Troponin Testing for Assessing Sepsis-Induced Myocardial Dysfunction in Patients with Septic Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Patients and Definition of Sepsis
2.3. Clinical and Laboratory Data
2.4. High-Sensitivity Troponin I Assay
2.5. Echocardiographic Variables
2.6. Statistics
3. Results
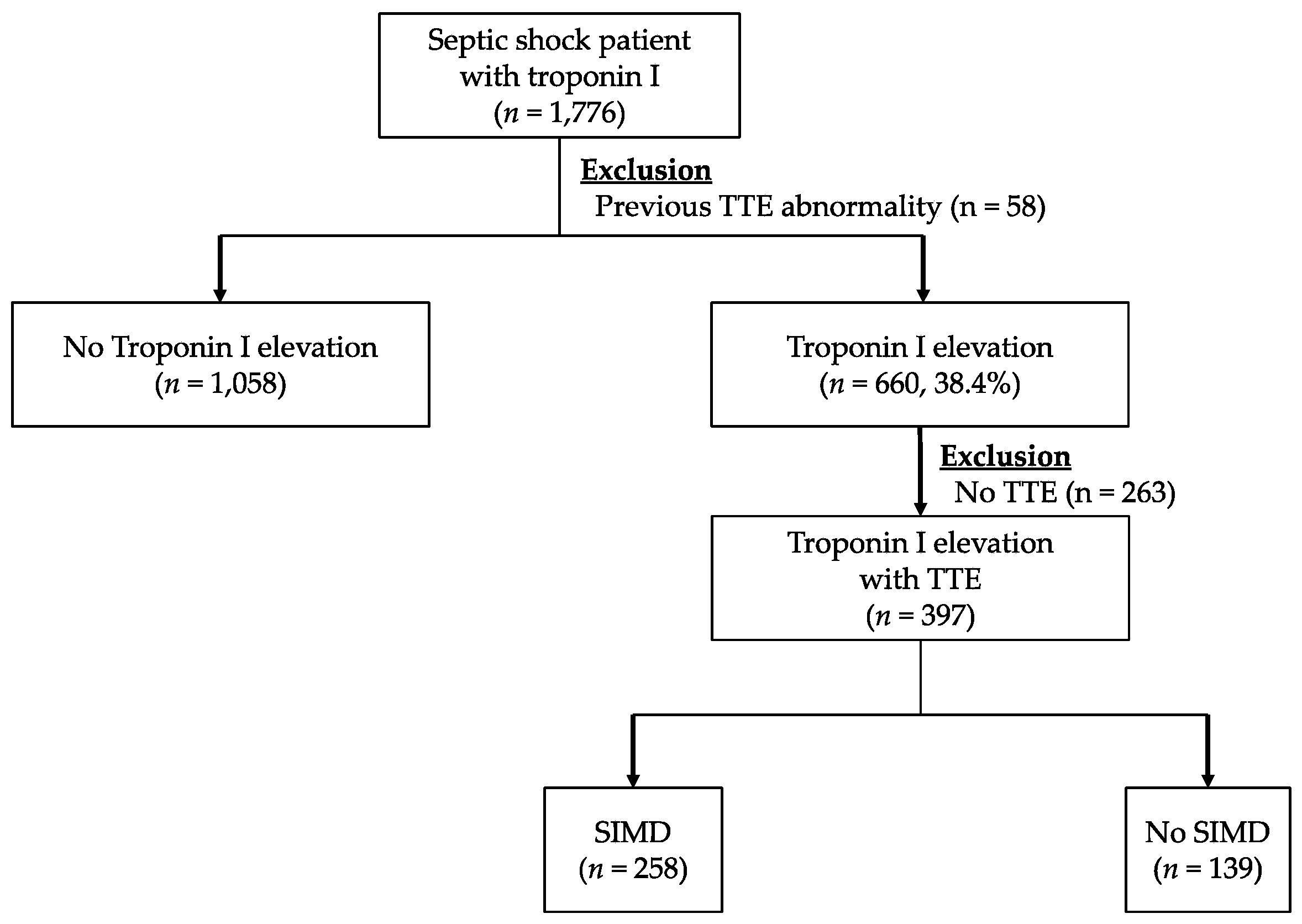
3.1. Study Population
3.2. Baseline Characteristics and Frequency of Cardiac Dysfunction
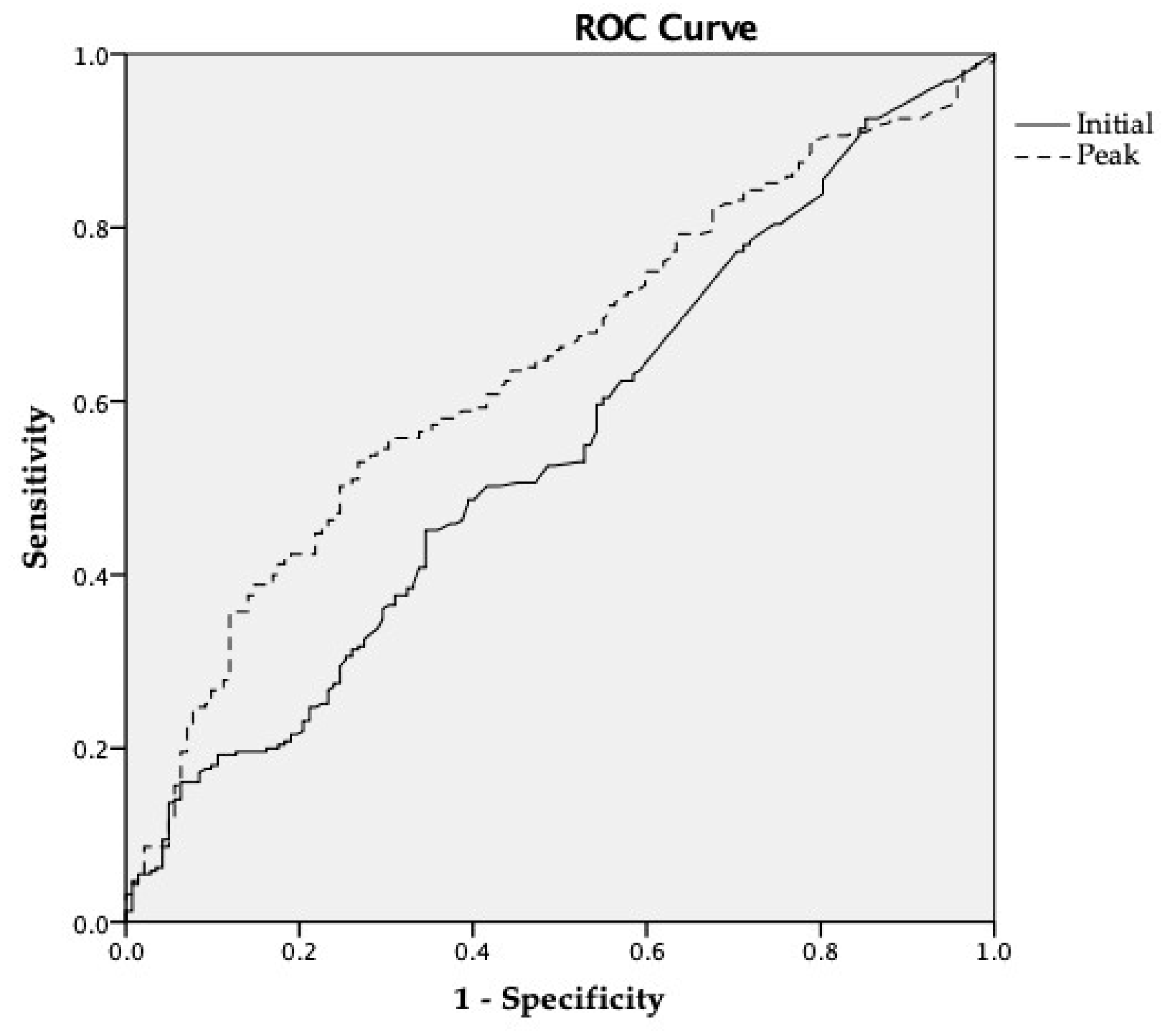
3.3. Relationship Between Troponin-I and TTE Variables
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Meyer, N.; Harhay, M.O.; Small, D.S.; Prescott, H.C.; Bowles, K.H.; Gaieski, D.F.; Mikkelsen, M.E. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit. Care Med. 2018, 46, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.J.; Rice, D.A.; Simpson, K.N.; Ford, D.W. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit. Care Med. 2015, 43, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Vieillard-Baron, A. Septic cardiomyopathy. Ann. Intensive Care 2011, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Kuriyama, A.; Takada, T.; Nasu, M.; Luthe, S.K. Prevalence and risk factors of sepsis-induced cardiomyopathy. Medicine 2016, 95, e5031. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.F.; Donker, D.W.; Spitoni, C.; Koster-Brouwer, M.E.; Soliman, I.W.; Ong, D.S.Y.; Horn, J.; van der Poll, T.; van Klei, W.A.; Bonten, M.J.M.; et al. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ. Cardiovasc. Qual Outcome 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Corredor, C.; Arcadipane, A.; Landesberg, G.; Vieillard-Baron, A.; Cecconi, M.; Fletcher, N. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: a systematic review and meta-analysis. Br. J. Anaesth. 2017, 119, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Babuin, L.; Vasile, V.C.; Rio Perez, J.A.; Alegria, J.R.; Chai, H.