Oral Bicarbonate Therapy in Non-Haemodialysis Dependent Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias
2.6. Outcome Measures and Data Synthesis
2.7. Subgroup Analysis
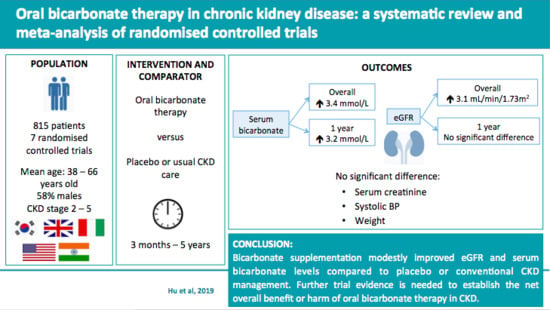
3. Results
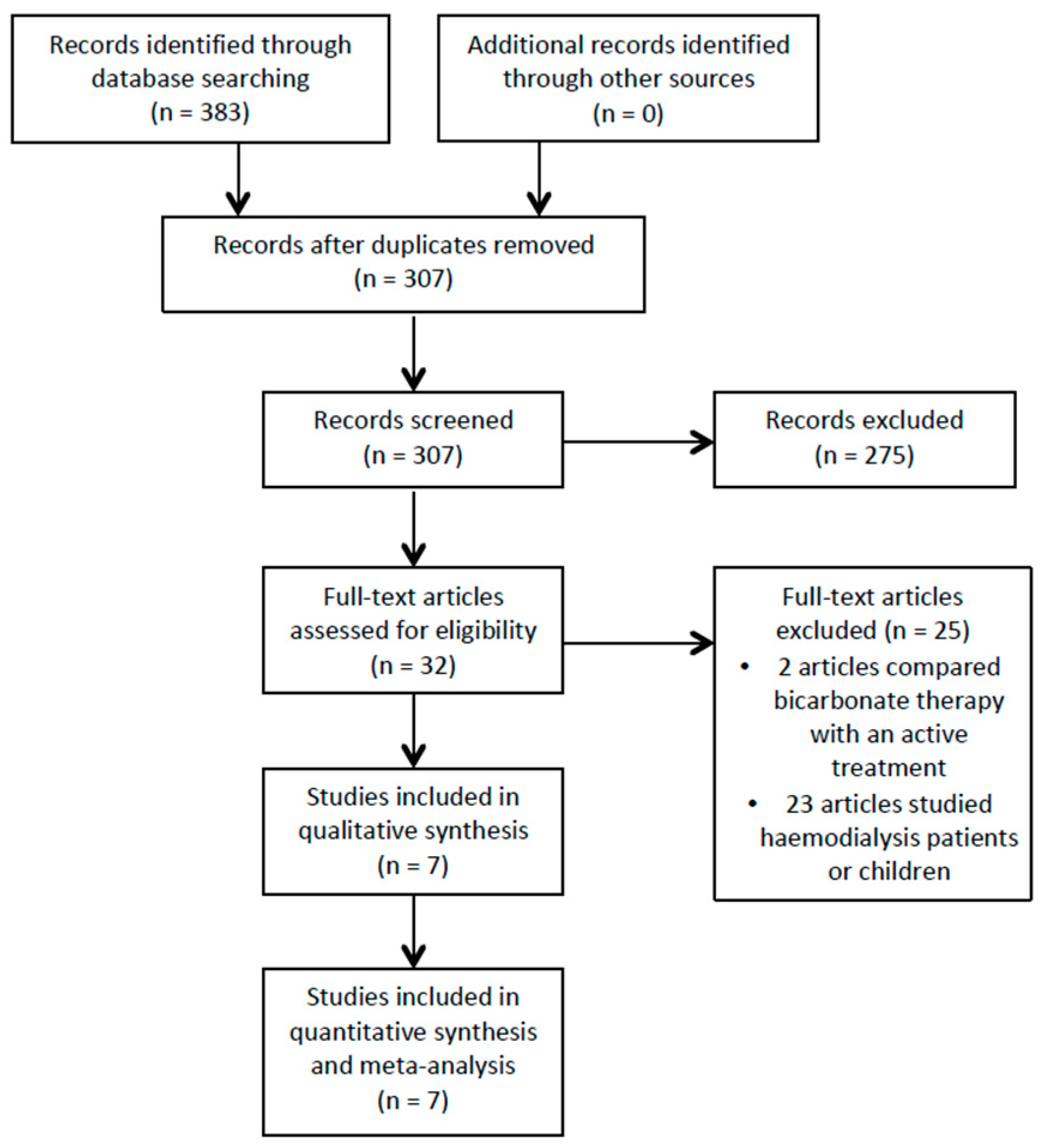
3.1. Study Selection
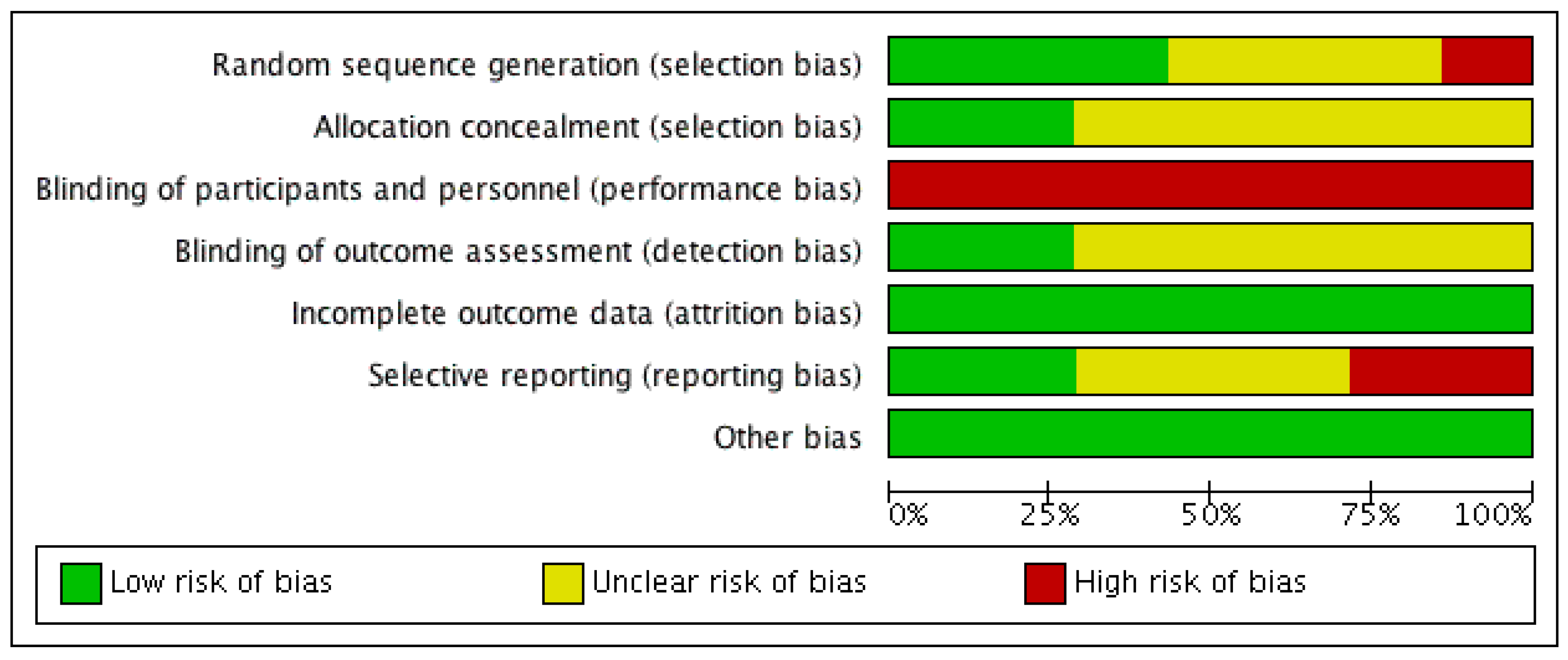
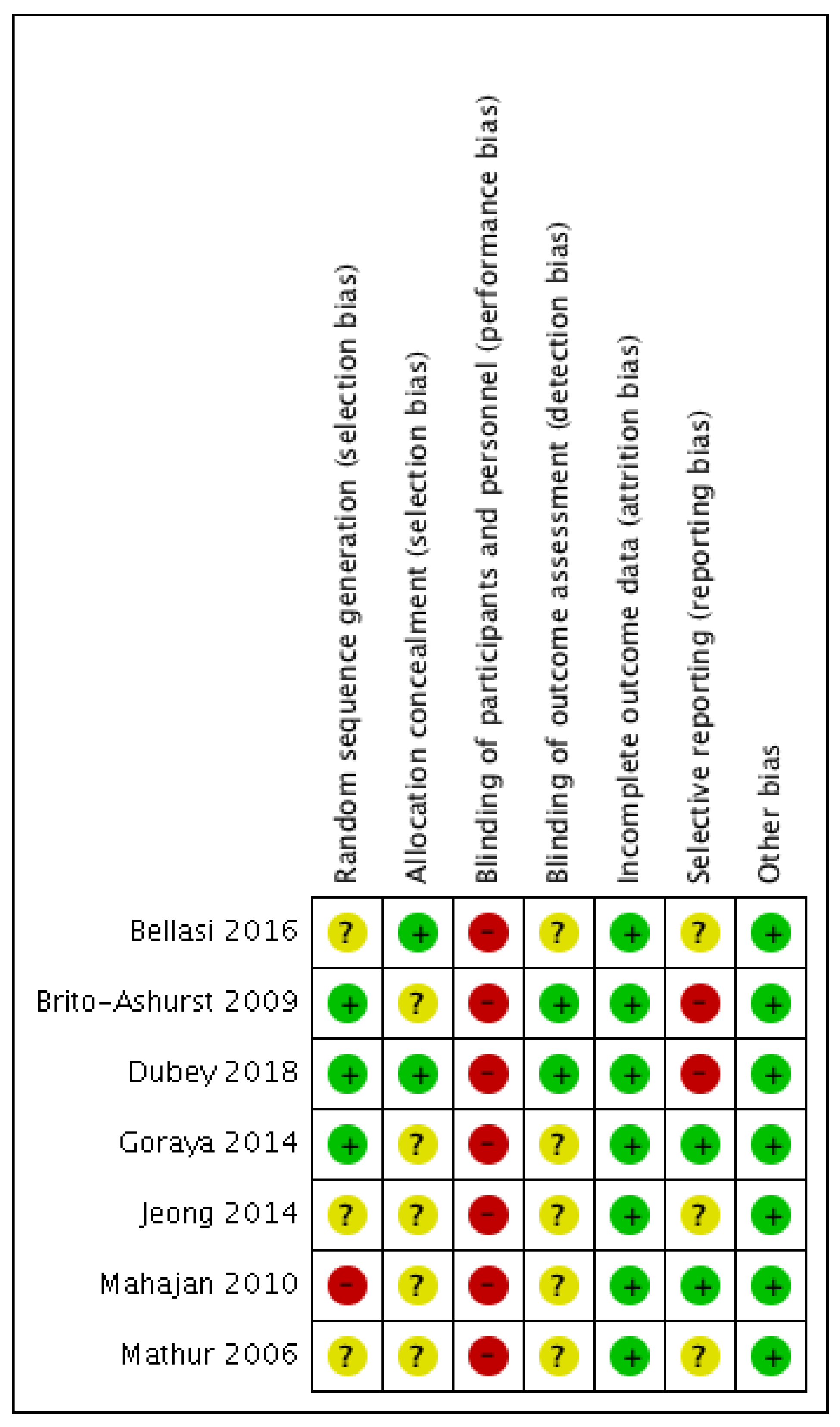
3.2. Risk of Bias Analysis
3.3. Outcomes
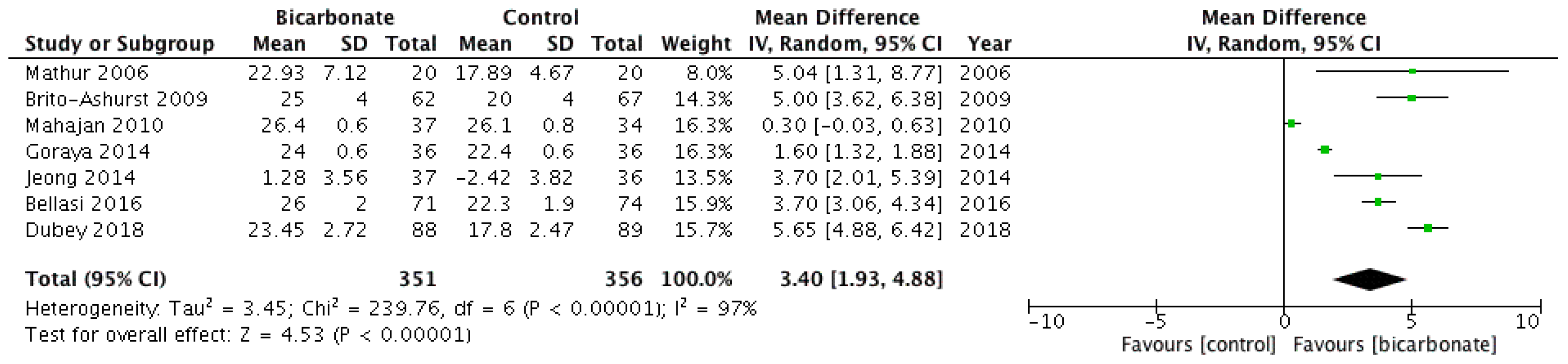
3.3.1. Serum Bicarbonate
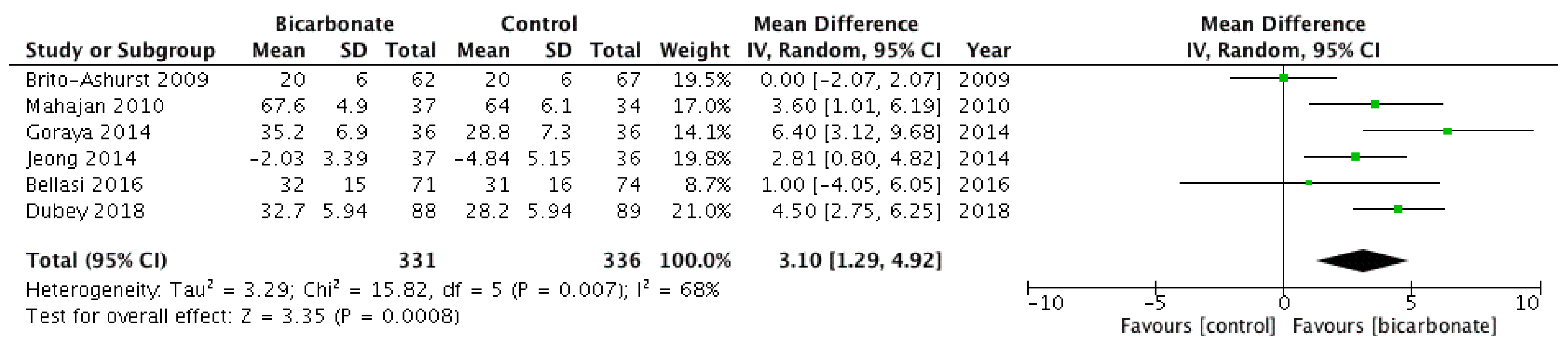
3.3.2. eGFR and Serum Creatinine
