Effects of Caloric Restriction with Protein Supplementation on Plasma Protein Profiles in Middle-Aged Women with Metabolic Syndrome—A Preliminary Open Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
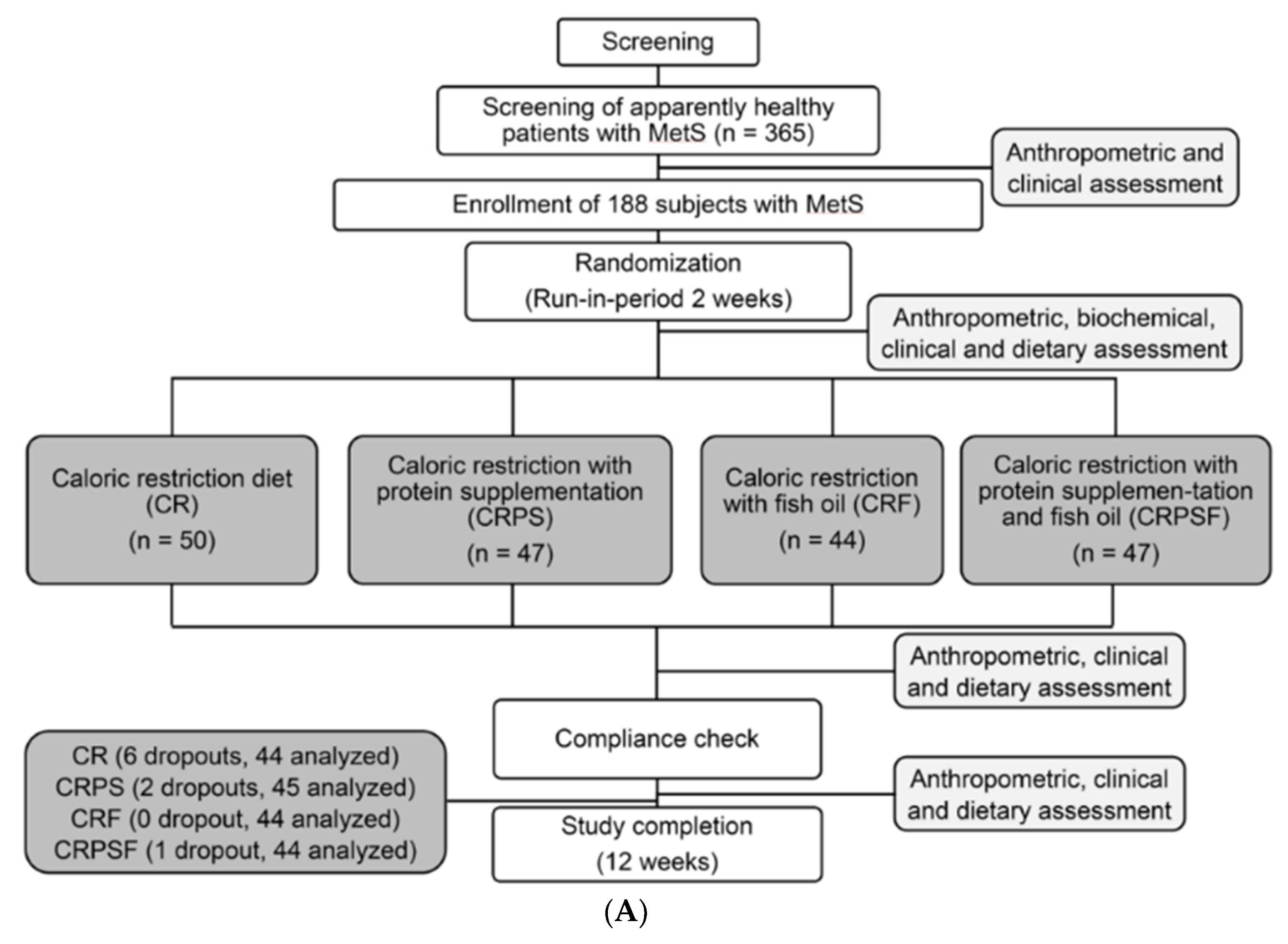
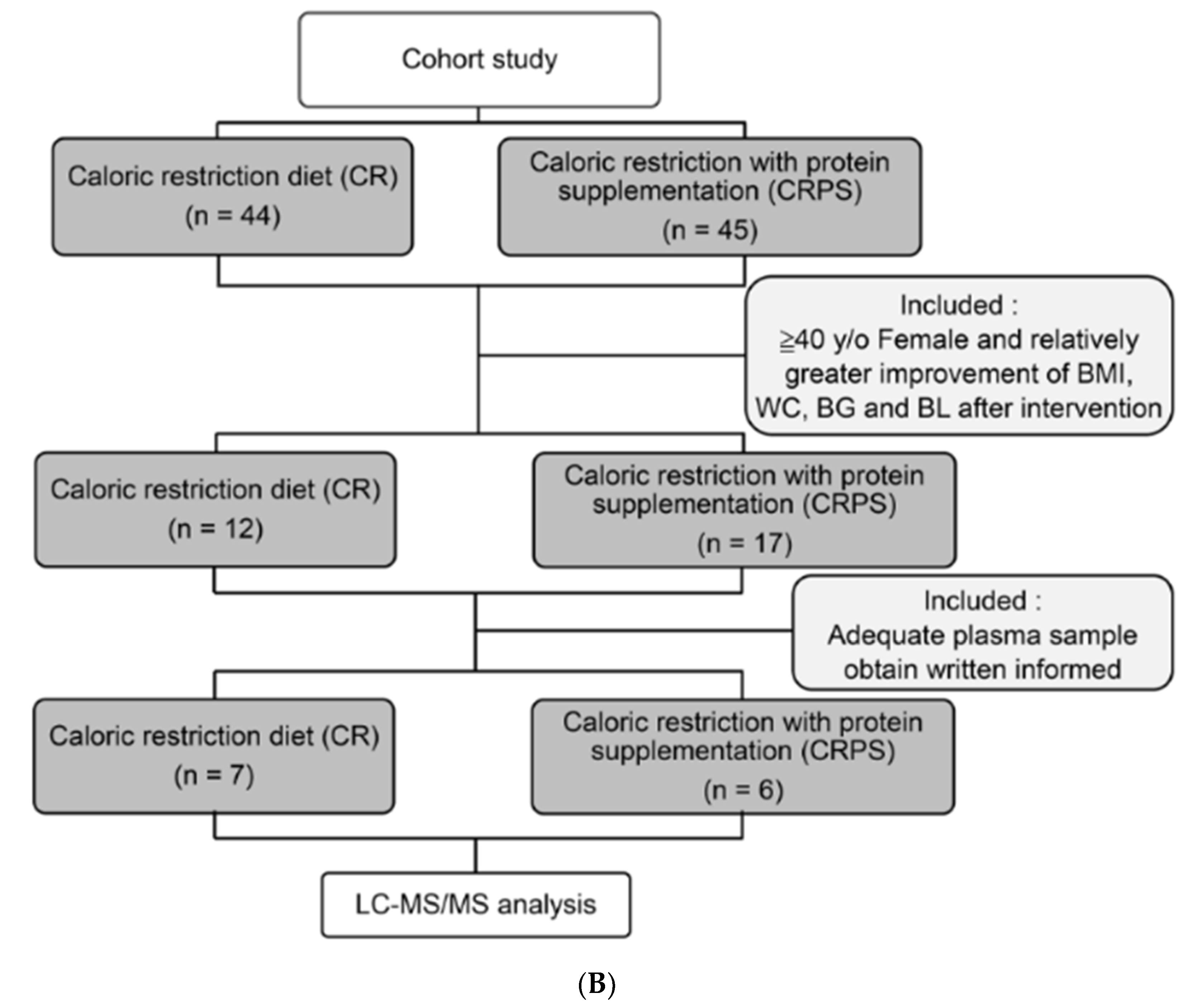
2.2. Study Design
2.3. Anthropometry and Analysis of Clinical and Biochemical Variables
2.4. Liquid Chromatography–Tandem Mass Spectrometry
2.5. Protein Identification
2.6. ELISA Analysis of Selected Protein
2.7. Statistical Analysis