-S.; Afessa, B.; Jaffe, A.S. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Crit. Care Med. 2008, 36, 759–765. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; De Gaudio, A.R. Cardiorenal syndromes and sepsis. Int. J. Nephrol. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Flynn, A.; Mani, B.C.; Mather, P.J. Sepsis-induced cardiomyopathy: A review of pathophysiologic mechanisms. Heart Fail. Rev. 2010, 15, 605–611. [Google Scholar] [CrossRef]
- Sheyin, O.; Davies, O.; Duan, W.; Perez, X. The prognostic significance of troponin elevation in patients with sepsis: A meta-analysis. Heart Lung J. Acute Crit. Care 2015, 44, 75–81. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, H.; Park, J.S.; Kim, S.J.; Cho, Y.-J.; Yoon, H.I.; Lee, J.H.; Lee, C.-T.; Park, J.S. Cardiac troponin I as a prognostic factor in critically ill pneumonia patients in the absence of acute coronary syndrome. J. Crit. Care 2015, 30, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Klouche, K.; Pommet, S.; Amigues, L.; Bargnoux, A.S.; Dupuy, A.M.; Machado, S.; Serveaux-Delous, M.; Morena, M.; Jonquet, O.; Cristol, J.P. Plasma Brain Natriuretic Peptide and Troponin Levels in Severe Sepsis and Septic Shock. J. Intensive. Care Med. 2012, 29, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Harmankaya, A.; Akilli, H.; Gul, M.; Akilli, N.B.; Ergin, M.; Arıbas, A.; Cander, B. Assessment of right ventricular functions in patients with sepsis, severe sepsis and septic shock and its prognostic importance: A tissue Doppler study. J. Crit. Care 2013, 28, 1111.e7–1111.e11. [Google Scholar] [CrossRef] [PubMed]