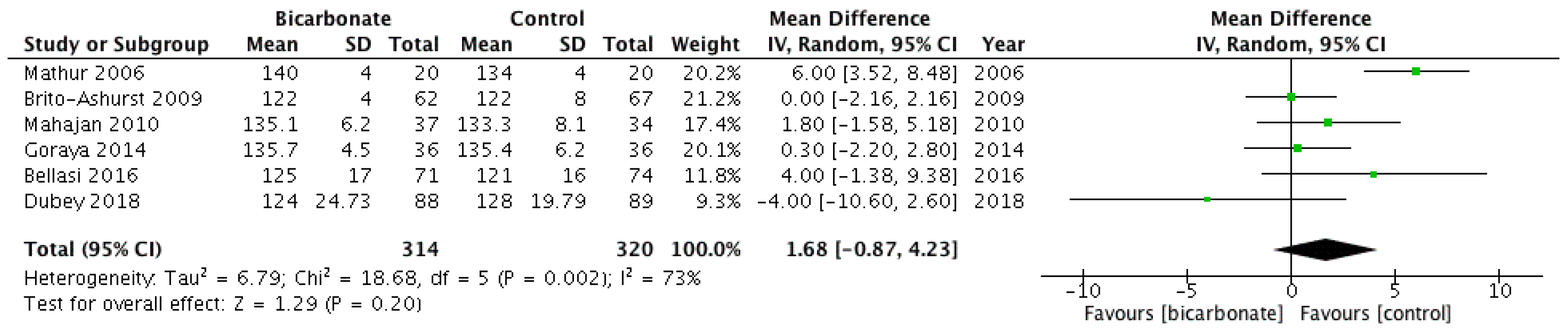
3.3.3. Systolic Blood Pressure
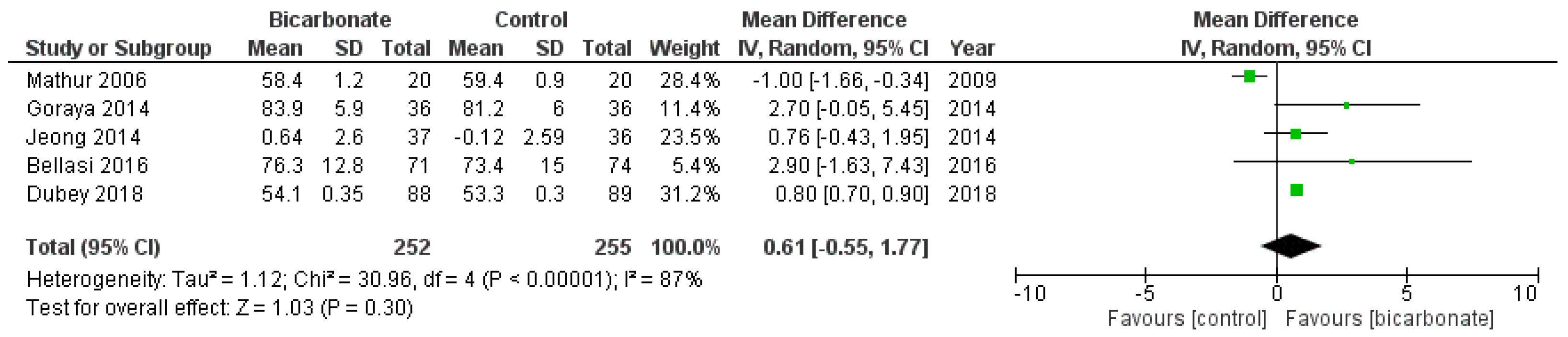
3.3.4. Weight
3.3.5. Other Outcomes
3.4. Subgroup Analyses
4. Discussion
4.1. Outcomes
4.2. Potential Adverse Effects of Bicarbonate Oral Therapy
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Search Strategy
| 1 | exp Bicarbonates [MeSH] or exp Sodium Bicarbonate [MeSH] or bicarbonates [tiab] | 24,367 |
| 2 | Chronic Kidney Disease or CKD [mp] or exp Renal Insufficiency, Chronic [MESH] or or Chronic Renal Insufficiency [tiab] | 127,737 |