3. Results
3.1. Anthropometric and Clinical Characteristics
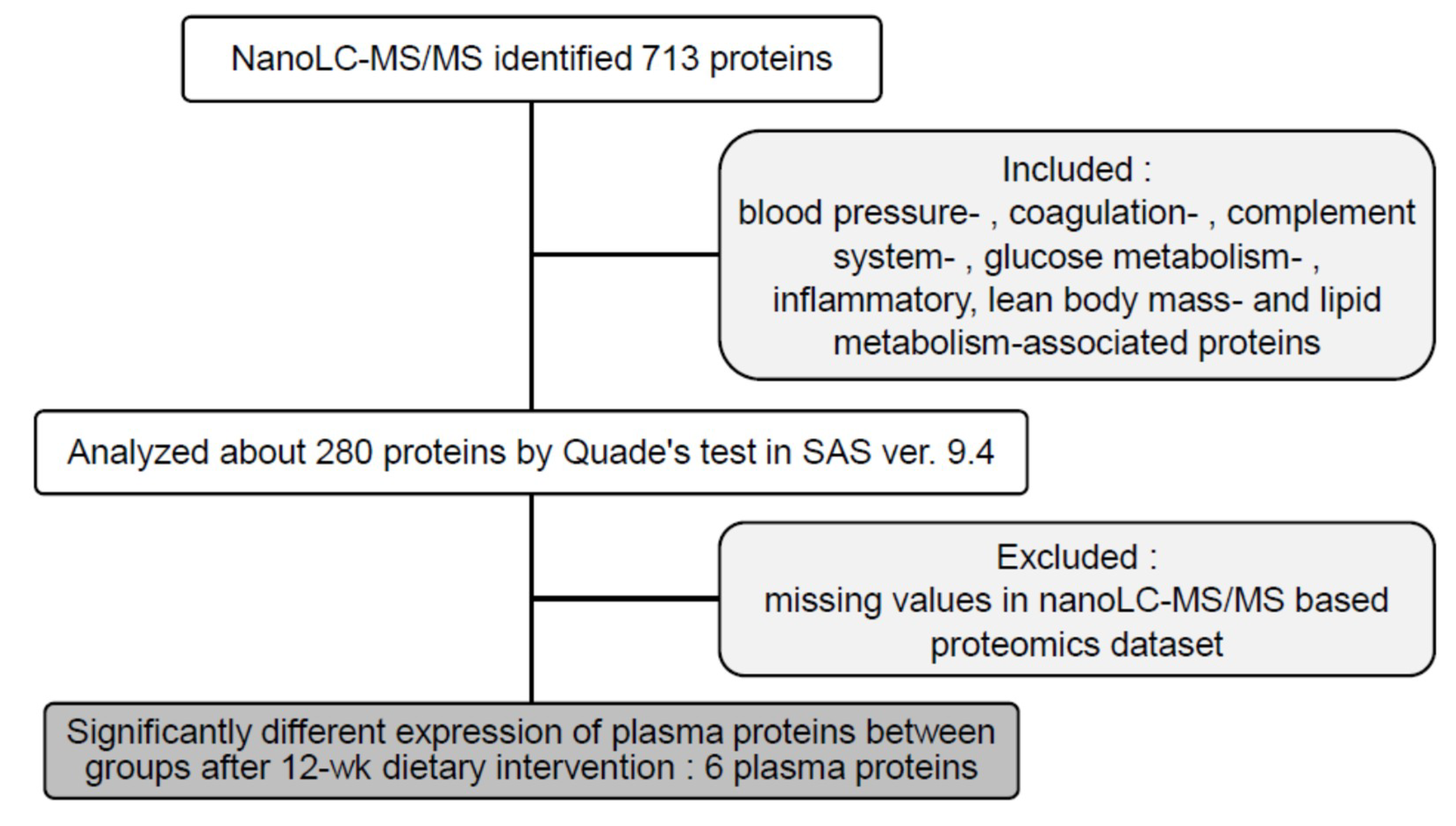
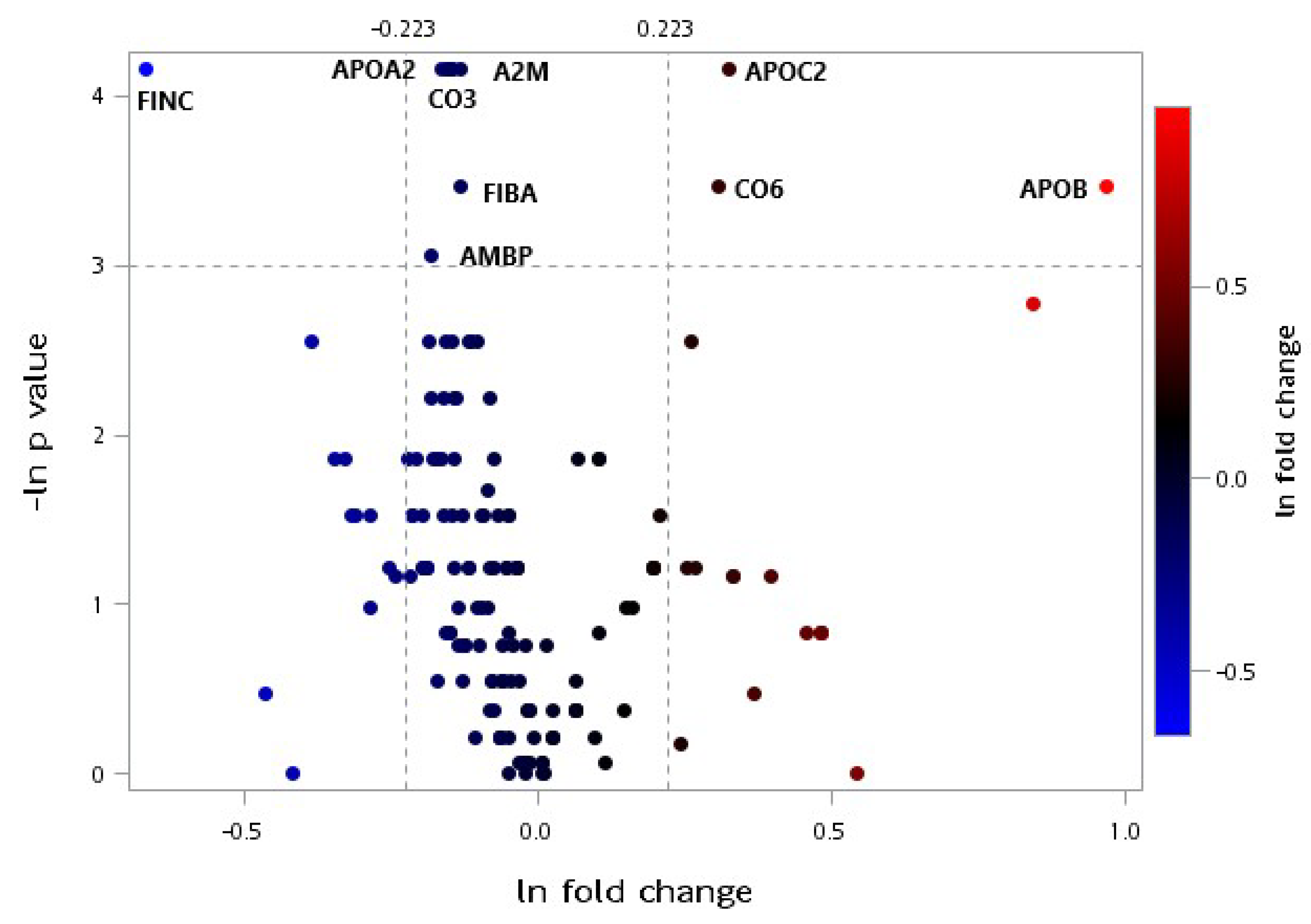
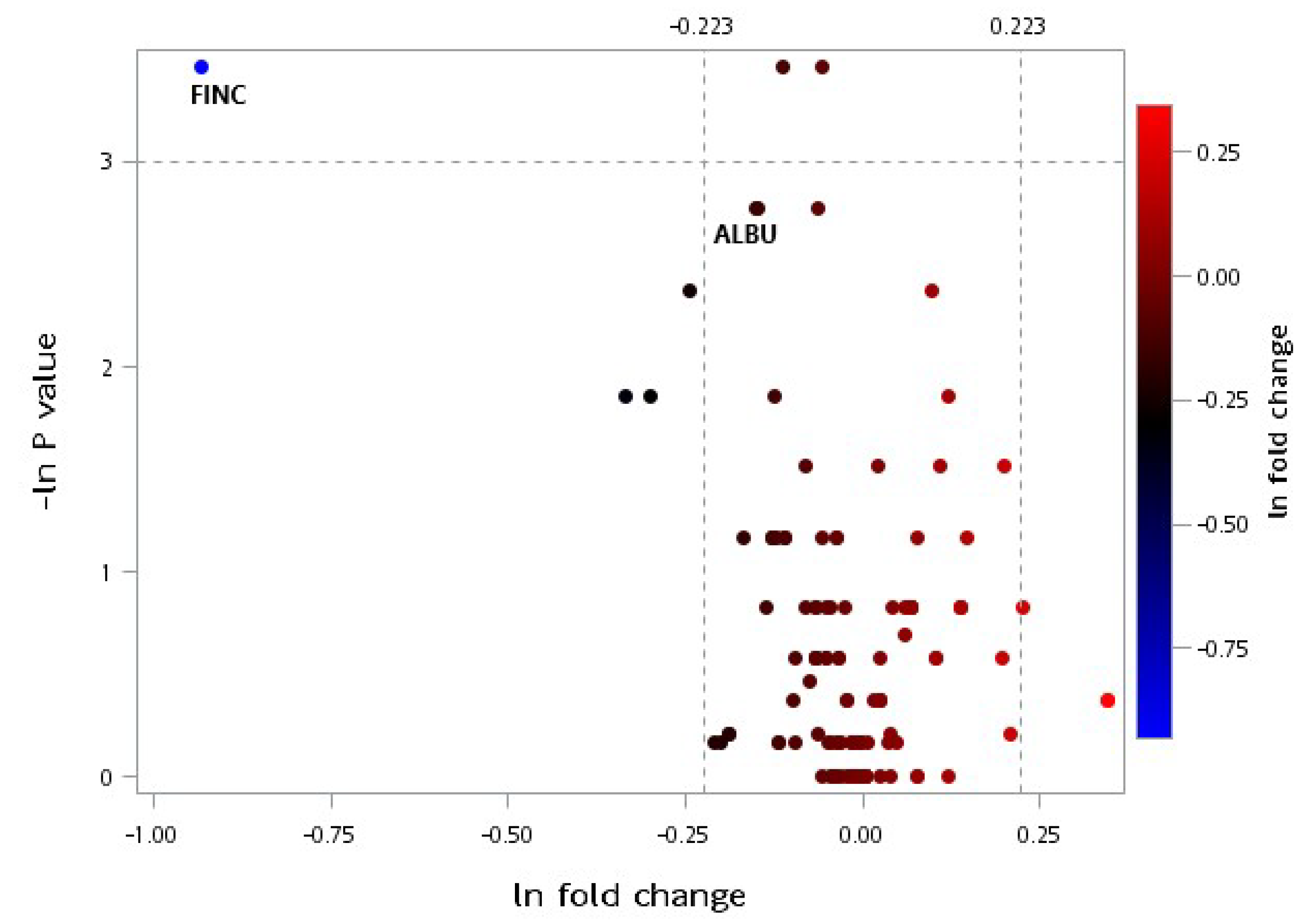
3.2. LC-MS/MS Proteomic Analysis
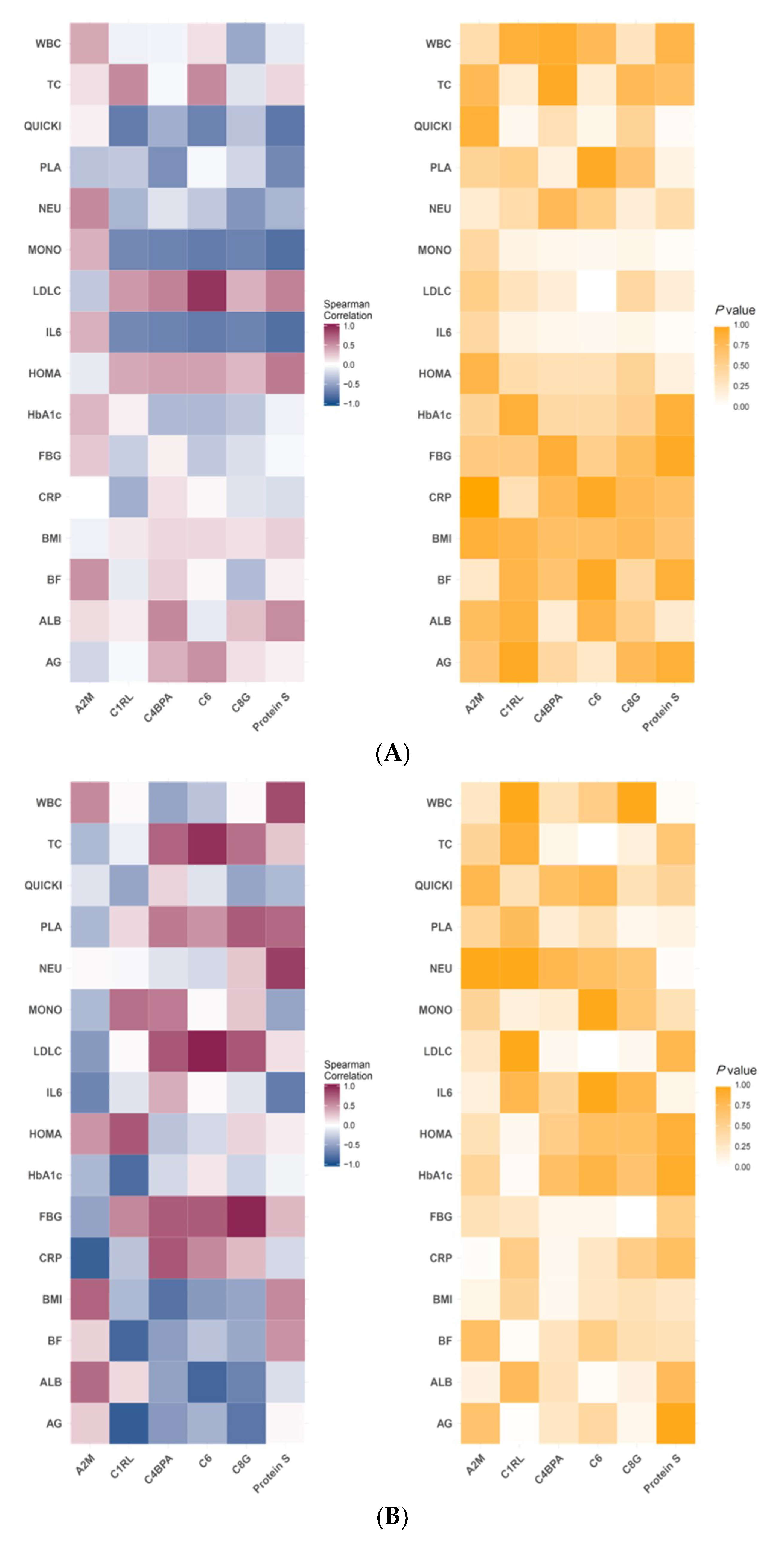
3.3. Correlation of Specific Protein Candidates with Biochemical Variables
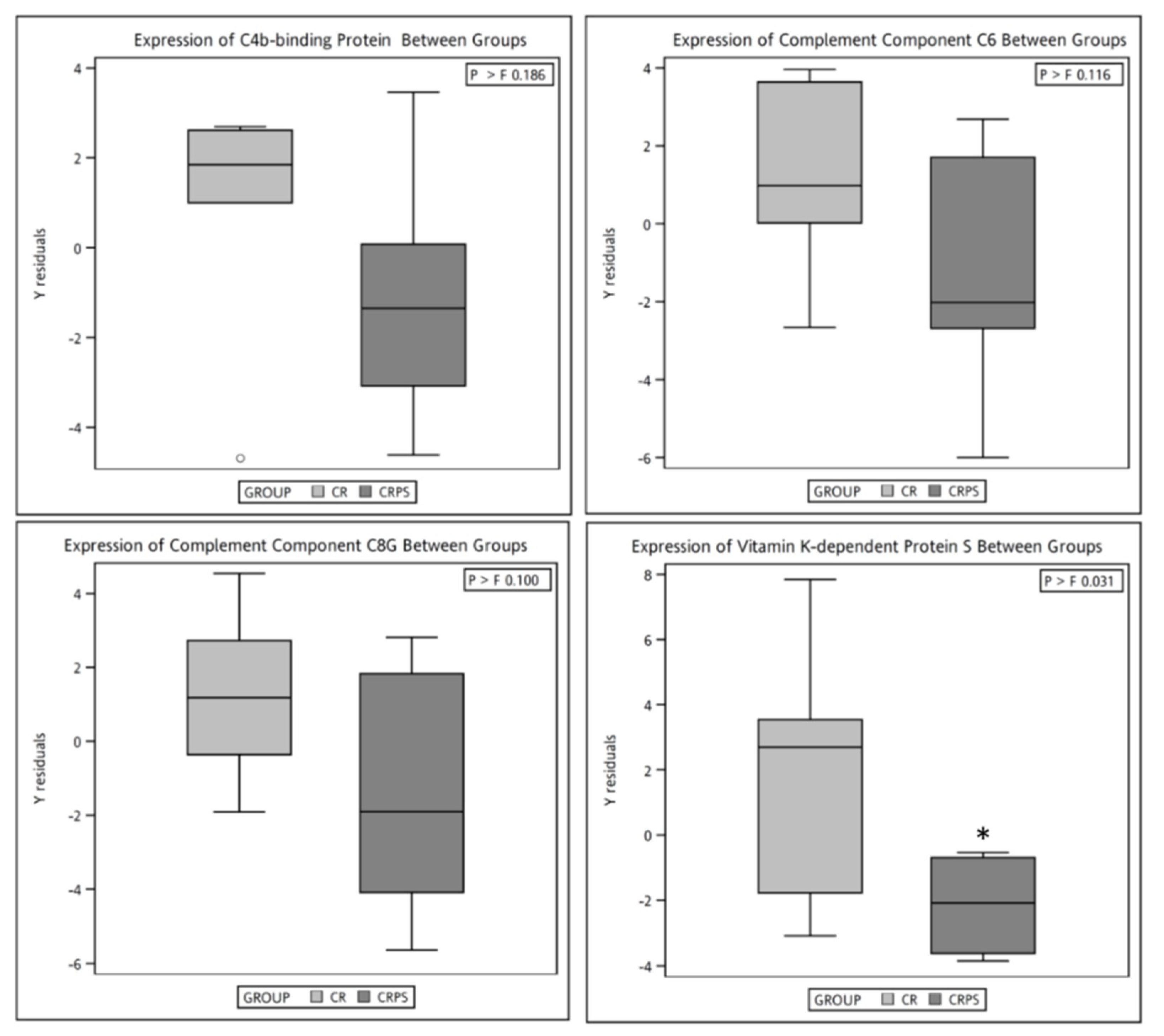
3.4. Plasma Proteomic Measurements
4. Discussion
4.1. Effects of the Dietary Intervention on the Clinical Variables
