Interventions to Promote Patient Utilization of Cardiac Rehabilitation: Cochrane Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Methods for Identification of Studies
2.2. Inclusion and Exclusion Criteria
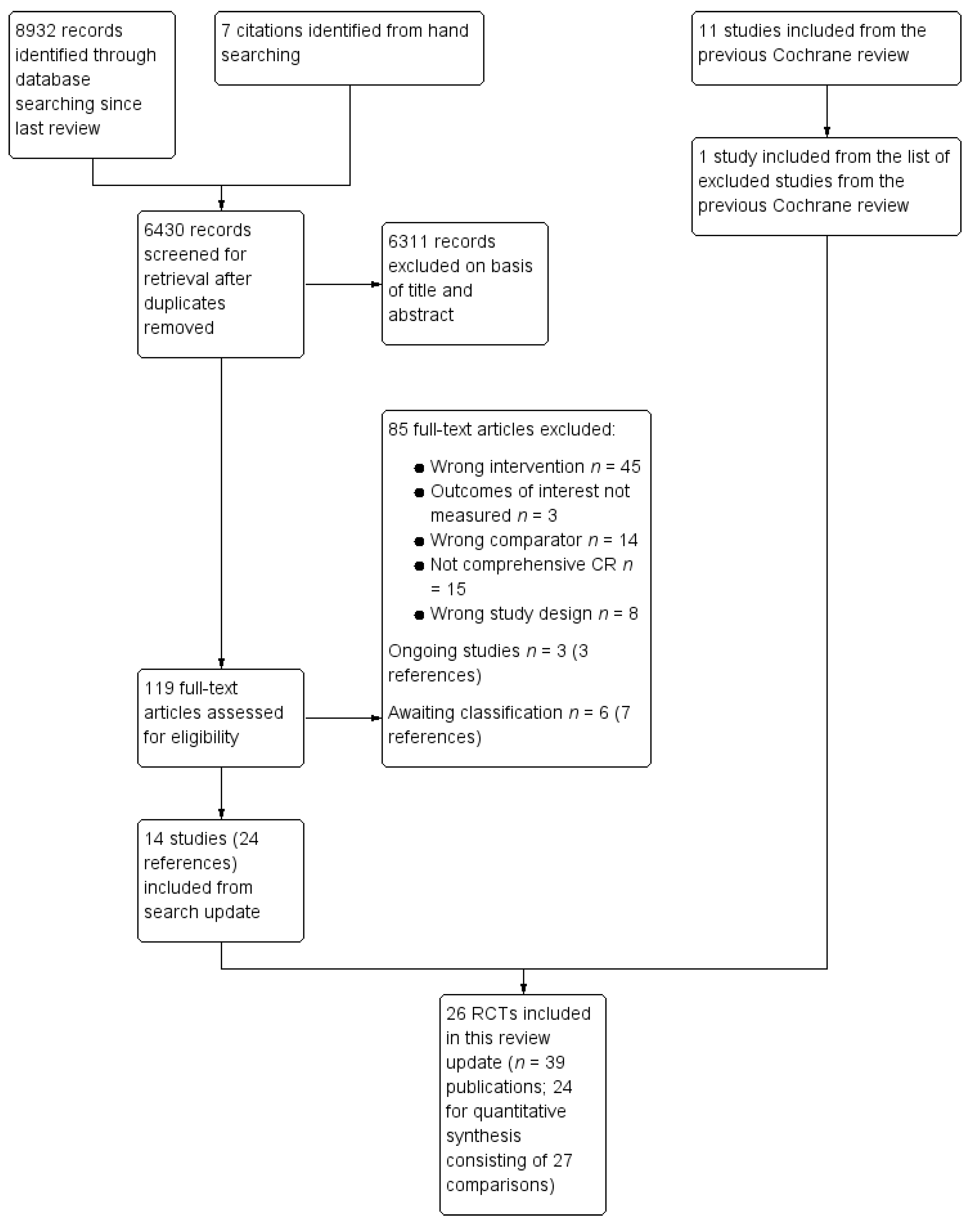
2.3. Selection of Studies for Inclusion
2.4. Data Extraction and Management
2.5. Assessment of Potential Bias
2.6. Data Synthesis and Analysis
3. Results
3.1. Included Studies by Outcome
3.2. Characteristics of Included Studies
3.3. Intervention Characteristics
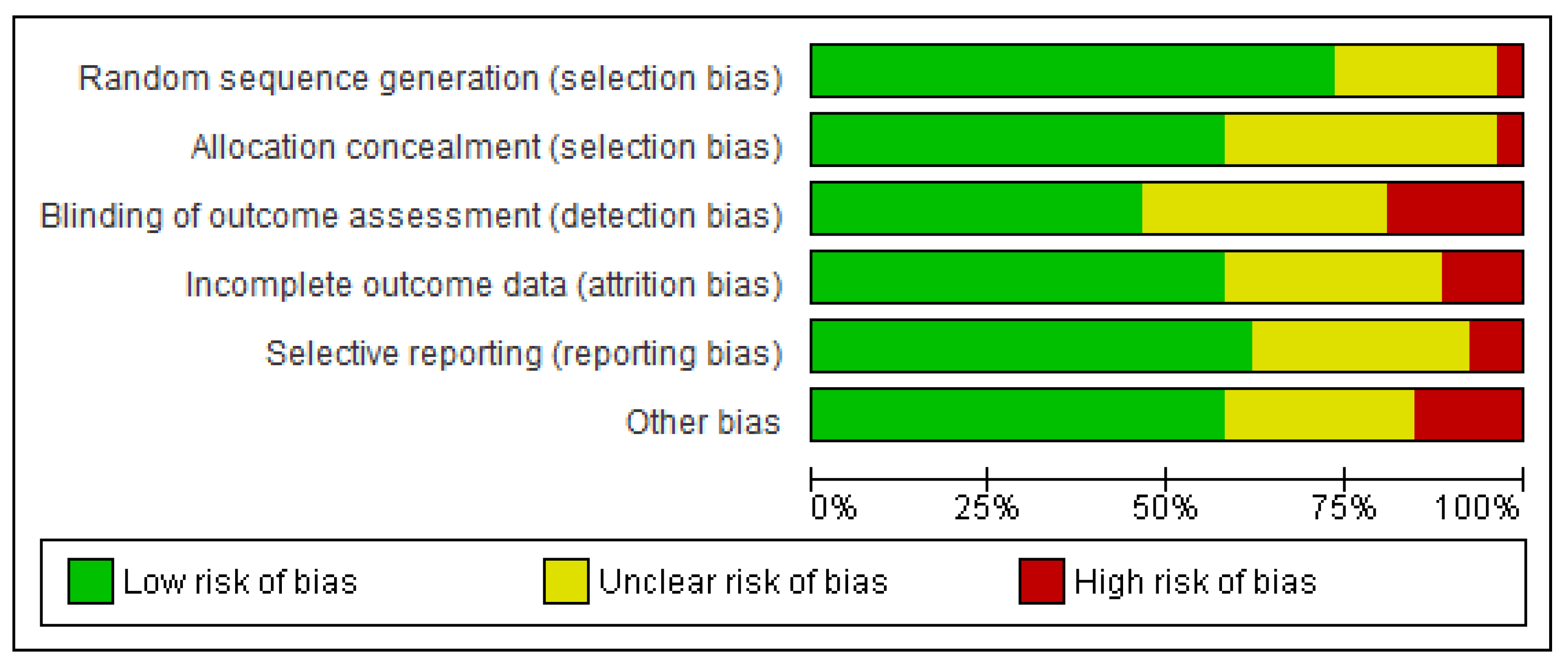
3.4. Risk of Bias in Included Studies
3.5. Effects of Interventions
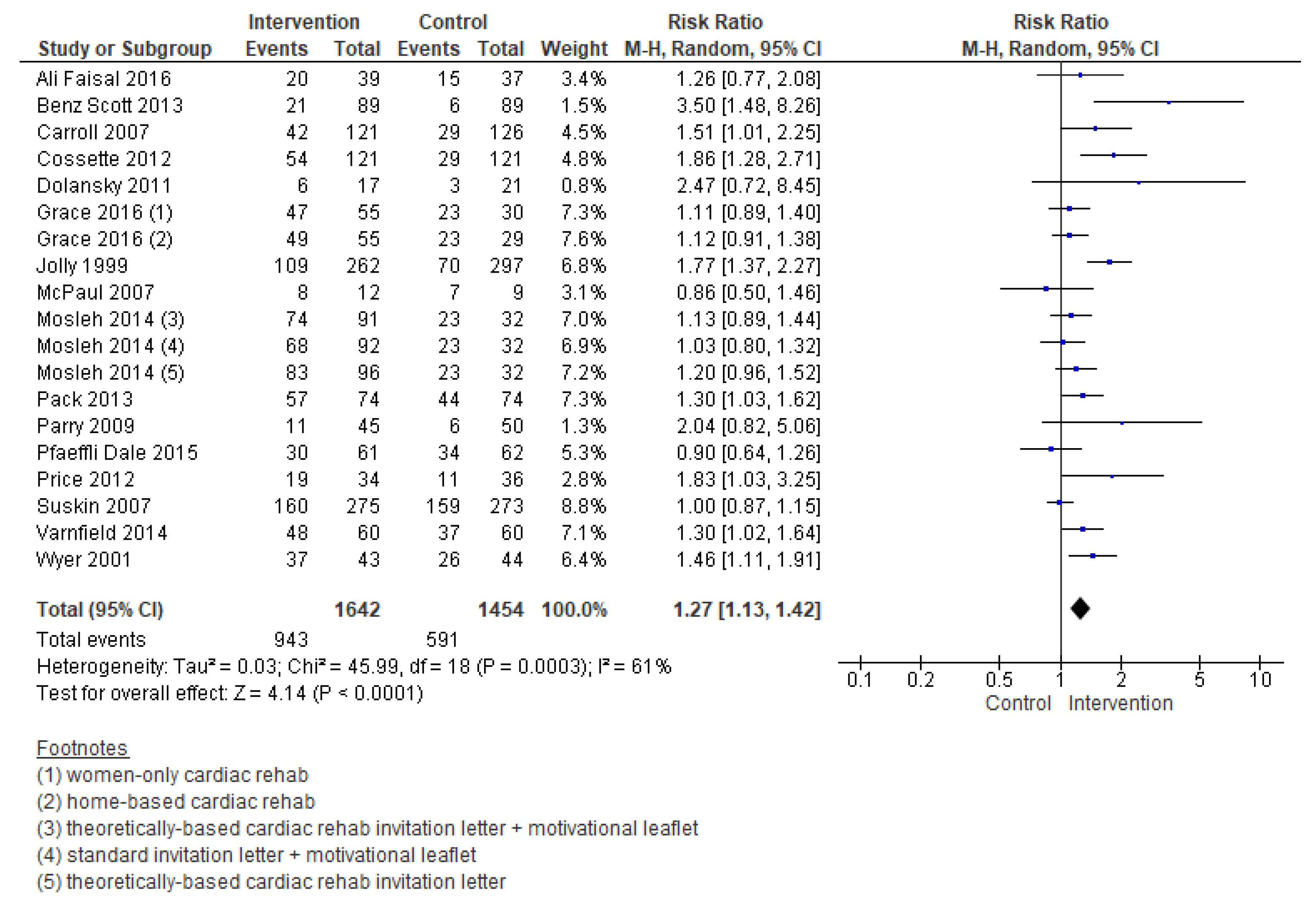
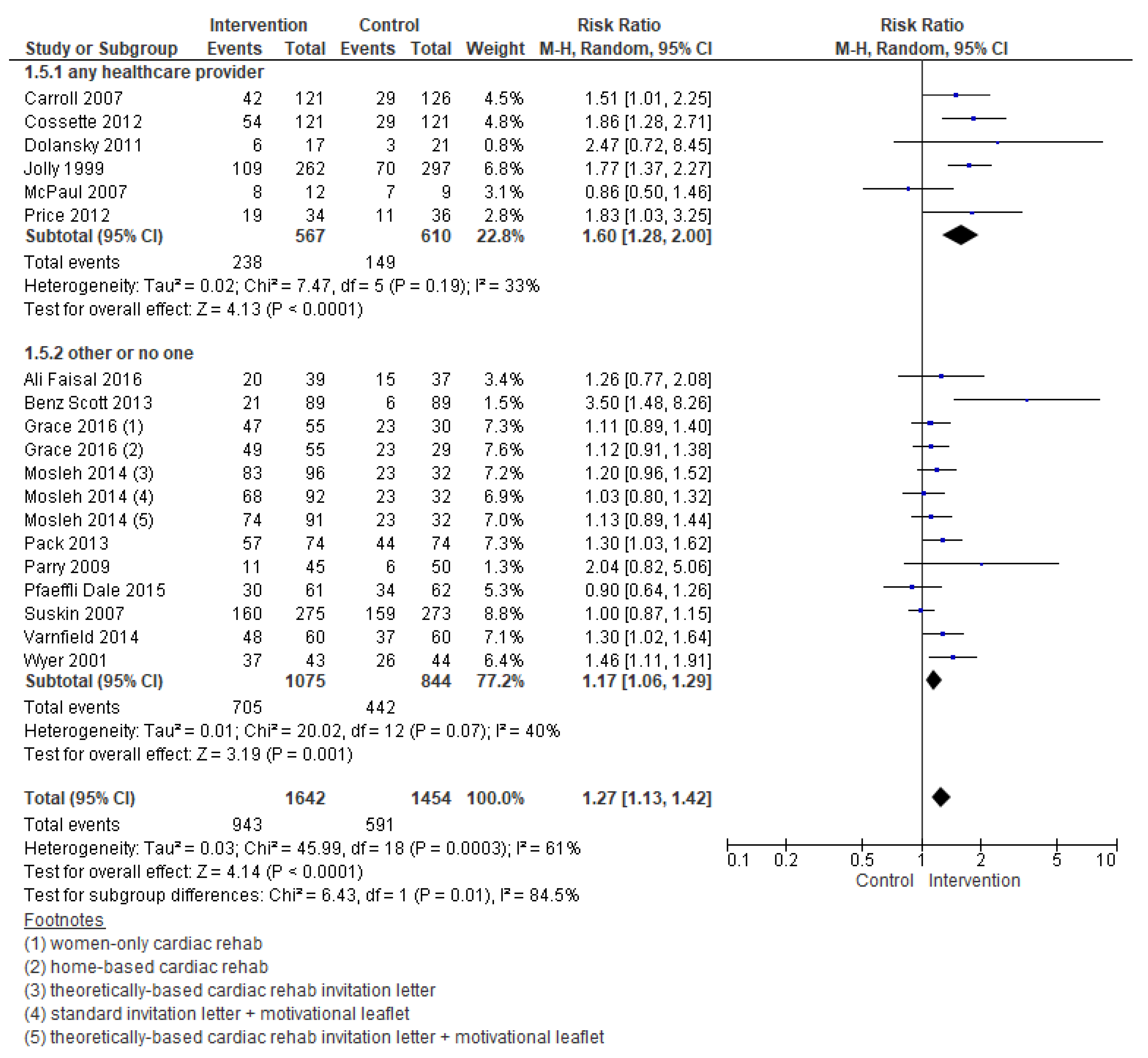
3.5.1. Enrolment
3.5.2. Adherence
3.5.3. Completion
3.5.4. Secondary Outcomes
3.6. Publication Bias
3.7. Overall Quality of Evidence
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Picatoste, B.; Badimón, J.J. Pathophysiology of Acute Coronary Syndrome. Curr. Atheroscler. Rep. 2014, 16, 401. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.L.; Turk-Adawi, K.I.; Contractor, A.; Atrey, A.; Campbell, N.; Derman, W.; Ghisi, G.L.M.; Oldridge, N.; Sarkar, B.K.; Yeo, T.J.; et al. Cardiac rehabilitation delivery model for low-resource settings. Heart 2016, 102, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.L.; Turk-Adawi, K.I.; Contractor, A.; Atrey, A.; Campbell, N.R.C.; Derman, W.; Ghisi, G.L.M.; Sarkar, B.K.; Yeo, T.J.T.J.; Lopez-Jimenez, F.; et al. Cardiac Rehabilitation Delivery Model for Low-Resource Settings: An International Council of Cardiovascular Prevention and Rehabilitation consensus statement. Prog. Cardiovasc. Dis. 2016, 59, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Santiago de Araujo Pio, C.; Marzolini, S.; Pakosh, M.; Grace, S.L. Dose of Cardiac Rehabilitation Across the Globe. In Proceedings of the Toronto General Research Institute Research Day, Toronto, ON, Canada, 6 October 2016. [Google Scholar]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.-D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: Executive summary. J. Am. Coll. Cardiol. 2012, 60, 2564–2603. [Google Scholar] [CrossRef]
- Smith, S.C.; Benjamin, E.J.; Bonow, R.O.; Braun, L.T.; Creager, M.A.; Franklin, B.A.; Gibbons, R.J.; Grundy, S.M.; Hiratzka, L.F.; Jones, D.W.; et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011, 124, 2458–2473. [Google Scholar] [CrossRef]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the american heart association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2016, 133, 1135–1147. [Google Scholar] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- Santiago de Araújo Pio, C.; Marzolini, S.; Pakosh, M.; Grace, S.L. Effect of Cardiac Rehabilitation Dose on Mortality and Morbidity: A Systematic Review and Meta-regression Analysis. Mayo Clin. Proc. 2017, 92, 1644–1659. [Google Scholar] [CrossRef]
- British Heart Foundation National Audit of Cardiac Rehabilitation (NACR). Annual Statistical Report 2017; NACR: York, UK, 2017. [Google Scholar]