- Berrios, R.A.S.; O’Horo, J.C.; Velagapudi, V.; Pulido, J.N. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: A systematic review and meta-analysis. J. Crit. Care 2014, 29, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Derwall, M.; Zoubi, A.S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The septic heart current understanding of molecular mechanisms and clinical implications. Chest 2018, 1–25. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, Y.-J.; Ryoo, S.M.; Sohn, C.H.; Seo, D.W.; Ahn, S.; Lim, K.S.; Kim, W.Y. One-Year Progression and Risk Factors for the Development of Chronic Kidney Disease in Septic Shock Patients with Acute Kidney Injury: A Single-Centre Retrospective Cohort Study. J. Clin. Med. 2018, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Smith, S.W.; Pearce, L.A.; Ler, R.; Murakami, M.M. Use of the Centaur TnI-Ultra Assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin. Chem. 2008, 54, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dai, H.; Yu, Y.; Yang, J.; Chen, J.; Wu, L. Elevated pregnancy-associated plasma protein A predicts myocardial dysfunction and death in severe sepsis. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2013, 51, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ammann, P.; Maggiorini, M.; Bertel, O.; Haenseler, E.; Joller-Jemelka, H.I.; Oechslin, E.; Minder, E.I.; Rickli, H.; Fehr, T. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J. Am. Coll. Cardiol. 2003, 41, 2004–2009. [Google Scholar] [CrossRef]
- Wilhelm, J.; Hettwer, S.; Schuermann, M.; Bagger, S.; Gerhardt, F.; Mundt, S.; Muschik, S.; Zimmermann, J.; Amoury, M.; Ebelt, H.; et al. Elevated troponin in septic patients in the emergency department: frequency, causes, and prognostic implications. Clin. Res. Cardiol. 2014, 103, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Caironi, P.; Fanizza, C.; Carrer, S.; Caricato, A.; Fassini, P.; Vago, T.; Romero, M.; Tognoni, G.; Gattinoni, L.; et al. Sequential N-terminal pro-B-type natriuretic peptide and high-sensitivity cardiac troponin measurements