Effects of a Short-Term Cycling Interval Session and Active Recovery on Non-Linear Dynamics of Cardiac Autonomic Activity in Endurance Trained Cyclists
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hottenrott, K.; Hoos, O. Heart rate variability analysis in exercise physiology. In ECG Time Series Analysis: Engineering to Medicine; Jelinek, H., Khandoker, A., Cornforth, D., Eds.; CRC Press: London, UK, 2017; pp. 245–257. [Google Scholar]
- Michael, S.; Graham, K.S.; Davis, G.M. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—A review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.B. Modulation of cardiovascular control mechanisms and their interaction. Physiol. Rev. 1996, 76, 193–244. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, G.R.H.; Brodie, D.A. The use of heart rate variability measures to assess autonomic control during exercise. Scand. J. Med. Sci. Sports 2006, 16, 302–313. [Google Scholar] [CrossRef]
- Hottenrott, K.; Hoos, O.; Esperer, H.D. Heart rate variability and physical exercise. Current status. Herz 2006, 31, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Schulz, S.; Schroeder, R.; Baumert, M.; Caminal, P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos. Trans. A Math. Phys. Eng. Sci. 2008, 367, 277–296. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Perkiömäki, J.S.; Maestri, R.; Pinna, G.D. Clinical impact of evaluation of cardiovascular control by novel methods of heart rate dynamics. Philos. Trans. R. Soc. A 2009, 367, 1223–1238. [Google Scholar] [CrossRef]
- Peng, C.K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef]
- Platisa, M.M.; Gal, V. Correlation properties of heartbeat dynamics. Eur. Biophys. J. 2008, 37, 1247–1252. [Google Scholar] [CrossRef]
- Vanderlei, L.C.M.; Pastre, C.M.; Júnior, I.F.F.; de Godoy, M.F. Fractal correlation of heart rate variability in obese children. Auton. Neurosci. 2010, 155, 125–129. [Google Scholar] [CrossRef]
- De Godoy, M.F. Nonlinear analysis of heart rate variability: A comprehensive review. J. Cardiol. Ther. 2016, 3, 528–533. [Google Scholar]
- Kauffman, S.A. At Home in the Universe: The Search for Laws of Self-Organization and Complexity; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Iyengar, N.; Peng, C.K.; Morin, R.; Goldberger, A.L.; Lipsitz, L.A. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996, 271, R1078–R1084. [Google Scholar] [CrossRef] [PubMed]