- Kotseva, K.; Wood, D.; De Backer, G.; De Bacquer, D. Use and effects of cardiac rehabilitation in patients with coronary heart disease: Results from the EUROASPIRE III survey. Eur. J. Prev. Cardiol. 2013, 20, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.L.; Truong, M.; Schopfer, D.W.; Shen, H.; Bachmann, J.M.; Whooley, M.A. Geographic Variation in Cardiac Rehabilitation Participation in Medicare and Veterans Affairs Populations: An Opportunity for Improvement? Circulation 2018, 137, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Turk-Adawi, K.I.; Grace, S.L. Narrative Review Comparing the Benefits of and Participation in Cardiac Rehabilitation in High-, Middle- and Low-Income Countries. Hear. Lung Circ. 2015, 24, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; King-Shier, K.M.; Duncan, A.; Spaling, M.; Stone, J.A.; Jaglal, S.; Angus, J. Factors influencing referral to cardiac rehabilitation and secondary prevention programs: A systematic review. Eur. J. Prev. Cardiol. 2013, 20, 692–700. [Google Scholar] [CrossRef]
- Oosenbrug, E.; Marinho, R.P.; Zhang, J.; Marzolini, S.; Colella, T.J.F.; Pakosh, M.; Grace, S.L. Sex differences in cardiac rehabilitation adherence: A meta-analysis. Can. J. Cardiol. 2016, 32, 1316–1324. [Google Scholar] [CrossRef]
- Clark, A.M.; King-Shier, K.M.; Thompson, D.R.; Spaling, M.A.; Duncan, A.S.; Stone, J.A.; Jaglal, S.B.; Angus, J.E. A qualitative systematic review of influences on attendance at cardiac rehabilitation programs after referral. Am. Heart J. 2012, 164, 835–845. [Google Scholar] [CrossRef]
- Karmali, K.N.; Davies, P.; Taylor, F.; Beswick, A.; Martin, N.; Ebrahim, S. Promoting patient uptake and adherence in cardiac rehabilitation. Cochrane Database Syst. Rev. 2014, 6, CD007131. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook; GRADE Working Group: Melbourne, Australia, 2013. [Google Scholar]
- Taylor, R.S.; Sagar, V.A.; Davies, E.J.; Briscoe, S.; Coats, A.J.; Dalal, H.; Lough, F.; Rees, K.; Singh, S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst. Rev. 2014, 4, CD003331. [Google Scholar] [CrossRef]
- Whalley, B.; Rees, K.; Davies, P.; Bennett, P.; Ebrahim, S.; Liu, Z.; West, R.; Moxham, T.; Thompson, D.R.; Taylor, R.S. Psychological interventions for coronary heart disease. Cochrane Database Syst. Rev. 2011, 8, CD002902. [Google Scholar]
- Matata, B.M.; Williamson, S.A. A Review of Interventions to Improve Enrolment and Adherence to Cardiac Rehabilitation Among Patients Aged 65 Years or Above. Curr. Cardiol. Rev. 2017, 13, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.L.; Poirier, P.; Norris, C.M.; Oakes, G.H.; Somanader, D.S.; Suskin, N. Pan-Canadian development of cardiac rehabilitation and secondary prevention quality indicators. Can. J. Cardiol. 2014, 30, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- GRADEpro Guideline Development Tool; McMaster University: Hamilton, ON, Canada, 2015.
