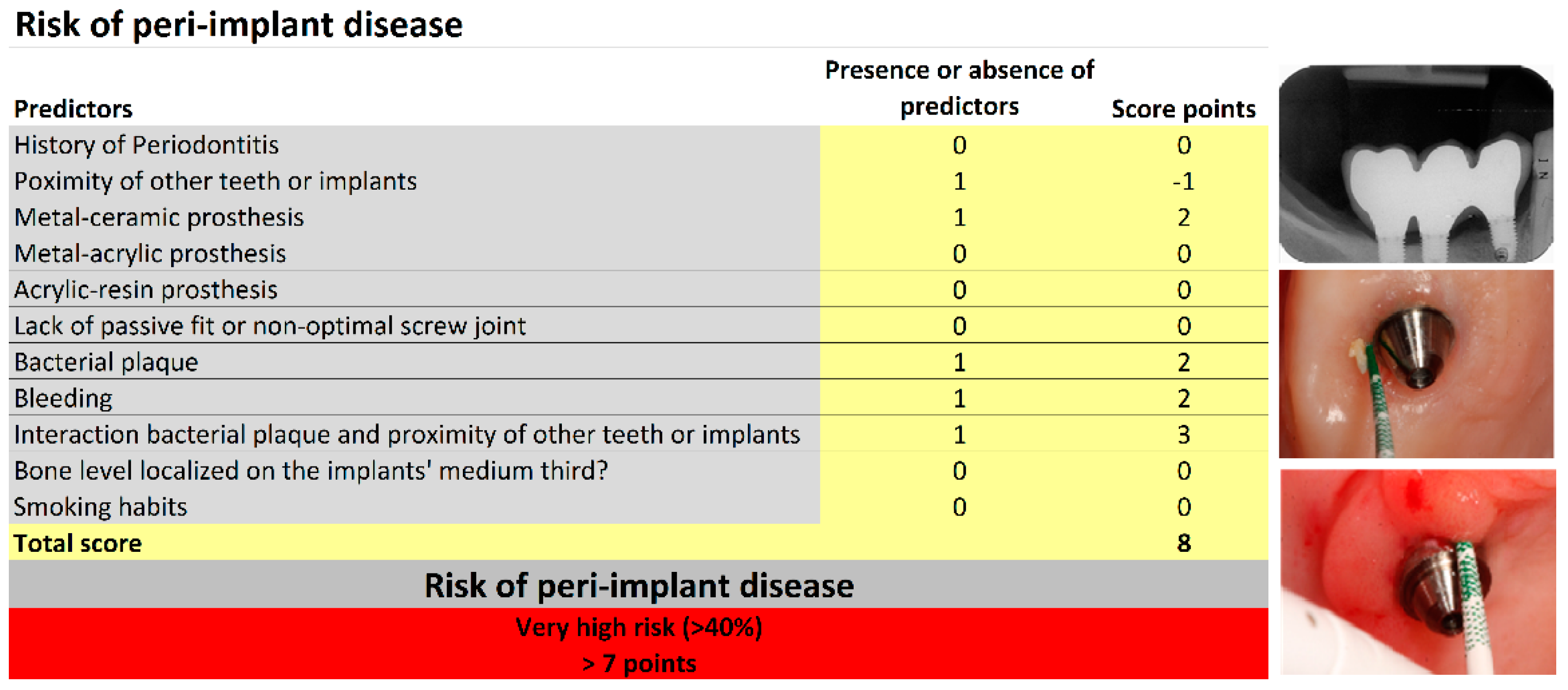
| Variable | Total (n = 353) n (%) | Risk Score Points * | ||
|---|---|---|---|---|
| History of Periodontitis | 137 (38.8) | 5 | ||
| Bone level located on the implants’ middle third | 20 (5.7) | 5 | ||
| Lack of prosthetic fit or non-optimal screw joint (passive misfit abutment screw loosening, prosthetic screw loosening, prosthetic decementations) | 20 (5.7) | 3 | ||
| Bacterial plaque | 187 (53) | 2 | ||
| Bleeding | 175 (49.6) | 2 | ||
| Type of material used in the restoration | ||||
| Ceramic | 79 (22.4) | 0 | ||
| Metal–ceramic | 186 (52.7) | 2 | ||
| Metal–acrylic | 104 (29.5) | −2 | ||
| Acrylic | 42 (11.9) | 1 | ||
| Adjacent implants or teeth present | 214 (60.6) | −1 | ||
| Simultaneous presence of bacterial plaque and adjacent implants or teeth present | 90 (25.5) | 3 | ||
| Smoker | 83 (23.5) | 0 | ||
| Risk profile groups obtained from the sum of risk score points for each variable | ||||
| Risk profile | Low risk | Moderate risk | High risk | Very high risk |
| Number of points | <2 points | 2–4 points | 5–7 points | >7 points |
| Variables | Low Risk Total of 102 Patients | Moderate Risk Total of 68 Patients | High Risk Total of 77 Patients | Very High Risk Total of 108 Patients |
|---|---|---|---|---|
| Demographics | N (within group %) | N (within group %) | N (within group %) | N (within group %) |
| Average age in years | 49.5 | 52.7 | 53.5 | 54.6 |
| Sex | ||||
| Female | 56 (54.9%) | 50 (73.5%) | 45 (58.4%) | 60 (56.6%) |
| Male | 46 (45.1%) | 18 (26.5%) | 32 (41.6%) | 46 (43.4%) |
| Predictors | N (within group %) | N (within group %) | N (within group %) | N (within group %) |
| History of Periodontitis | 0 (0%) | 13 (19.1%) | 43 (55.8%) | 81 (76.4%) |
| Bone level located on the implants’ middle third | 0 (0%) | 0 (0%) | 2 (2.6%) | 18 (17%) |
| Lack of prosthetic fit or non-optimal screw joint | 0 (0%) | 2 (2.9%) | 2 (2.6%) | 16 (15.1%) |
| Bacterial plaque | 6 (5.9%) | 37 (54.4%) | 49 (63.6%) | 95 (89.6%) |
| Bleeding | 11 (10.8%) | 35 (51.5%) | 42 (54.5%) | 87 (82.1%) |
| Type of restoration material | ||||
| Ceramic | 39 (38.2%) | 13 (19.1%) | 15 (19.5%) | 12 (11.3%) |
| Metal–ceramic | 45 (44.1%) | 26 (38.2%) | 39 (50.7%) | 76 (71.7%) |