| 3 | (exp Bicarbonates [MeSH] or exp Sodium Bicarbonate [MESH] or bicarbonates [tiab]) and (Chronic Kidney Disease or CKD [mp] or exp Renal Insufficiency, Chronic [MESH] or or Chronic Renal Insufficiency [tiab]) | 812 |
| 4 | Limit 3 to RCT and English language | 76 |
| 1 | exp Bicarbonate [MeSH] or Bicarbonate.mp | 69,535 |
| 2 | exp Chronic Kidney Failure [MeSH] or (chronic kidney disease or chronic kidney failure or chronic kidney insufficiency or chronic renal disease or chronic renal failure or chronic renal insufficiency).mp | 116,647 |
| 3 | (exp Bicarbonate [MeSH] or Bicarbonate.mp) and (exp Chronic Kidney Failure [MeSH] or (chronic kidney disease or chronic kidney failure or chronic kidney insufficiency or chronic renal disease or chronic renal failure or chronic renal insufficiency).mp) | 2323 |
| 4 | Limit 3 to English language and exclude Medline journals and RCT | 3 |
| 1 | MeSH descriptor. [Bicarbonates] explode all trees | 1201 |
| 2 | MeSH descriptor: [Sodium Bicarbonate] explode all trees | 610 |
| 3 | #1 or #2 or (Bicarbonate *) | 3123 |
| 4 | MeSH descriptor: [Renal Insufficiency, Chronic] explode all trees | 5705 |
| 5 | Chronic kidney disease or chronic kidney failure or chronic kidney insufficiency or chronic renal disease or chronic renal failure or chronic renal insufficiency | 13,858 |
| 6 | #4 or #5 | 13,524 |
| 7 | #3 and #6 in Trials | 304 |
References
- Kerr, M. Chronic Kidney Disease in England: The Human and Financial Cost; NHS Kidney Care: Newcastle upon Tyne, UK, 2012. [Google Scholar]
- Barron, E. Chronic Kidney Disease Prevalence Model; Report number: 2014386; Public Health England: London, UK, 2014.