- The Cochrane Collaboration. Review Manager (RevMan); Version 5.3.; The Nordic Cochrane Centre: Copenhagen, Denmark, 2014. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC: College Station, TX, USA, 2017. [Google Scholar]
- Morton, S.C.; Adams, J.L.; Suttorp, M.J.; Shekelle, P.J. Meta-regression Approaches: What, Why, When, and How? Technical Review 8; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004.
- Ashe, E.D. The Effects of a Relapse Prevention Program on Adherence to a Phase II Cardiac Exercise Program. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 1995. [Google Scholar]
- Beckie, T.M.; Beckstead, J.W. Predicting cardiac rehabilitation attendance in a gender-tailored randomized clinical trial. J. Cardiopulm. Rehabil. Prev. 2010, 30, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Cossette, S.; Frasure-Smith, N.; Dupuis, J.; Juneau, M.; Guertin, M.-C.C.; Cossette, S. Randomized Controlled Trial of Tailored Nursing Interventions to Improve Cardiac Rehabilitation Enrollment. Nurs. Res. 2012, 61, 111–120. [Google Scholar] [CrossRef]
- Dolansky, M.A.; Zullo, M.D.; Boxer, R.S.; Moore, S.M. Initial Efficacy of a Cardiac Rehabilitation Transition Program: Cardiac TRUST. J. Gerontol. Nurs. 2011, 37, 36–44. [Google Scholar] [CrossRef]
- Jolly, K.; Bradley, F.; Sharp, S.; Smith, H.; Mant, D. Follow-up care in general practice of patients with myocardial infarction or angina pectoris: Initial results of the SHIP trial. Fam. Pract. 1998, 15, 548–555. [Google Scholar] [CrossRef]
- McPaul, J. Home Visit Versus Telephone Follow Up in Phase II Cardiac Rehabilitation Following Myocardial Infarction. Master’s Thesis, University of Chester, Chester, UK, 2007. [Google Scholar]
- Oldridge, N.B.; Jones, N. Improving patient compliance in cardiac exercise rehabilitation: Effects of written agreement and self monitoring. J. Card. Rehabil. 1983, 3, 257–262. [Google Scholar]
- Pack, Q.R.; Mansour, M.; Barboza, J.S.; Hibner, B.A.; Mahan, M.G.; Ehrman, J.K.; Vanzant, M.A.; Schairer, J.R.; Keteyian, S.J. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: A randomized, single-blind, controlled trial. Circulation 2013, 127, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.; Watt-Watson, J.; Hodnett, E.; Tranmer, J.; Dennis, C.L.; Brooks, D. Cardiac Home Education and Support Trial (CHEST): A pilot study. Can. J. Cardiol. 2009, 25, e393–e398. [Google Scholar] [CrossRef]
- Price, J. A Pilot Trial of a Coaching Intervention Designed to Increase Women’s Attendance at Cardiac Rehabilitation Intake. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2012. [Google Scholar]
- Wyer, S.J.; Earll, L.; Joseph, S.; Harrison, J.; Giles, M.; Johnston, M. Increasing attendance at a cardiac rehabilitation programme: An intervention study using the Theory of Planned Behaviour. Coron. Heal. Care 2001, 5, 154–159. [Google Scholar] [CrossRef]
