Comparison of Postoperative Opioid Consumption and Pain Scores in Primary Versus Repeat Cesarean Delivery in Opioid Naïve Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
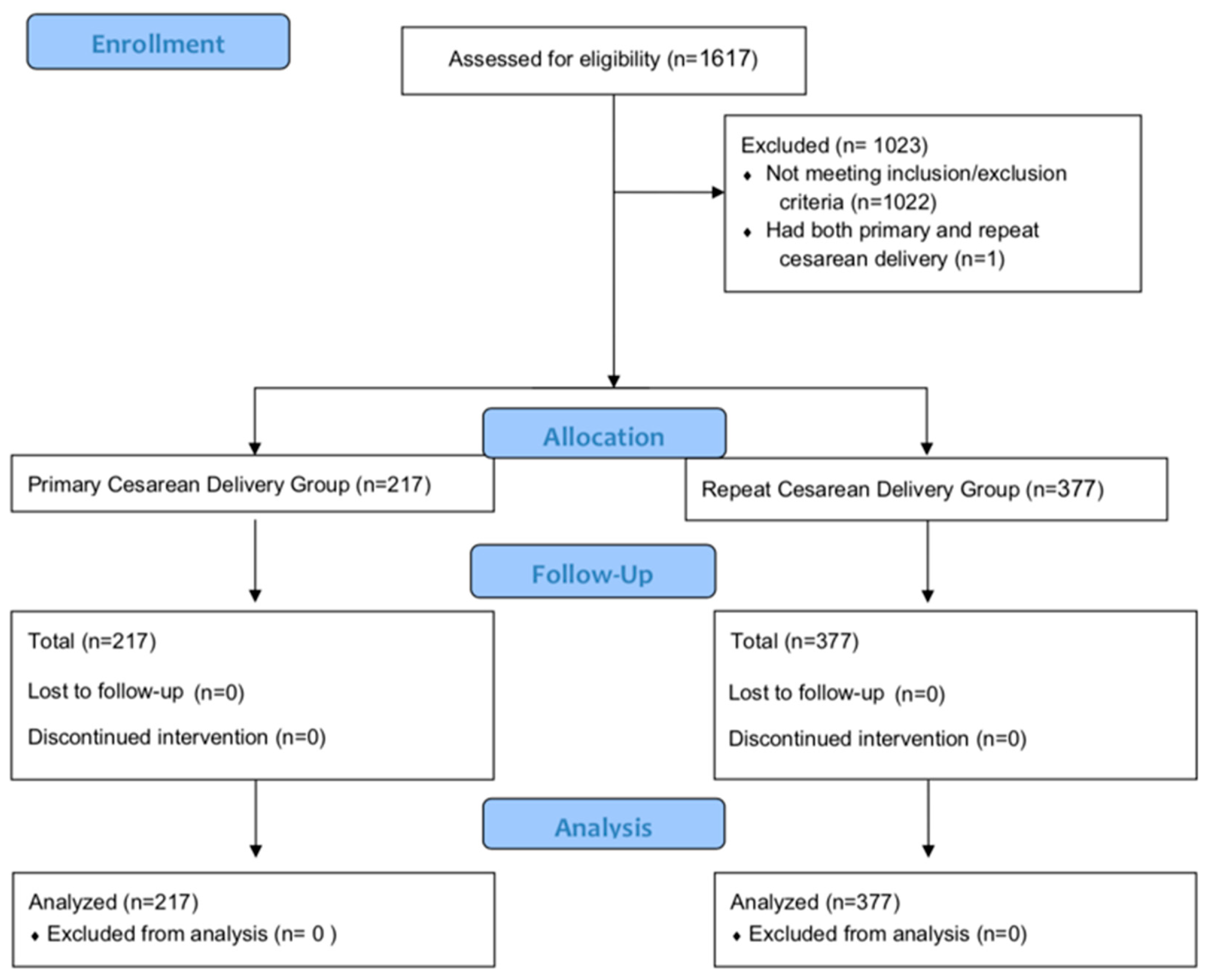
2.2. Participants
2.3. Variables
2.4. Data Sources
2.5. Bias
2.6. Study Size
2.7. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K.; Drake, P. Births: Final data for 2016. Natl. Vital. Stat. Rep. 2018, 67, 1–50. [Google Scholar] [PubMed]
- Mylonas, I.; Friese, K. Indications for and risks of elective cesarean section. Dtsch. Arztebl. Int. 2015, 112, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlstrom, A.; Engstrom-Olofsson, R.; Norbergh, K.G.; Sjoling, M.; Hildingsson, I. Postoperative pain after cesarean birth affects breastfeeding and infant care. J. Obste.t Gynecol. Neonatal Nurs. 2007, 36, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, J.C.; Pan, P.H.; Smiley, R.; Lavand’homme, P.; Landau, R.; Houle, T.T. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain 2008, 140, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, B.; Georgsson Ohman, S.; Segerdahl, M.; Blanck, A. Risk factors for persistent pain and its influence on maternal wellbeing after cesarean section. Acta Obstet. Gynecol. Scand. 2015, 94, 622–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 742: Postpartum pain management. Obstet. Gynecol. 2018, 132, e35–e43. [Google Scholar] [CrossRef] [PubMed]
- Osmundson, S.S.; Schornack, L.A.; Grasch, J.L.; Zuckerwise, L.C.; Young, J.L.; Richardson, M.G. Postdischarge opioid use after cesarean delivery. Obstet. Gynecol. 2017, 130, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gamez, B.H.; Habib, A.S. Predicting severity of acute pain after cesarean delivery: A narrative review. Anesth. Analg. 2018, 126, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Wanderer, J.P.; Nathan, N. A pain in the Abs: Predicting post-cesarean analgesia. Anesth. Analg. 2018, 126, 1432. [Google Scholar] [CrossRef] [PubMed]