- Makikallio, T.H.; Høiber, S.; Køber, L.; Torp-Pedersen, C.; Peng, C.K.; Goldberger, A.L.; Huikuri, H.V. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. Am. J. Cardiol. 1999, 83, 836–839. [Google Scholar] [CrossRef]
- Goldberger, A.L. Fractal variability versus pathologic periodicity: Complexity loss and stereotypy in disease. Perspect. Biol. Med. 1997, 40, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Casties, J.; Mottet, D.; Le Gallais, D. Non-linear analyses of heart rate variability during heavy exercise and recovery in cyclists. Int. J. Sports Med. 2006, 27, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Hautala, A.J.; Makikallio, T.H.; Seppanen, T.; Huikuri, H.V.; Tulppo, M.P. Short-term correlation properties of R-R interval dynamics at different exercise intensity levels. Clin. Physiol. Funct. Imaging 2003, 23, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Platisa, M.M.; Mazic, S.; Nestorovic, Z.; Gal, V. Complexity of heartbeat interval series in young healthy trained and untrained men. Physiol. Meas. 2008, 29, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Tulppo, M.P.; Hughson, R.L.; Makikallio, T.H.; Airaksinen, K.E.; Seppanen, T.; Huikuri, H.V. Effects of exercise and passive head-up tilt on fractal and complexity properties of heart rate dynamics. Am. J. Physiol. 2001, 280, H1081–H1087. [Google Scholar] [CrossRef]
- Blasco-Lafarga, C.; Camarena, B.; Mateo-March, M. Cardiovascular and Autonomic Responses to a Maximal Exercise Test in Elite Youngsters. Int. J. Sports Med. 2017, 38, 666–674. [Google Scholar] [CrossRef]
- Gronwald, T.; Hoos, O.; Ludyga, S.; Hottenrott, K. Non-linear dynamics of heart rate variability during incremental cycling exercise. Res. Sports Med. 2019, 27, 88–89. [Google Scholar] [CrossRef]
- Gronwald, T.; Ludyga, S.; Hoos, O.; Hottenrott, K. Non-linear dynamics of cardiac autonomic activity during cycling exercise with varied cadence. Hum. Mov. Sci. 2018, 60, 225–233. [Google Scholar] [CrossRef]
- Peçanha, T.; Bartels, R.; Brito, L.C.; Paula-Ribeiro, M.; Oliveira, R.S.; Goldberger, J.J. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017, 227, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.R.; del Rosso, S.; Leicht, A.S.; Hautala, A.J.; Boullosa, D.A. Methods of assessment of the post-exercise cardiac autonomic recovery: Additional important factors to be considered. Int. J. Cardiol. 2017, 239, 23. [Google Scholar] [CrossRef] [PubMed]