- Carroll, D.L.; Rankin, S.H.; Cooper, B.A. The Effects of a Collaborative Peer Advisor/Advanced Practice Nurse Intervention. J. Cardiovasc. Nurs. 2007, 22, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Santiago de Araujo Pio, C.; Chaves, G.S.S.; Davies, P.; Taylor, R.S.; Grace, S.L. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst. Rev. 2019, CD007131, 1–145. [Google Scholar] [CrossRef]
- Ali-Faisal, S.F.; Scott, L.B.; Johnston, L.; Grace, S.L. Cardiac rehabilitation referral and enrolment across an academic health sciences centre with eReferral and peer navigation: A randomised controlled pilot trial. BMJ Open 2016, 6, e010214. [Google Scholar] [CrossRef]
- Bertelsen, J.B.; Refsgaard, J.; Kanstrup, H.; Johnsen, S.P.; Qvist, I.; Christensen, B.; Christensen, K.L. Cardiac rehabilitation after acute coronary syndrome comparing adherence and risk factor modification in a community-based shared care model versus hospital-based care in a randomised controlled trial with 12 months of follow-up. Eur. J. Cardiovasc. Nurs. 2017, 16, 334–343. [Google Scholar] [CrossRef]
- Focht, B.C.; Brawley, L.R.; Rejeski, W.J.; Ambrosius, W.T. Group-mediated activity counseling and traditional exercise therapy programs: Effects on health-related quality of life among older adults in cardiac rehabilitation. Ann. Behav. Med. 2004, 28, 52–61. [Google Scholar] [CrossRef]
- Farias-Godoy, A. Design, Implementation and Evaluation of a Reduced Cardiac Rehabilitation Program. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2013. [Google Scholar]
- Grace, S.L.; Midence, L.; Oh, P.; Brister, S.; Chessex, C.; Stewart, D.E.; Arthur, H.M. Cardiac Rehabilitation Program Adherence and Functional Capacity among Women: A Randomized Controlled Trial. Mayo Clin. Proc. 2016, 91, 140–148. [Google Scholar] [CrossRef]
- Hwang, R.; Bruning, J.; Morris, N.R.; Mandrusiak, A.; Russell, T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: A randomised trial. J. Physiother. 2017, 63, 101–107. [Google Scholar] [CrossRef]
- Kraal, J.J.; Peek, N.; Van den Akker-Van Marle, M.E.; Kemps, H.M. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: Short-term results of the FIT@Home study. Eur. J. Prev. Cardiol. 2014, 21, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lynggaard, V.; Nielsen, C.V.; Zwisler, A.D.; Taylor, R.S.; May, O. The patient education—Learning and Coping Strategies—Improves adherence in cardiac rehabilitation (LC-REHAB): A randomised controlled trial. Int. J. Cardiol. 2017, 236, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, S.M.; Bond, C.M.; Lee, A.J.; Kiger, A.; Campbell, N.C. Effectiveness of theory-based invitations to improve attendance at cardiac rehabilitation: A randomized controlled trial. Eur. J. Cardiovasc. Nurs. 2014, 13, 201–210. [Google Scholar] [CrossRef]
- Pfaeffli Dale, L.; Whittaker, R.; Jiang, Y.; Stewart, R.; Rolleston, A.; Maddison, R. Text Message and Internet Support for Coronary Heart Disease Self-Management: Results From the Text4Heart Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e237. [Google Scholar] [CrossRef] [PubMed]