4.2. Effect of Protein Supplementation on Plasma Protein Profiles
4.3. Spearman’s Correlation of Selected Plasma Protein Candidates with Biochemical Variables
4.4. Effects of CRPS on Plasma Protein Profiles
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A/G% | android-to-gynoid ratio |
| APP | acute phase protein |
| BMI | body mass index |
| C1RL | complement C1r subcomponent–like protein |
| C4BP | plasma C4b-binding protein |
| C6 | complement component C6 |
| C8G | complement component C8 gamma chain |
| CR | caloric restriction |
| CRPS | caloric restriction with protein supplementation |
| DM | diabetes mellitus |
| FBG | fasting blood glucose |
| HbA1c | haemoglobin A1c |
| HDL-C | high-density lipoprotein cholesterol |
| IL6 | interleukin 6 |
| LDL-C | low-density lipoprotein cholesterol |
| MetS | metabolic syndrome |
| PROS | vitamin K–dependent protein S |
| TC | total cholesterol |
| TG | triglyceride |
References
- Desroches, S.; Lamarche, B. The evolving definitions and increasing prevalence of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Salpea, K.D.; Mikhailidis, D.P. The prevalence of metabolic syndrome in various populations. Am. J. Med. Sci. 2007, 333, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.J.; Shaw, J.E.; Zimmet, P.Z. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol. Metab. Clin. N. Am. 2004, 33, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare, 2017 Taiwan Health and Welfare Report. Available online: http://www.arteck.com.tw/2017mohw/ebook_en/mobile/index.html#p=1 (accessed on 11 December 2018).