during albumin replacement in patients with severe sepsis or septic shock. Crit. Care Med. 2015, 44, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Sakhuja, A.; Geske, J.B.; Kumar, M.; Poterucha, J.T.; Kashyap, R.; Kashani, K.; Jaffe, A.S.; Jentzer, J.C. Role of admission troponin-T and serial troponin-T testing in predicting outcomes in severe sepsis and septic shock. J. Am. Heart Assoc. 2017, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.J.; Khan, I.A.; Gupta, V.; Jani, K.; Gowda, R.M.; Smith, P.R. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int. J. Cardiol. 2004, 95, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Elsk, K.M.; Spapen, H.D.; Nguyen, D.N.; Garbar, C.; Huyghens, L.P.; Gorus, F.K. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin. Chem. 2000, 46, 650–657. [Google Scholar]
- Røsjø, H.; Varpula, M.; Hagve, T.-A.; Karlsson, S.; Ruokonen, E.; Pettilä, V.; Omland, T.; The FINNSEPSIS Study Group. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2010, 37, 77–85. [Google Scholar]
- Wu, A.H.B. Release of cardiac troponin from healthy and damaged myocardium. Front. Lab. Med. 2017, 1, 144–150. [Google Scholar] [CrossRef]
- Cimolai, M.; Alvarez, S.; Bode, C.; Bugger, H. Mitochondrial mechanisms in septic cardiomyopathy. IJMS 2015, 16, 17763–17778. [Google Scholar] [CrossRef]
- Omar, A.A.; El-Shahat, N.; Ramadan, M.M. Cardiac functions in patients with sepsis and septic shock. Egypt. Heart J. 2012, 64, 191–196. [Google Scholar] [CrossRef]
- Bouhemad, B.; Nicolas-Robin, A.; Arbelot, C.; Arthaud, M.; Féger, F.; Rouby, J.-J. Acute left ventricular dilatation and shock-induced myocardial dysfunction. Crit. Care Med. 2009, 37, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Gilon, D.; Meroz, Y.; Georgieva, M.; Levin, P.D.; Goodman, S.; Avidan, A.; Beeri, R.; Weissman, C.; Jaffe, A.S.; et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur. Heart J. 2012, 33, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Pulido, J.N.; Afessa, B.; Masaki, M.; Yuasa, T.; Gillespie, S.; Herasevich, V.; Brown, D.R.; Oh, J.K. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. JMCP 2012, 87, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Ehrman, R.R.; Sullivan, A.N.; Favot, M.J.; Sherwin, R.L.; Reynolds, C.A.; Abidov, A.; Levy, P.D. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit. Care 2018, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Jaffe, A.S.; Gilon, D.; Levin, P.D.; Goodman, S.; Abu-Baih, A.; Beeri, R.; Weissman, C.; Sprung, C.L.; Landesberg, A.; et al. Troponin Elevation in Severe Sepsis and Septic Shock. Crit. Care Med. 2014, 42, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Tritapepe, L.; Lorini, F.L.; Arcadipane, A.; Vieillard-Baron, A.; Cecconi, M. Left ventricular systolic function evaluated by strain echocardiography and relationship with mortality in patients with severe sepsis or septic shock: a systematic review and meta-analysis. Crit. Care 2018, 22, 183. [Google Scholar] [CrossRef] [PubMed]
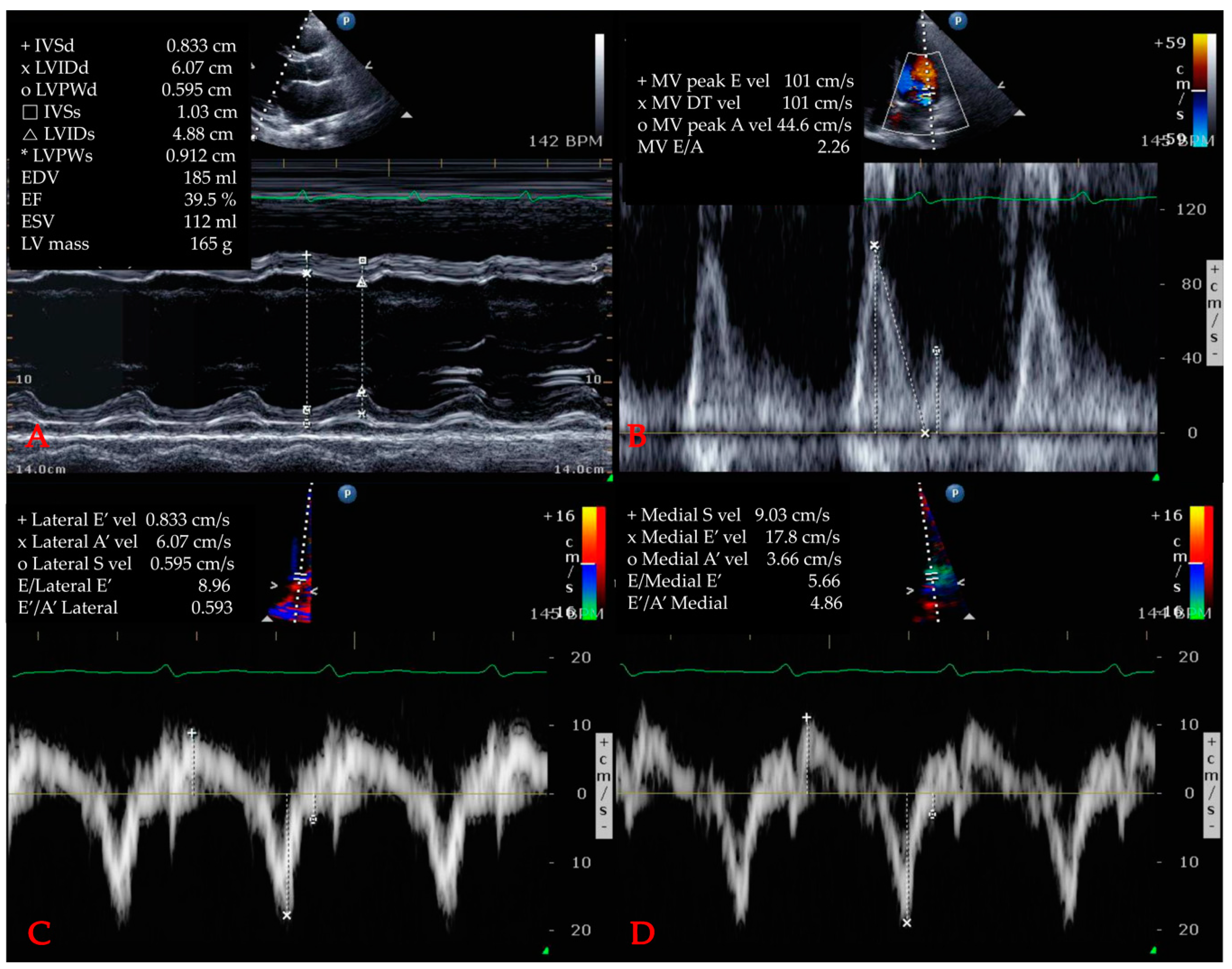
| Characteristics | Total n = 397 | SIMD (−) Based on TTE n = 139 | SIMD (+) Based on TTE n = 258 | p-Value |
|---|---|---|---|---|
| Age | 67.0 (58.0–75.0) | 66.0 (54.0–75.0) | 69.0 (60.0–76.0) | 0.270 |
| Male | 224 (56.4) | 83 (59.7) | 141 (54.7) | 0.342 |
| Past illness | ||||
| HTN | 131 (33.0) | 37 (26.6) | 94 (36.4) | 0.057 |
| DM | 114 (28.8) | 32 (23.0) | 82 (31.9) | 0.064 |
| CAD | 60 (15.1) | 14 (10.1) | 46 (17.8) | 0.041 * |
| CKD | 53 (13.4) | 13 (9.4) | 40 (15.5) | 0.091 |
| Pulmonary | 58 (14.6) | 21 (15.1) | 37 (14.3) | 0.882 |
| Malignancy | 130 (32.7) | 50 (36.0) | 80 (31.0) | 0.370 |
| Heart failure | 25 (6.3) | 5 (3.6) | 20 (7.8) | 0.130 |