- Benz-Scott, L.; Gravely, S.; Sexton, T.R.; Brzostek, S.; Brown, D.L. Examining the effect of a patient navigation intervention on outpatient cardiac rehabilitation awareness and enrollment. J. Cardiopulm. Rehabil. Prev. 2013, 33, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Suskin, N.; Irvine, J.; Arnold, J.M.O.; Turner, R.; Zandri, J.; Prior, P.; Unsworth, K. Improving cardiac rehabilitation (CR) participation in women and men, the CR participation study. J. Cardiopulm. Rehabil. Prev. 2007, 27, 342. [Google Scholar] [CrossRef]
- Varnfield, M.; Karunanithi, M.; Lee, C.-K.; Honeyman, E.; Arnold, D.; Ding, H.; Smith, C.; Walters, D.L. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: Results from a randomised controlled trial. Heart 2014, 100, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- McGrady, A.; McGinnis, R.; Badenhop, D.; Bentle, M.; Rajput, M. Effects of depression and anxiety on adherence to cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev. 2009, 29, 358–364. [Google Scholar] [CrossRef]
- McGrady, A.; Burkes, R.; Badenhop, D.; McGinnis, R. Effects of a brief intervention on retention of patients in a cardiac rehabilitation program. Appl. Psychophysiol. Biofeedback 2014, 39, 163–170. [Google Scholar] [CrossRef]
- Rejeski, W.J.; Brawley, L.R.; Ambrosius, W.T.; Brubaker, P.H.; Focht, B.C.; Foy, C.G.; Fox, L.D. Older adults with chronic disease: Benefits of group-mediated counseling in the promotion of physically active lifestyles. Heal. Psychol. 2003, 22, 414–423. [Google Scholar] [CrossRef]
- Buckingham, S.A.; Taylor, R.S.; Jolly, K.; Zawada, A.; Dean, S.G.; Cowie, A.; Norton, R.J.; Dalal, H.M. Home-based versus centre-based cardiac rehabilitation: Abridged Cochrane systematic review and meta-analysis. Open Hear. 2016, 3, e000463. [Google Scholar] [CrossRef] [PubMed]
- Beckie, T.M.; Beckstead, J.W. The effects of a cardiac rehabilitation program tailored for women on global quality of life: A randomized clinical trial. J. Womens. Health 2010, 19, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Sharp, G.A.; Norton, R.J.; Dalal, H.; Dean, S.G.; Jolly, K.; Cowie, A.; Zawada, A.; Taylor, R.S. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst. Rev. 2017, 6, CD007130. [Google Scholar] [CrossRef] [PubMed]
- Lima de Melo Ghisi, G.; Pesah, E.; Turk-Adawi, K.; Supervia, M.; Lopez Jimenez, F.; Grace, S.; Lima de Melo Ghisi, G.; Pesah, E.; Turk-Adawi, K.; Supervia, M.; et al. Cardiac Rehabilitation Models around the Globe. J. Clin. Med. 2018, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Pourhabib, S.; Kentner, A.C.; Grace, S.L. The impact of patient-healthcare provider discussions on enrollment in cardiovascular rehabilitation. J. Rehabil. Med. 2014, 46, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Sherry Grace—York University. Available online: http://www. http://sgrace.info.yorku.ca/ (accessed on 1 February 2019).
- Cardiac Rehabilitation Change Package. Available online: https://millionhearts.hhs.gov/tools-protocols/action-guides/cardiac-change-package/index.html (accessed on 15 December 2018).