| Metal–acrylic | 22 (21.6%) | 35 (51.5%) | 29 (37.7%) | 18 (17%) |
| Acrylic | 6 (5.9%) | 4 (5.9%) | 9 (11.7%) | 23 (21.7%) |
| Adjacent implants/teeth present | 84 (82.4%) | 26 (38.2%) | 41 (53.2%) | 63 (59.4%) |
| Interaction presence of bacterial plaque * adjacent implants/teeth present | 0 (0%) | 9 (13.2%) | 25 (32.5%) | 56 (52.8%) |
| Smoker | 19 (18.6%) | 12 (17.6%) | 22 (28.6%) | 30 (28.3%) |
| Variable | B | Standard Error | Significance (p-Value) | Relative Risk (RR) | 95% Confidence Interval for RR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Risk Group | 1.71 | 0.212 | p < 0.001 | 5.52 | 3.64 | 8.35 |
| Constant | −6.41 | 0.753 | p < 0.001 | 0.002 | ||
| Variable | B | Standard Error | Significance (p-Value) | Relative Risk (RR) | 95% Confidence Interval for RR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| >6 months between maintenance appointments | 0.118 | 0.344 | p = 0.732 | 1.13 | 0.57 | 2.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo Nobre, M.; Salvado, F.; Nogueira, P.; Rocha, E.; Ilg, P.; Maló, P. A Peri-Implant Disease Risk Score for Patients with Dental Implants: Validation and the Influence of the Interval between Maintenance Appointments. J. Clin. Med. 2019, 8, 252. https://doi.org/10.3390/jcm8020252
de Araújo Nobre M, Salvado F, Nogueira P, Rocha E, Ilg P, Maló P. A Peri-Implant Disease Risk Score for Patients with Dental Implants: Validation and the Influence of the Interval between Maintenance Appointments. Journal of Clinical Medicine. 2019; 8(2):252. https://doi.org/10.3390/jcm8020252
Chicago/Turabian Stylede Araújo Nobre, Miguel, Francisco Salvado, Paulo Nogueira, Evangelista Rocha, Peter Ilg, and Paulo Maló. 2019. "A Peri-Implant Disease Risk Score for Patients with Dental Implants: Validation and the Influence of the Interval between Maintenance Appointments" Journal of Clinical Medicine 8, no. 2: 252. https://doi.org/10.3390/jcm8020252
APA Stylede Araújo Nobre, M., Salvado, F., Nogueira, P., Rocha, E., Ilg, P., & Maló, P. (2019). A Peri-Implant Disease Risk Score for Patients with Dental Implants: Validation and the Influence of the Interval between Maintenance Appointments. Journal of Clinical Medicine, 8(2), 252. https://doi.org/10.3390/jcm8020252