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Forbes, G.B. Weight loss during fasting: Implications for the obese. Am. J. Clin. Nutr. 1970, 23, 1212–1219. [Google Scholar] [CrossRef]
- Barzilai, N.; Banerjee, S.; Hawkins, M.; Chen, W.; Rossetti, L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J. Clin. Investig. 1998, 101, 1353–1361. [Google Scholar] [CrossRef]
- Havel, P.J. Control of energy homeostasis and insulin action by adipocyte hormones: Leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 2002, 13, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]
- Josse, A.R.; Atkinson, S.A.; Tarnopolsky, M.A.; Phillips, S.M. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J. Nutr. 2011, 141, 1626–1634. [Google Scholar] [CrossRef]
- Selsby, J.T.; Judge, A.R.; Yimlamai, T.; Leeuwenburgh, C.; Dodd, S.L. Life long calorie restriction increases heat shock proteins and proteasome activity in soleus muscles of fisher 344 rats. Exp. Gerontol. 2005, 40, 37–42. [Google Scholar] [CrossRef]
- Peart, J.N.; See Hoe, L.; Pepe, S.; Johnson, P.; Headrick, J.P. Opposing effects of age and calorie restriction on molecular determinants of myocardial ischemic tolerance. Rejuvenation Res. 2012, 15, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Matthews, K.A.; Kuller, L.H.; Meilahn, E.N.; Plantinga, P.L. Weight gain at the time of menopause. Arch. Intern. Med. (Chic) 1991, 151, 97–102. [Google Scholar] [CrossRef]
- Verkouter, I.; Noordam, R.; de Roos, A.; Lamb, H.J.; Rosendaal, F.R.; van Heemst, D.; de Mutsert, R. Adult weight change in relation to visceral fat and liver fat at middle age: The netherlands epidemiology of obesity study. Int. J. Obes. (Lond.) 2018. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Lee, H.C.; Cheng, W.Y.; Huang, S.Y. A calorie-restriction diet supplemented with fish oil and high-protein powder is associated with reduced severity of metabolic syndrome in obese women. Eur. J. Clin. Nutr. 2015, 69, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Cheng, W.-Y.; Hsu, Y.-H.; Su, H.-Y.; Huang, B.E.T.G.; Lin, Y.-K.; Chan, W.P.; Su, C.-T.; Huang, S.-Y. Effects of calorie restriction with n-3 long-chain polyunsaturated fatty acids on metabolic syndrome severity in obese subjects: A randomize-controlled trial. J. Funct. Foods 2015, 19, 929–940. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Available online: https://apps.who.int/iris/bitstream/handle/10665/66040/WHO_NCD_NCS_99.2.pdf;jsessionid=8B779DB8836D3734FA7A235F5BB0AFCB?sequence=1 (accessed on 11 December 2018).
- Flechtner-Mors, M.; Boehm, B.O.; Wittmann, R.; Thoma, U.; Ditschuneit, H.H. Enhanced weight loss with protein-enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab. Res. Rev. 2010, 26, 393–405. [Google Scholar] [CrossRef]
- Rehman, A.A.; Ahsan, H.; Khan, F.H. Alpha-2-macroglobulin: A physiological guardian. J. Cell. Physiol. 2013, 228, 1665–1675. [Google Scholar] [CrossRef]
- Schelp, F.-P.; Pongpaew, P. Protection against cancer through nutritionally-induced increase of endogenous proteinase inhibitors—A hypothesis. Int. J. Epidemiol. 1988, 17, 287–292. [Google Scholar] [CrossRef]
- Bode, J.G.; Albrecht, U.; Häussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins–regulation by IL-6-and IL-1-type cytokines involving stat3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef]
- Maruna, P.; Gurlich, R.; Frasko, R.; Haluzik, M. Serum leptin levels in septic men correlate well with the C-reactive protein (CRP) and TNF-alpha but not with BMI. Physiol. Res. 2001, 50, 589–594. [Google Scholar] [PubMed]
- Tungtrongchitr, R.; Pongpaew, P.; Vudhivai, N.; Changbumrung, S.; Tungtrongchitr, A.; Phonrat, B.; Viroonudomphol, D.; Pooudong, S.; Schelp, F.P. Relationship between alpha-2-macroglobulin, anthropometric parameters and lipid profiles in thai overweight and obese in Bangkok. Nutr. Res. 2003, 23, 1143–1152. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, C.; Yang, P. Association of single nucleotide polymorphisms in the 5′ upstream region of the C4BPA gene with essential hypertension in a northeastern Han Chinese population. Mol. Med. Rep. 2017, 16, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Zoller, B.; Li, X.; Sundquist, J.; Sundquist, K. Autoimmune diseases and venous thromboembolism: A review of the literature. Am. J. Cardiovasc. Dis. 2012, 2, 171–183. [Google Scholar] [PubMed]
- Buil, A.; Tregouet, D.A.; Souto, J.C.; Saut, N.; Germain, M.; Rotival, M.; Tiret, L.; Cambien, F.; Lathrop, M.; Zeller, T.; et al. C4BPB/C4BPA is a new susceptibility locus for venous thrombosis with unknown protein S-independent mechanism: Results from genome-wide association and gene expression analyses followed by case-control studies. Blood 2010, 115, 4644–4650. [Google Scholar] [CrossRef]