- Pirruccello, J.P.; Traynor, K.C.; Natarajan, P.; Brown, C.; Hidrue, M.K.; Rosenfield, K.A.; Kathiresan, S.; Wasfy, J.H. An electronic cardiac rehabilitation referral system increases cardiac rehabilitation referrals. Coron. Artery Dis. 2017, 28, 342–345. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Sex-Specific Reporting of Scientific Research: A Workshop Summary; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Supervía, M.; Medina-Inojosa, J.R.; Yeung, C.; Lopez-Jimenez, F.; Squires, R.W.; Pérez-Terzic, C.M.; Brewer, L.C.; Leth, S.E.; Thomas, R.J. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clin. Proc. 2017, 92, 565–577. [Google Scholar] [CrossRef]
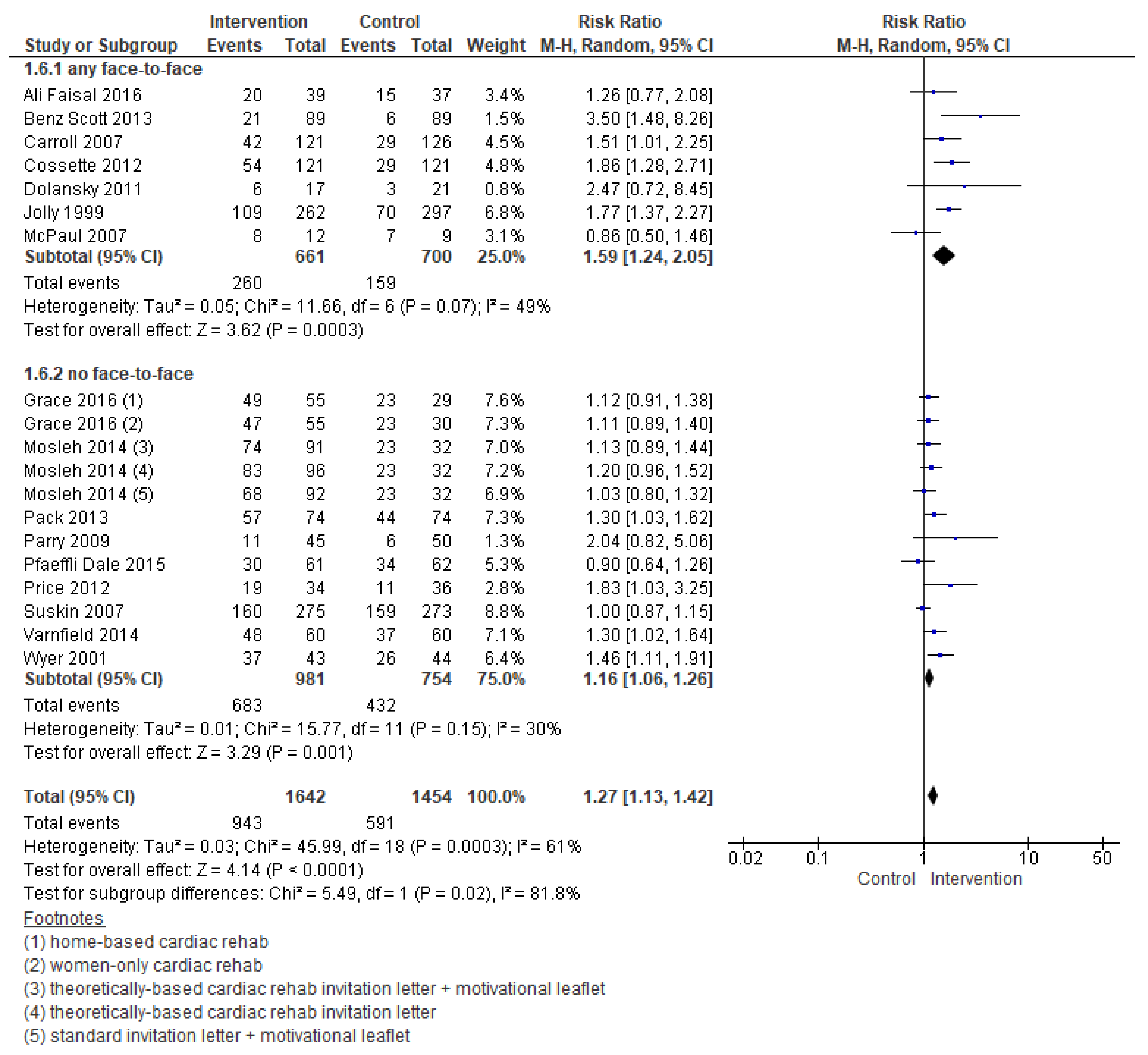

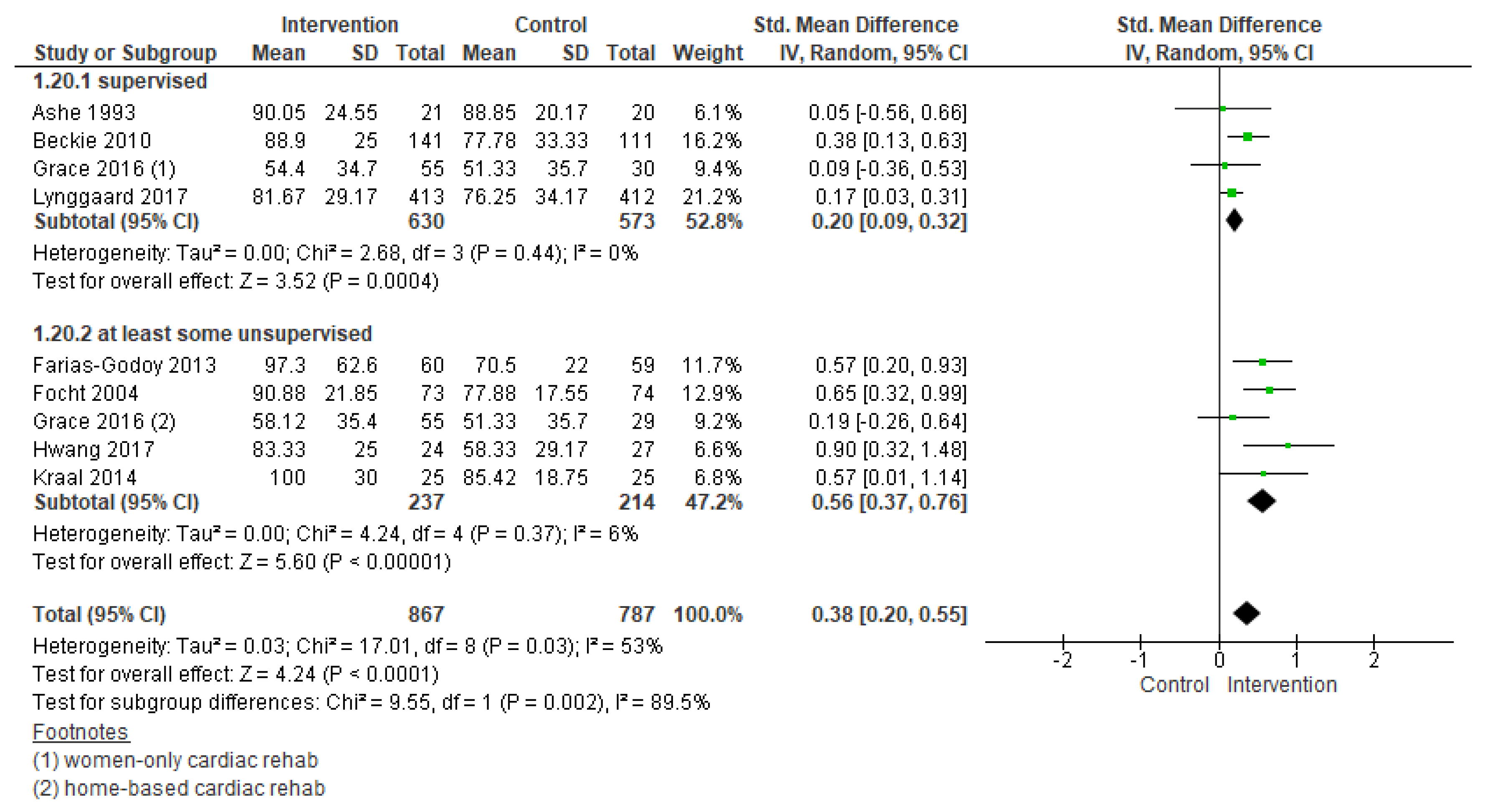
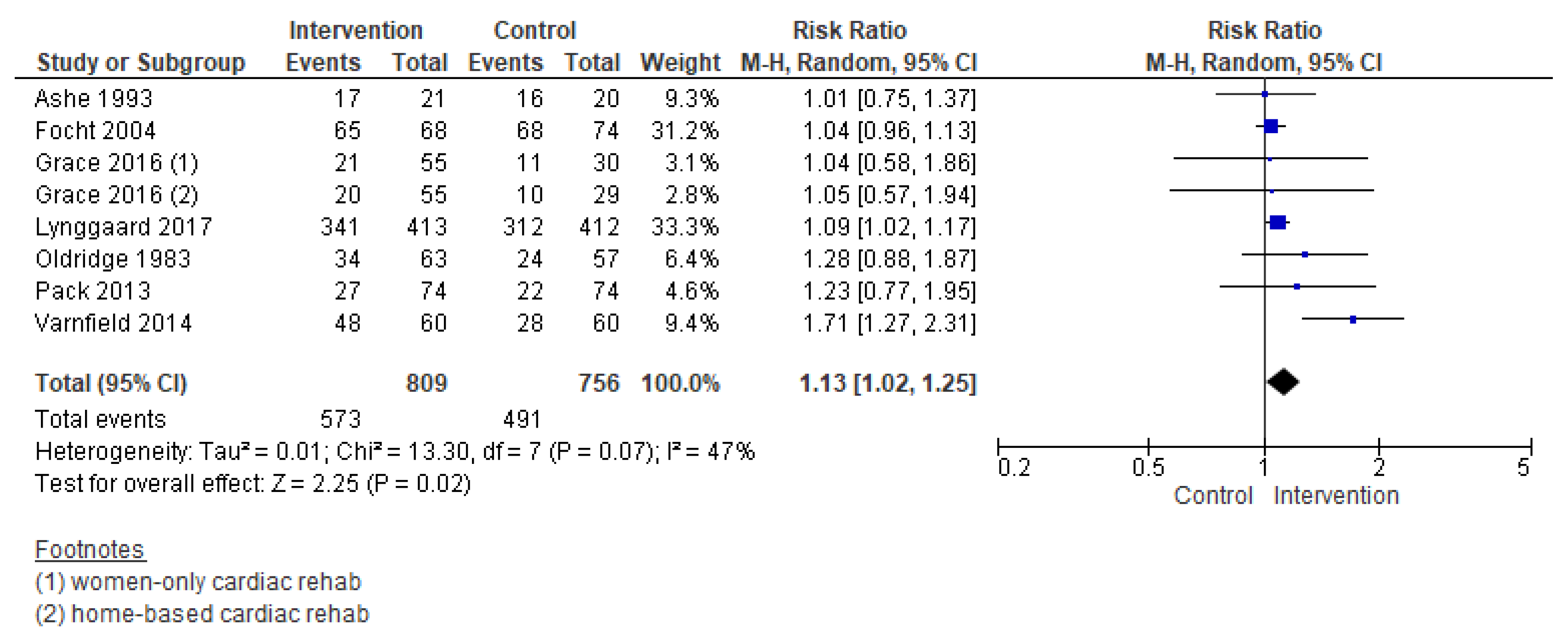

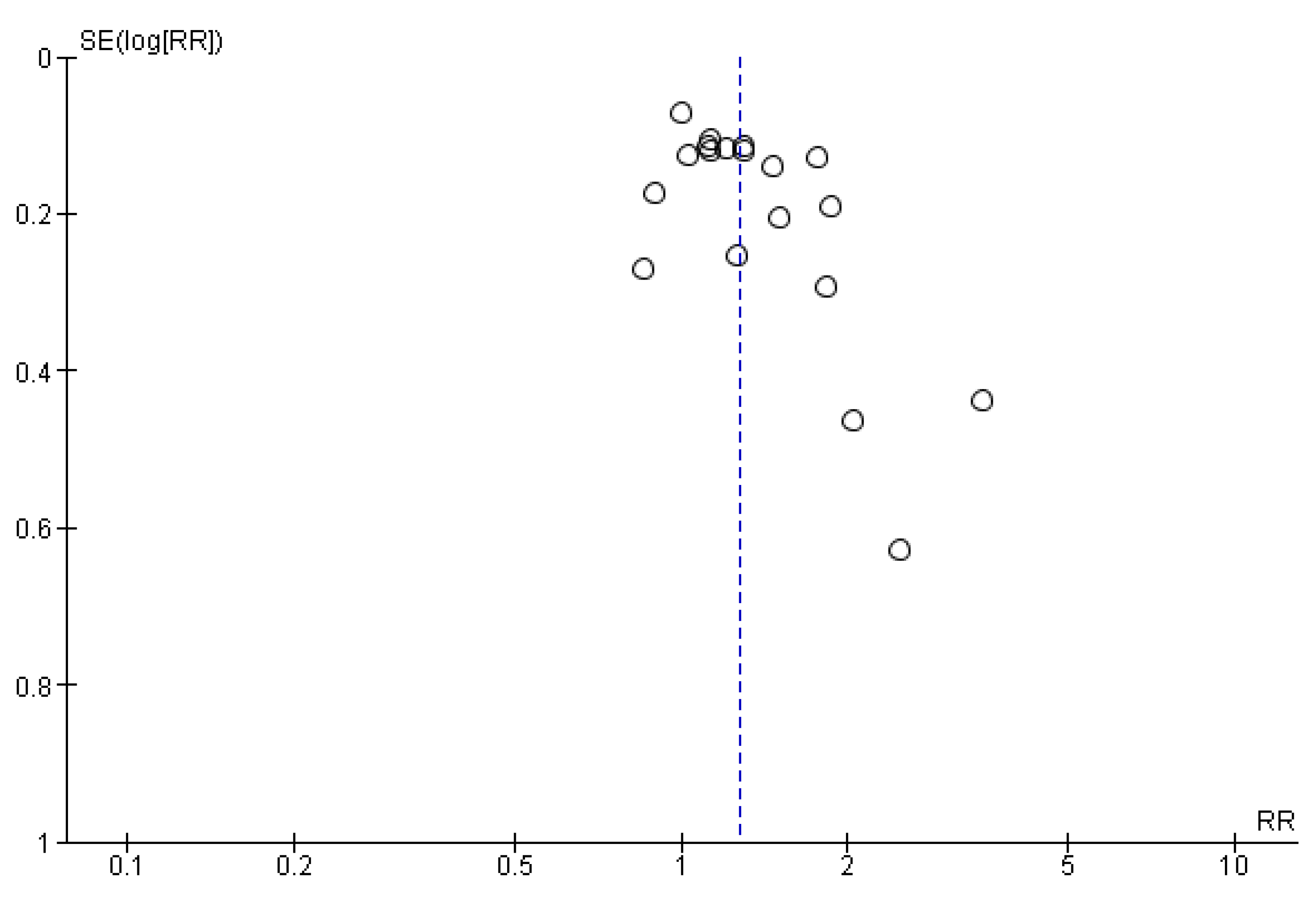
| Outcomes | № of Participants (Studies) Follow Up | Certainty of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects * (95% CI) | |
|---|---|---|---|---|---|
| Risk with No Interventions to Promote Utilization of CR | Risk Difference WITH Interventions to Promote Utilization of CR | ||||
| Enrolment | 3096 (19 RCTs) – 11 weeks | ⊕⊕⊝⊝LOW 1,2 | RR 1.27 (1.13 to 1.42) | Study population | |