| Arrhythmia | 46 (11.6) | 14 (10.1) | 32 (12.4) | 0.517 |
| EKG STTC | 200 (50.4) | 55 (39.6) | 145 (56.2) | 0.006 ** |
| Vital signs | ||||
| Systolic BP | 96.0 (79.0–121.0) | 99.0 (80.0–123.5) | 98.5 (79.0–127.0) | 0.891 |
| Diastolic BP | 59.0 (49.0–76.0) | 59.0 (49.0–73.0) | 62.0 (48.8–80.0) | 0.557 |
| Heart rate | 110.0 (90.0–128.0) | 111.0 (91.5–125.0) | 107.0 (89.0–128.0) | 0.430 |
| SOFA | 9.0 (7.0–12.0) | 9.0 (8.0–12.0) | 9.0 (7.0–12.0) | 0.427 |
| Laboratory | ||||
| WBC (×103/µL) | 10.4 (4.2–16.9) | 9.0 (3.9–16.9) | 11.6 (5.1–17.0) | 0.282 |
| Hb (g/dL) | 11.1 (9.2–13.1) | 11.2 (9.1–13.5) | 11.15 (9.2–13.1) | 0.484 |
| BUN (mg/dL) | 29.0 (19.0–46.0) | 31.0 (20.5–47.0) | 29.0 (19.0–43.0) | 0.463 |
| Creatinine (mg/dL) | 1.72 (1.10–2.97) | 1.79 (1.11–3.16) | 1.69 (1.08–3.01) | 0.420 |
| CRP (mg/dL) | 13.9 (5.4–21.8) | 15.7 (4.3–25.3) | 13.3 (4.9–20.5) | 0.433 |
| Lactate (mmol/L) | 4.1 (2.4–6.4) | 4.2 (2.7–6.3) | 3.9 (2.4–6.0) | 0.424 |
| Initial hs-TnI (ng/mL) | 0.110 (0.044–0.318) | 0.100 (0.040–0.258) | 0.110 (0.050–0.480) | 0.165 |
| Peak hs-TnI (ng/mL) | 0.539 (0.178–2.293) | 0.390 (0.168–1.145) | 1.535 (0.329–7.103) | <0.001 *** |
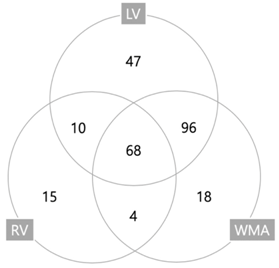
| Type of Cardiac Dysfunctions | Frequency (%) |
|---|---|
| LV dysfunction | 221 (85.7%) |
| Systolic dysfunction | 163 (63.2%) |
| Diastolic dysfunction | 104 (40.3%) |
| RV dysfunction | 97 (37.6%) |
| Wall motion abnormalities | 186 (72.1%) |
| Parameter | Total n = 397 | SIMD (−) on TTE n = 139 | SIMD (+) on TTE n = 258 | p-Value |
|---|---|---|---|---|
| LVIDs (mm) | 33.0 (27.0–39.0) | 29.0 (25.0–33.0) | 35.0 (28.8–40.3) | <0.001 *** |
| LVIDd (mm) | 48.0 (42.3–52.0) | 45.5 (41.0–50.0) | 59.0 (44.0–54.0) | <0.001 *** |
| LA (mm) | 36.0 (32.0–41.0) | 36.0 (31.0–38.8) | 37.0 (33.8–41.0) | 0.013 * |
| Aorta (mm) | 34.0 (31.0–36.0) | 34.0 (31.0–36.0) | 34.0 (31.0–37.0) | 0.566 |