- Boullosa, D.A.; Barros, E.S.; del Rosso, S.; Nakamura, F.Y.; Leicht, A.S. Reliability of heart rate measures during walking before and after running maximal efforts. Int. J. Sports Med. 2014, 35, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Weippert, M.; Kumar, M.; Kreuzfeld, S.; Arndt, D.; Rieger, A.; Stoll, R. Comparison of three mobile devices for measuring R–R intervals and heart rate variability: Polar S810i, Suunto t6 and an ambulatory ECG system. Eur. J. Appl. Physiol. 2010, 109, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Heffernan, K.S.; Rossow, L.; Guerra, M.; Pereira, F.D.; Fernhall, B. Sex differences in linear and nonlinear heart rate variability during early recovery from supramaximal exercise. Appl. Physiol. Nutr. Metab. 2010, 35, 439–446. [Google Scholar] [CrossRef]
- Faude, O.; Meyer, T. Methodische Aspekte der Laktatbestimmung [Methodological Aspects of Lactate Determination]. Dtsch. Z. Sportmed. 2008, 59, 305–309. [Google Scholar]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sport Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Kannankeril, P.J.; Goldberger, J.J. Parasympathetic effects on cardiac electrophysiology during exercise and recovery. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2091–H2098. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Garcia, V.; García-Massó, X.; Salvá, P.; Escobar, R. The use of heart rate variability in assessing precompetitive stress in high-standard judo athletes. Int. J. Sports Med. 2013, 34, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Millar, P.J.; Rakobowchuk, M.; McCartney, N.; MacDonald, M.J. Heart rate variability and nonlinear analysis of heart rate dynamics following single and multiple Wingate bouts. Appl. Physiol. Nutr. Metab. 2009, 34, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Goya-Esteban, R.; Barquero-Pérez, O.; Sarabia-Cachadina, E.; de la Cruz-Torres, B.; Naranjo-Orellana, J.; Rojo-Alvarez, J.L. Heart rate variability non linear dynamics in intense exercise. Comput. Cardiol. 2012, 39, 177–180. [Google Scholar]
- Blasco-Lafarga, C.; Martínez-Navarro, I.; Mateo-March, M. Is baseline cardiac autonomic modulation related to performance and physiological responses following a supramaximal Judo test? PLoS ONE 2013, 8, e78584. [Google Scholar] [CrossRef]
- Blasco-Lafarga, C.; Martínez-Navarro, I.; Mateo, M.; Pablos, C.; Carratalá, V. Non-Linear Approach to Cardiac Autonomic Recovery Following an Upper-Limb Judo Test. Int. J. Mot. Learn. Sport Perf. 2011, 2, 72–79. [Google Scholar]