- Bassig, B.A.; Zheng, T.; Zhang, Y.; Berndt, S.I.; Holford, T.R.; Hosgood, H.D., 3rd; Hu, W.; Leaderer, B.; Yeager, M.; Menashe, I.; et al. Polymorphisms in complement system genes and risk of non-Hodgkin lymphoma. Environ. Mol. Mutagen. 2012, 53, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Ariga, S.K.; Barbeiro, H.V.; de Lima, T.M.; de Paula Silva, E.; Barbeiro, D.F.; Machado, M.C.C.; Nizet, V.; Pinheiro da Silva, F. Cathelicidin-deficient mice exhibit increased survival and upregulation of key inflammatory response genes following cecal ligation and puncture. J. Mol. Med. (Berl.) 2017, 95, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef]
- Morgan, B.P. The membrane attack complex as an inflammatory trigger. Immunobiology 2016, 221, 747–751. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Ricklin, D.; Ward, P.A.; Lambris, J.D. Interactions between coagulation and complement—Their role in inflammation. Semin. Immunopathol. 2012, 34, 151–165. [Google Scholar] [CrossRef]
- Baldwin, W.M., 3rd; Qian, Z.; Ota, H.; Samaniego, M.; Wasowska, B.; Sanfilippo, F.; Hruban, R.H. Complement as a mediator of vascular inflammation and activation in allografts. J. Heart Lung Transplant. 2000, 19, 723–730. [Google Scholar] [CrossRef]
- Rezende, S.M.; Simmonds, R.E.; Lane, D.A. Coagulation, inflammation, and apoptosis: Different roles for protein S and the protein S-C4B binding protein complex. Blood 2004, 103, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, J.H.; van der Poll, T.; van’t Veer, C. Tam receptors, GAS6, and protein S: Roles in inflammation and hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef]
- Alberio, L.; Lammle, B.; Esmon, C.T. Protein C replacement in severe meningococcemia: Rationale and clinical experience. Clin. Infect. Dis. 2001, 32, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Hiramori, K.; Imaizumi, T.; Kitabatake, A.; Hishida, H.; Nomura, M.; Fujii, T.; Sakuma, I.; Fukami, K.; Honda, T.; et al. Intravascular ultrasound evaluation of coronary plaque regression by low density lipoprotein-apheresis in familial hypercholesterolemia: The low density lipoprotein-apheresis coronary morphology and reserve trial (LACMART). J. Am. Coll. Cardiol. 2002, 40, 220–227. [Google Scholar] [CrossRef]
- Yuasa, Y.; Osaki, T.; Makino, H.; Iwamoto, N.; Kishimoto, I.; Usami, M.; Minamino, N.; Harada-Shiba, M. Proteomic analysis of proteins eliminated by low-density lipoprotein apheresis. Ther. Apher. Dial. 2014, 18, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Dejgaard, A.; Andersen, T.; Christoffersen, P.; Clemmensen, I.; Gluud, C. Plasma fibronectin concentrations in morbidly obese patients. Scand. J. Clin. Lab. Investig. 1984, 44, 207–210. [Google Scholar] [CrossRef]
- Mutch, D.M.; Pers, T.H.; Temanni, M.R.; Pelloux, V.; Marquez-Quinones, A.; Holst, C.; Martinez, J.A.; Babalis, D.; van Baak, M.A.; Handjieva-Darlenska, T.; et al. A distinct adipose tissue gene expression response to caloric restriction predicts 6-mo weight maintenance in obese subjects. Am. J. Clin. Nutr. 2011, 94, 1399–1409. [Google Scholar] [CrossRef]
- Ekaidem, I.S.; Bolarin, D.M.; Udoh, A.E.; Etuk, S.J.; Udiong, C.E. Plasma fibronectin concentration in obese/overweight pregnant women: A possible risk factor for preeclampsia. Indian J. Clin. Biochem. 2011, 26, 187–192. [Google Scholar] [CrossRef]
- Kanters, S.D.; Banga, J.-D.; Algra, A.; Frijns, R.C.; Beutler, J.J.; Fijnheer, R. Plasma levels of cellular fibronectin in diabetes. Diabetes Care 2001, 24, 323–327. [Google Scholar] [CrossRef]
- Song, K.S.; Kim, H.K.; Shim, W.; Jee, S.H. Plasma fibronectin levels in ischemic heart disease. Atherosclerosis 2001, 154, 449–453. [Google Scholar] [CrossRef]
- Waalkes, S.; Atschekzei, F.; Kramer, M.W.; Hennenlotter, J.; Vetter, G.; Becker, J.U.; Stenzl, A.; Merseburger, A.S.; Schrader, A.J.; Kuczyk, M.A. Fibronectin 1 mRNA expression correlates with advanced disease in renal cancer. BMC Cancer 2010, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Örem, C.; Çelik, S.ü.; Örem, A.; Calapoğlu, M.; Erdöl, C. Increased plasma fibronectin levels in patients with acute myocardial infarction complicated with left ventricular thrombus. Thromb. Res. 2002, 105, 37–41. [Google Scholar] [CrossRef]
- Örem, C.; Durmus, I.; Klç, K.; Baykan, M.; Gökçe, M.; Örem, A.; Topbas, M. Plasma fibronectin level and its association with coronary artery disease and carotid intima-media thickness. Coron. Artery Dis. 2003, 14, 219–224. [Google Scholar] [CrossRef] [PubMed]