| LVPWs (mm) | 13.0 (12.0–15.0) | 14.0 (12.3–15.0) | 13.0 (12.0–15.0) | <0.001 *** |
| LVPWd (mm) | 9.0 (8.0–10.0) | 9.0 (8.0–10.0) | 9.0 (8.0–10.0) | 0.116 |
| ESV (mL) | 42.0 (28.0–66.0) | 31.5 (25.0–40.0) | 49.5 (33.0–74.0) | <0.001 *** |
| EDV (mL) | 90.0 (69.0–113.5) | 81.5 (66.3–97.8) | 98.5 (76.0–125.3) | <0.001 *** |
| IVSs (mm) | 13.0 (11.0–14.0) | 13.5 (12.0–15.0) | 13.0 (11.0–14.0) | <0.001 *** |
| IVSd (mm) | 9.0 (8.0–10.0) | 9.0 (8.0–10.0) | 9.0 (8.0–10.0) | 0.173 |
| LV mass (g) | 148.6 (114.5–175.6) | 144.8 (111.1–164.5) | 159.0 (128.6–189.2) | 0.029 * |
| LVEF (%) | 55.0 (40.0–62.0) | 61.0 (57.0–65.0) | 49.0 (35.0–59.0) | <0.001 *** |
| Peak E velocity (cm/s) | 69.0 (54.0–90.0) | 62.0 (52.0–77.8) | 69.0 (53.8–86.8) | 0.090 |
| Peak A velocity (cm/s) | 77.0 (62.0–92.0) | 75.0 (63.3–87.5) | 75.0 (59.0–93.0) | 0.111 |
| Deceleration time (ms) | 174.0 (140.0–213.0) | 199.0 (155.3–234.3) | 172.0 (139.5–207.5) | <0.001 *** |
| E/A ratio | 0.84 (0.65–1.13) | 0.87 (0.65–1.13) | 0.86 (0.72–1.21) | 0.707 |
| E/e’ ratio | 12.0 (9.0–15.0) | 10.0 (9.0–12.0) | 14.0 (10.0–17.0) | <0.001 *** |
| SIMD | hs-TnI (ng/mL) | SIMD (−) | SIMD (+) | p |
|---|---|---|---|---|
| Any | Initial | 0.100 (0.040–0.258) | 0.110 (0.050–0.480) | 0.165 |
| Peak | 0.390 (0.168–1.145) | 1.535 (0.329–7.103) | <0.001 *** | |
| LV systolic | Initial | 0.100 (0.040–0.323) | 0.110 (0.050–0.527) | 0.228 |
| Peak | 0.540 (0.233–14.240) | 1.609 (0.341–34.649) | <0.001 *** | |
| LV diastolic | Initial | 0.085 (0.312–0.289) | 0.160 (0.050–0.513) | 0.019 * |
| Peak | 0.739 (0.236–3.519) | 0.903 (0.306–5.230) | 0.177 | |
| RV | Initial | 0.101 (0.040–0.305) | 0.094 (0.050–0.574) | 0.272 |
| Peak | 0.546 (0.222–2.672) | 2.478 (0.547–25.845) | <0.001 *** | |
| WMA | Initial | 0.100 (0.040–0.288) | 0.110 (0.050–0.523) | 0.869 |