- Kidney Care UK. Facts and Stats. Available online: https://www.kidneycareuk.org/news-and-campaigns/facts-and-stats/ (accessed on 1 December 2018).
- Ahmed, A.R.; Lappin, D. Oral alkali therapy and the management of metabolic acidosis of chronic kidney disease: A narrative literature review. World J. Nephrol. 2018, 7, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.J.; Haymann, J.P.; M’rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Metabolic acidosis of CKD: An Update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Pathogenesis, Consequences and Treatment of Metabolic Acidosis in Chronic Kidney Disease. Available online: https://www.uptodate.com/contents/pathogenesis-consequences-and-treatment-of-metabolic-acidosis-in-chronic-kidney-disease (accessed on 1 December 2018).
- Krieger, N.S.; Frick, K.K.; Bushinsky, D.A. Mechanism of acid-induced bone resorption. Curr. Opin. Nephrol. Hypertens. 2004, 13, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Mehrotra, R.; Fouque, D.; Kopple, J.D. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin. Dial. 2004, 17, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Wildenthal, K.; Johnson, R.L., Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972, 1, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Rahman, M.; Hostetter, T.H. Current status of bicarbonate in CKD. J. Am. Soc. Nephrol. 2015, 26, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Anderson, J.E.; Kalantar-Zadeh, K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol. Dial. Transplant. 2009, 24, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Witham, D.M.; Band, M.M.; Littleford, R.C.; Avenell, A.; Soiza, R.L.; McMurdo, M.E.; Sumukadas, D.; Ogston, S.A.; Lamb, E.J.; Hampson, G.; et al. Does oral sodium bicarbonate therapy improve function and quality of life in older patients with chronic kidney disease and low-grade acidosis (the BiCARB trial)? Study protocol for a randomized controlled trial. Trials 2015, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health Research. Does Sodium Bicarbonate Improve Health Outcomes in Patients with Chronic Kidney Disease and Metabolic Acidosis? Systematic Review and Meta-Analysis of Randomised Controlled Trials. PROSPERO International Prospective Register of Systematic Reviews. Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.php? ID=CRD42018112908 (accessed on 7 February 2019).
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; The Cochrane Collaboration: Oxford, UK, 2011; Available online: http://handbook.cochrane.org (accessed on 2 December 2018).
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. Available online: https://community.cochrane.org/help/tools-and-software/revman-5/revman-5-download (accessed on 20 October 2018).
- Mathur, R.P.; Dash, S.C.; Gupta, N.; Prakash, S.; Saxena, S.; Bhowmik, D. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: A prospective randomized single blind controlled trial. Ren. Fail. 2006, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- de Brito-Ashurst, I.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 2009, 20, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Simoni, J.; Sheather, S.J.; Broglio, K.R.; Rajab, M.H.; Wesson, D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010, 78, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kwon, S.K.; Kim, H.Y. Effect of bicarbonate supplementation on renal function and nutritional indices in predialysis advanced chronic kidney disease. Electrolytes Blood Press. 2014, 12, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Di Micco, L.; Santoro, D.; Marzocco, S.; De Simone, E.; Cozzolino, M.; Di Lullo, L.; Guastaferro, P.; Di Iorio, B. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016, 17, 158. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Sahoo, J.; Vairappan, B.; Haridasan, S.; Parameswaran, S.; Priyamvada, P.S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial. Nephrol. Dial. Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Aucella, F.; Conte, G.; Cupisti, A.; Santoro, D. A prospective, multicenter, randomized, controlled study: The correction of metabolic acidosis with use of bicarbonate in chronic renal insufficiency (UBI) study. J. Nephrol. 2012, 25, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Gaggl, M.; Cejka, D.; Plischke, M.; Heinze, G.; Fraunschiel, M.; Schmidt, A.; Hörl, W.H.; Sunder-Plassmann, G. Effect of oral sodium bicarbonate supplementation on progression of chronic kidney disease in patients with chronic metabolic acidosis: Study protocol for a randomized controlled trial (SoBic-Study). Trials 2013, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- US National Library of Medicine. Placebo-Controlled Randomized Clinical Trial of Alkali Therapy in Patients with Chronic Kidney Disease (BASE Study). Available online: https://clinicaltrials.gov/ct2/show/NCT01452412 (accessed on 1 December 2018).