- Martínez-Navarro, I.; Chiva-Bartoll, O.; Hernando, B.; Collado, E.; Porcar, V.; Hernando, C. Hydration Status, Executive Function, and Response to Orthostatism After a 118-km Mountain Race: Are They Interrelated? J. Strength Cond. Res. 2018, 32, 441–449. [Google Scholar]
- Karasik, R.; Sapir, N.; Ashkenazy, Y.; Ivanov, P.C.; Dvir, I.; Lavie, P.; Havlin, S. Correlation differences in heartbeat fluctuations during rest and exercise. Phys. Rev. E 2002, 66, 062902. [Google Scholar] [CrossRef]
- Lewis, M.J.; Short, A.L. Exercise and cardiac regulation: What can electrocardiographic time series tell us? Scand. J. Med. Sci. Sports 2010, 20, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Smirmaul, B.P.; Fontes, E.B.; Noakes, T.D. Afferent feedback from fatigued locomotor muscles is important, but not limiting, for endurance exercise performance. J. Appl. Physiol. 2010, 108, 458. [Google Scholar] [PubMed]
- Marino, F.E.; Gard, M.; Drinkwater, E.J. The limits to exercise performance and the future of fatigue research. Br. J. Sports. Med. 2011, 45, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Venhorst, A.; Micklewright, D.; Noakes, T.D. Towards a three-dimensional framework of centrally regulated and goal-directed exercise behaviour: A narrative review. Br. J. Sports Med. 2018, 52, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.B.; Wagner, C.D. General principles of chaotic dynamics. Cardiovasc. Res. 1996, 31, 332–341. [Google Scholar] [CrossRef]
- Tulppo, M.P.; Kiviniemi, A.M.; Hautala, A.J.; Kallio, M.; Seppänen, T.; Mäkikallio, T.H.; Huikuri, H.V. Physiological background of the loss of fractal heart rate dynamics. Circulation 2005, 112, 314–319. [Google Scholar] [CrossRef]
- Massaro, S.; Pecchia, L. Heart rate variability (HRV) analysis: A methodology for organizational neuroscience. Organ. Res. Methods 2019, 22, 354–393. [Google Scholar] [CrossRef]
- Perakakis, P.; Taylor, M.; Martinez-Nieto, E.; Revithi, I.; Vila, J. Breathing frequency bias in fractal analysis of heart rate variability. Biol. Psychol. 2009, 82, 82–88. [Google Scholar] [CrossRef]
- Weippert, M.; Behrens, K.; Rieger, A.; Kumar, M.; Behrens, M. Effects of breathing patterns and light exercise on linear and nonlinear heart rate variability. Appl. Physiol. Nutr. Metab. 2015, 40, 762–768. [Google Scholar] [CrossRef] [PubMed]
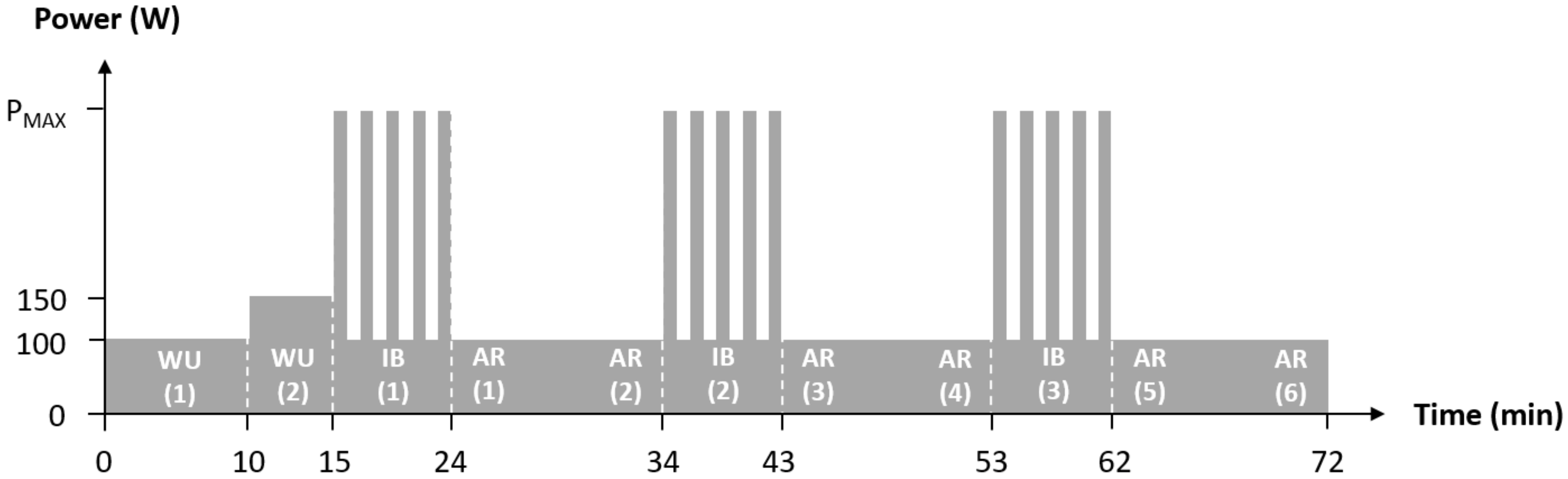