| 406 per 1000 | 110 more per 1000 (53 more to 171 more) | ||||
| Adherence | 1654 (9 RCTs) – 18 weeks | ⊕⊕⊝⊝LOW 1,2 | - | SMD 0.38 SD higher (0.20 higher to 0.55 higher) | |
| Completion | 1565 (8 RCTs) – 24 weeks | ⊕⊕⊕⊝MODERATE 2 | RR 1.13 (1.02 to 1.25) | Study population | |
| 649 per 1000 | 84 more per 1000 (13 more to 162 more) | ||||
| Subgroup | n | Odds Ratio (95% CI) | p | Residual I2 * |
|---|---|---|---|---|
| Delivery format (any face-to-face or no face-to-face) | 3096 | 0.73 (0.57 to 0.93) | 0.01 | 37% |
| Theory-based (yes or no) | 3096 | 0.98 (0.75 to 1.27) | 0.86 | 60% |
| Outcome ascertainment (self-report or chart-report) | 1835 | 0.99 (0.99 to 1.00) | 0.74 | 53% |
| Number of sites (multi-site or single-centre) | 943 | 0.90 (0.69 to 1.17) | 0.40 | 60% |
| Region (North America or other) | 3096 | 0.91 (0.70 to 1.17) | 0.44 | 60% |
| Intervention Intensity (<5 contacts or ≥5 contacts) | 2659 | 0.99 (0.99 to 1.00) | 0.23 | 66% |
| Peer navigation (yes or no) | 3096 | 0.74 (0.50 to 1.10) | 0.13 | 55% |
| Intervention deliverer (nurse or allied health care professional or no one) | 3096 | 0.73 (0.56 to 0.94) | 0.02 | 37% |
| Intervention target (patient or other) | 3096 | 1.49 (0.98 to 2.28) | 0.06 | 46% |