| Variable | CR (n = 7) | CRPS (n = 6) | Post-Intervention | ||
|---|---|---|---|---|---|
| Baseline | 12-Week | Baseline | WK12 | p-Value b | |
| Age (y/o) | 61.97 (64.61) | - | 55.84 (63.87) | - | 0.52 |
| Anthropometrics | |||||
| BW (kg) | 66.10 (75.40) | 59.80 (68.90) * | 67.90 (77.35) | 60.60 (72.23) * | 0.94 |
| BMI (kg/m2) | 28.21 (31.59) | 25.46 (28.40) * | 27.03 (28.25) | 24.50 (26.09) * | 0.62 |
| WC (cm) | 85.00 (94.00) | 79.00 (86.00) * | 85.50 (93.50) | 76.00 (83.75) * | 0.43 |
| Body Composition | |||||
| Android (%) | 53.50 (55.00) | 46.30 (53.10) * | 47.95 (51.83) | 44.20 (48.65) * | 0.52 |
| Gynoid (%) | 48.40 (49.30) | 43.30 (45.90) * | 43.60 (49.05) | 39.65 (43.80) * | 0.23 |
| TBF (%) | 43.20 (46.90) | 40.00 (44.10) * | 40.45 (43.20) | 34.85 (40.93) * | 0.35 |
| Blood Pressure | |||||
| SBP (mmHg) | 144.0 (162.0) | 116.0 (149.0) * | 147.5 (152.3) | 123.0 (130.0) * | 1.00 |
| DBP (mmHg) | 84.00 (93.00) | 75.00 (86.00) * | 81.50 (85.25) | 70.50 (76.50) | 0.32 |
| Laboratory Data | |||||
| GOT (U/L) | 29.00 (33.00) | 24.00 (31.00) | 22.00 (26.25) | 22.50 (25.50) | 0.22 |
| GPT (U/L) | 36.00 (42.00) | 24.00 (27.00) * | 16.50 (21.00) | 18.00 (21.50) | 0.20 |
| BUN (mg/dL) | 15.00 (16.00) | 12.90 (19.40) | 13.50 (16.35) | 13.05 (16.93) | 0.62 |
| CR (mg/dL) | 0.72 (0.73) | 0.64 (0.75) | 0.63 (0.67) | 0.62 (0.69) | 0.28 |
| ALB (g/dL) | 4.20 (4.50) | 4.40 (4.60) | 4.30 (4.30) | 4.25 (4.33) | 0.07 |
| Glucose Metabolism | |||||
| FBG (mg/dL) | 103.0 (105.0) | 97.0 (101.0) * | 102.5 (127.0) | 91.0 (97.8) * | 0.06 |
| PC (mg/dL) | 132.0 (155.0) | 121.0 (138.0) | 146.5 (198.8) | 119.5 (135.0) | 0.43 |
| Insulin (μIU/mL) | 11.00 (14.70) | 7.86 (14.40) | 10.09 (12.58) | 7.43 (9.16) * | 0.28 |
| HbA1c (%) | 5.70 (5.90) | 5.60 (5.90) | 5.75 (6.55) | 5.70 (6.48) | 0.94 |
| HOMA-IR | 2.85 (3.70) | 1.86 (3.45) | 2.60 (3.58) | 1.60 (2.07) * | 0.18 |
| QUICKI | 0.33 (0.33) | 0.35 (0.36) | 0.33 (0.34) | 0.36 (0.37) * | 0.22 |
| Blood Lipids | |||||
| TG (mg/dL) | 167.0 (184.0) | 100.0 (137.0) * | 122.0 (155.3) | 96.0 (108.3) | 0.78 |
| TC (mg/dL) | 209.0 (233.0) | 184.0 (207.0) | 202.0 (210.3) | 180.5 (217.0) | 1.00 |
| HDL-C (mg/dL) | 49.40 (58.00) | 44.70 (54.90) | 45.25 (63.50) | 41.95 (73.13) | 0.78 |
| LDL-C (mg/dL) | 129.0 (150.0) | 127.0 (138.0) | 117.0 (138.8) | 117.0 (124.5) | 0.52 |
| Inflammatory Status | |||||
| CRP (mg/dL) | 0.36 (0.77) | 0.22 (0.58) | 0.22 (0.51) | 0.21 (0.30) | 0.83 |
| IL-6 (pg/mL) | 2.42 (3.80) | 1.71 (2.59) * | 3.50 (4.96) | 1.68 (2.86) | 0.89 |
| Accession | Entry Name | Protein Name | CR (n = 7) | CRPS (n = 6) | p-Value b |
|---|---|---|---|---|---|
| P01023 | A2MG_HUMAN | Alpha-2-macroglobulin (A2M) | 92.0 (94.1) | 105.0 (116.0) | 0.01 |
| P04003 | C4BPA_HUMAN | C4b-binding protein alpha chain (C4BPA) | 7.55 (8.71) | 4.19 (6.45) | 0.04 |
| Q9NZP8 | C1RL_HUMAN | Complement C1r subcomponent-like protein (C1RL) | 1.74 (3.05) | 0.80 (1.79) | 0.02 |
| P13671 | CO6_HUMAN | Complement component C6 (C6) | 8.22 (9.94) | 5.39 (7.50) | 0.02 |
| P07360 | CO8G_HUMAN | Complement component C8 gamma chain (C8) | 1.59 (2.17) | 0.20 (1.26) | 0.04 |
| P07225 | PROS_HUMAN | Vitamin K-dependent protein S (PROS) | 4.73 (5.62) | 3.50 (4.48) | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Tung, Y.-T.; Lin, Y.-K.; Liao, C.-C.; Chiu, C.-F.; Tung, T.-H.; Shabrina, A.; Huang, S.-Y. Effects of Caloric Restriction with Protein Supplementation on Plasma Protein Profiles in Middle-Aged Women with Metabolic Syndrome—A Preliminary Open Study. J. Clin. Med. 2019, 8, 195. https://doi.org/10.3390/jcm8020195
Chang C-Y, Tung Y-T, Lin Y-K, Liao C-C, Chiu C-F, Tung T-H, Shabrina A, Huang S-Y. Effects of Caloric Restriction with Protein Supplementation on Plasma Protein Profiles in Middle-Aged Women with Metabolic Syndrome—A Preliminary Open Study. Journal of Clinical Medicine. 2019; 8(2):195. https://doi.org/10.3390/jcm8020195
Chicago/Turabian StyleChang, Chia-Yu, Yu-Tang Tung, Yen-Kuang Lin, Chen-Chung Liao, Ching-Feng Chiu, Te-Hsuan Tung, Amalina Shabrina, and Shih-Yi Huang. 2019. "Effects of Caloric Restriction with Protein Supplementation on Plasma Protein Profiles in Middle-Aged Women with Metabolic Syndrome—A Preliminary Open Study" Journal of Clinical Medicine 8, no. 2: 195. https://doi.org/10.3390/jcm8020195
APA StyleChang, C.-Y., Tung, Y.-T., Lin, Y.-K., Liao, C.-C., Chiu, C.-F., Tung, T.-H., Shabrina, A., & Huang, S.-Y. (2019). Effects of Caloric Restriction with Protein Supplementation on Plasma Protein Profiles in Middle-Aged Women with Metabolic Syndrome—A Preliminary Open Study. Journal of Clinical Medicine, 8(2), 195. https://doi.org/10.3390/jcm8020195