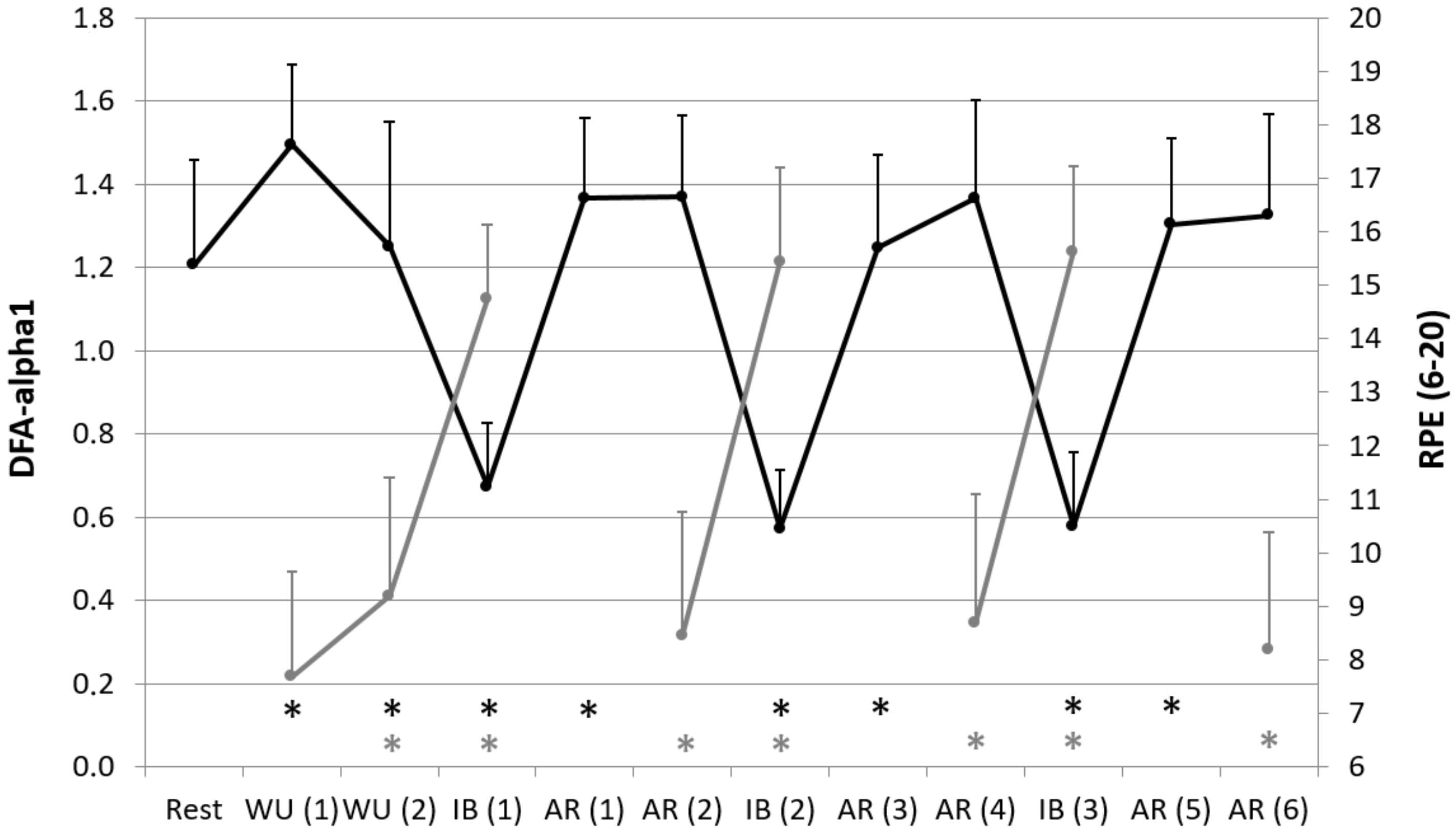
| Rest | WU (1) | WU (2) | IB (1) | AR (1) | AR (2) | IB (2) | AR (3) | AR (4) | IB (3) | AR (5) | AR (6) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (1/min) | 66.5 ± 8.2 | 106.4 * ± 7.4 | 119.6 * ± 9.4 | 154.5 * ± 8.4 | 118.4 * ± 10.3 | 114.1 * ± 10.2 | 157.0 * ± 8.4 | 121.6 * ± 11.6 | 116.1 * ± 10.8 | 157.9 * ± 9.1 | 121.3 * ± 11.0 | 116.4 * ± 11.0 |
| La (mmol/L) | 0.91 ± 0.29 | 0.69 * ± 0.19 | 0.74 ± 0.28 | 3.81 * ± 1.28 | - | 1.09 * ± 0.53 | 3.54 * ± 1.18 | - | 1.16 * ± 0.52 | 3.48 * ± 1.25 | - | 1.23 * ± 0.60 |
| RPE (6–20) | - | 7.7 ± 2.0 | 9.2 * ± 2.2 | 14.8 * ± 1.4 | - | 8.4 * ± 2.3 | 15.4 * ± 1.8 | - | 8.7 * ± 2.4 | 15.6 * ± 1.6 | - | 8.2 * ± 2.2 |
| meanRR (ms) | 923 ± 108 | 566 * ± 39 | 505 * ± 38 | 390 * ± 22 | 510 * ± 43 | 530 * ± 46 | 384 * ± 22 | 498 * ± 47 | 521 * ± 46 | 381 * ± 23 | 499 * ± 43 | 519 * ± 46 |
| RMSSD (ms) | 56.3 ± 31.1 | 3.2 * ± 0.6 | 2.9 ± 0.7 | 2.5 * ± 0.5 | 3.1 * ± 0.4 | 2.8 ± 0.6 | 2.6 ± 0.5 | 3.0 ± 0.6 | 2.8 ± 0.6 | 2.6 ± 0.6 | 3.0 ± 0.6 | 2.8 ± 0.8 |
| DFA-alpha1 | 1.21 ± 0.25 | 1.49 * ± 0.19 | 1.25 * ± 0.30 | 0.67 * ± 0.15 | 1.37 * ± 0.19 | 1.37 ± 0.20 | 0.57 * ± 0.14 | 1.25 * ± 0.22 | 1.37 ± 0.23 | 0.58 * ± 0.18 | 1.30 * ± 0.21 | 1.32 ± 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gronwald, T.; Hoos, O.; Hottenrott, K. Effects of a Short-Term Cycling Interval Session and Active Recovery on Non-Linear Dynamics of Cardiac Autonomic Activity in Endurance Trained Cyclists. J. Clin. Med. 2019, 8, 194. https://doi.org/10.3390/jcm8020194
Gronwald T, Hoos O, Hottenrott K. Effects of a Short-Term Cycling Interval Session and Active Recovery on Non-Linear Dynamics of Cardiac Autonomic Activity in Endurance Trained Cyclists. Journal of Clinical Medicine. 2019; 8(2):194. https://doi.org/10.3390/jcm8020194
Chicago/Turabian StyleGronwald, Thomas, Olaf Hoos, and Kuno Hottenrott. 2019. "Effects of a Short-Term Cycling Interval Session and Active Recovery on Non-Linear Dynamics of Cardiac Autonomic Activity in Endurance Trained Cyclists" Journal of Clinical Medicine 8, no. 2: 194. https://doi.org/10.3390/jcm8020194
APA StyleGronwald, T., Hoos, O., & Hottenrott, K. (2019). Effects of a Short-Term Cycling Interval Session and Active Recovery on Non-Linear Dynamics of Cardiac Autonomic Activity in Endurance Trained Cyclists. Journal of Clinical Medicine, 8(2), 194. https://doi.org/10.3390/jcm8020194