| Cardiac indication (heart failure included or not) | 2196 | 0.83 (0.63 to 1.10) | 0.19 | 55% |
| CR setting (supervised or unsupervised) | 1650 | 1.03 (0.84 to 1.25) | 0.76 | 15% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago de Araújo Pio, C.; Chaves, G.; Davies, P.; Taylor, R.; Grace, S. Interventions to Promote Patient Utilization of Cardiac Rehabilitation: Cochrane Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 189. https://doi.org/10.3390/jcm8020189
Santiago de Araújo Pio C, Chaves G, Davies P, Taylor R, Grace S. Interventions to Promote Patient Utilization of Cardiac Rehabilitation: Cochrane Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(2):189. https://doi.org/10.3390/jcm8020189
Chicago/Turabian StyleSantiago de Araújo Pio, Carolina, Gabriela Chaves, Philippa Davies, Rod Taylor, and Sherry Grace. 2019. "Interventions to Promote Patient Utilization of Cardiac Rehabilitation: Cochrane Systematic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 2: 189. https://doi.org/10.3390/jcm8020189
APA StyleSantiago de Araújo Pio, C., Chaves, G., Davies, P., Taylor, R., & Grace, S. (2019). Interventions to Promote Patient Utilization of Cardiac Rehabilitation: Cochrane Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 8(2), 189. https://doi.org/10.3390/jcm8020189