- Dobre, M.; Yang, W.; Chen, J.; Drawz, P.; Hamm, L.L.; Horwitz, E.; Hostetter, T.; Jaar, B.; Lora, C.M.; Nessel, L.; et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis. 2013, 62, 670–678. [Google Scholar] [CrossRef] [PubMed]
| Study | Location | n | Mean Age (Years) | CKD Stage | Bicarbonate Level Entry Criterion | Intervention | Comparator | Duration | Primary Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Mathur et al., 2006 [17] | India | 40 | 41 | “Mild to moderate” CKD (creatinine < 442 μmol/L). CKD stage not specified | Not specified | Oral bicarbonate 1.2 mEq/kg in 3 divided doses, titrated to maintain serum bicarbonate in range 22–26 mmol/L | Placebo | 3 months | Not specified |
| De Brito-Ashurt et al., 2009 [18] | UK | 134 | 55 | 4 or 5 | 16 mmol/L < Bicarbonate < 19 mmol/L | Oral bicarbonate 600 mg 3×/day, increased as needed to maintain serum bicarbonate > 23 mmol/L | Usual care | 2 years | Decline in creatinine clearance of >3 mL/min/year |
| Mahajan et al., 2010 [19] | USA | 120 | 51 | 2 with hypertension and microalbuminuria | Total CO2 > 24.5 mmol/L | Oral bicarbonate 0.5 mEq/kg lean body weight | Placebo | 5 years | eGFR decline rate |
| Jeong et al., 2014 [20] | South Korea | 80 | 55 | 4 or 5 | Total CO2 < 22 mmol/L | Oral bicarbonate 1 g 3×/day, titrated to maintain serum bicarbonate > 22 mmol/L | Usual care | 12 months | eGFR |
| Goraya et al., 2014 [21] | USA | 108 | 54 | 3 | 22 mmol/L < Total CO2 < 24 mmol/L | Oral bicarbonate 0.3 mEq/Kg lean body weight in three divided doses | Usual care | 3 years | eGFR |
| Bellasi et al., 2016 [22] | Italy | 145 | 65 | 3b or 4; in patients with T2DM | Bicarbonate < 24mmol/L | Oral bicarbonate 0.5 mEq/kg twice daily, until serum bicarbonate 24–28 mmol/L | Usual care | 12 months | Insulin resistance |
| Dubey et al., 2018 [23] | India | 188 | 50 | 3 and 4 | Bicarbonate < 22mmol/L | Oral bicarbonate titrated with weekly monitoring | Usual care | 6 months | Mid-arm muscle circumference |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.K.; Witham, M.D.; Soiza, R.L. Oral Bicarbonate Therapy in Non-Haemodialysis Dependent Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Clin. Med. 2019, 8, 208. https://doi.org/10.3390/jcm8020208
Hu MK, Witham MD, Soiza RL. Oral Bicarbonate Therapy in Non-Haemodialysis Dependent Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Journal of Clinical Medicine. 2019; 8(2):208. https://doi.org/10.3390/jcm8020208
Chicago/Turabian StyleHu, May Khei, Miles D. Witham, and Roy L. Soiza. 2019. "Oral Bicarbonate Therapy in Non-Haemodialysis Dependent Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Journal of Clinical Medicine 8, no. 2: 208. https://doi.org/10.3390/jcm8020208
APA StyleHu, M. K., Witham, M. D., & Soiza, R. L. (2019). Oral Bicarbonate Therapy in Non-Haemodialysis Dependent Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Journal of Clinical Medicine, 8(2), 208. https://doi.org/10.3390/jcm8020208