| Peak | 0.455 (0.205–1.680) | 1.950 (0.374–9.741) | <0.001 *** |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| CAD | 1.595 | 0.820–3.105 | 0.169 | |||
| EKG STTC | 1.689 | 1.115–2.561 | 0.013 | 1.822 | 1.210–2.744 | 0.004 ** |
| Peak hs-TnI | 1.032 | 1.008–1.057 | 0.008 | 1.033 | 1.009–1.057 | 0.008 ** |
| Variables | Multivariate Analysis | ||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| Initial hs-TnI | |||
| Quartile 1 | Reference | ||
| Quartile 2 | 1.270 | 0.711–2.266 | 0.419 |
| Quartile 3 | 0.805 | 0.443–1.462 | 0.477 |
| Quartile 4 | 1.561 | 0.875–2.785 | 0.132 |
| Maximum hs-TnI | |||
| Quartile 1 | Reference | ||
| Quartile 2 | 1.241 | 0.671–2.295 | 0.491 |
| Quartile 3 | 1.667 | 0.881–3.151 | 0.116 |
| Quartile 4 | 5.496 | 2.843–10.624 | 0.001 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Kim, M.; Kim, Y.-J.; Ryoo, S.M.; Sohn, C.H.; Ahn, S.; Kim, W.Y. Troponin Testing for Assessing Sepsis-Induced Myocardial Dysfunction in Patients with Septic Shock. J. Clin. Med. 2019, 8, 239. https://doi.org/10.3390/jcm8020239
Kim J-S, Kim M, Kim Y-J, Ryoo SM, Sohn CH, Ahn S, Kim WY. Troponin Testing for Assessing Sepsis-Induced Myocardial Dysfunction in Patients with Septic Shock. Journal of Clinical Medicine. 2019; 8(2):239. https://doi.org/10.3390/jcm8020239
Chicago/Turabian StyleKim, June-Sung, Muyeol Kim, Youn-Jung Kim, Seung Mok Ryoo, Chang Hwan Sohn, Shin Ahn, and Won Young Kim. 2019. "Troponin Testing for Assessing Sepsis-Induced Myocardial Dysfunction in Patients with Septic Shock" Journal of Clinical Medicine 8, no. 2: 239. https://doi.org/10.3390/jcm8020239
APA StyleKim, J.-S., Kim, M., Kim, Y.-J., Ryoo, S. M., Sohn, C. H., Ahn, S., & Kim, W. Y. (2019). Troponin Testing for Assessing Sepsis-Induced Myocardial Dysfunction in Patients with Septic Shock. Journal of Clinical Medicine, 8(2), 239. https://doi.org/10.3390/jcm8020239