- Ortner, C.M.; Granot, M.; Richebe, P.; Cardoso, M.; Bollag, L.; Landau, R. Preoperative scar hyperalgesia is associated with post-operative pain in women undergoing a repeat Caesarean delivery. Eur. J. Pain 2013, 17, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathes, E.F.; Frieden, I.J.; Cho, C.S.; Boscardin, C.K. Randomized controlled trial of spaced education for pediatric residency education. J. Grad. Med. Educ. 2014, 6, 270–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opioid Calculator. Practical Pain Management. Available online: https://opioidcalculator.practicalpainmanagement.com (accessed on 14 December 2019).
- Peng, K.; Liu, H.Y.; Wu, S.R.; Cheng, H.; Ji, F.H. Effects of combining dexmedetomidine and opioids for postoperative intravenous patient-controlled analgesia: A systematic review and meta-analysis. Clin. J. Pain 2015, 31, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.H.; Sulaiman, S.; Khan, A.H.; Rajah, P.S. Factors affecting post caesarean pain intensity among women in the northern peninsular of malaysia. J. Clin. Diagn. Res. 2017, 11, IC07–IC11. [Google Scholar] [CrossRef] [PubMed]
- Bateman, B.T.; Cole, N.M.; Maeda, A.; Burns, S.M.; Houle, T.T.; Huybrechts, K.F.; Clancy, C.R.; Hopp, S.B.; Ecker, J.L.; Ende, H.; et al. Patterns of opioid prescription and use after cesarean delivery. Obstet. Gynecol. 2017, 130, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Poleshuck, E.L.; Andrus, C.H.; Hogan, L.A.; Jung, B.F.; Kulick, D.I.; Dworkin, R.H. Risk factors for acute pain and its persistence following breast cancer surgery. Pain 2005, 119, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.R.; McIntyre, T.; Ferrero, R.; Almeida, A.; Araujo-Soares, V. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. J. Pain 2013, 14, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.H.; Tonidandel, A.M.; Aschenbrenner, C.A.; Houle, T.T.; Harris, L.C.; Eisenach, J.C. Predicting acute pain after cesarean delivery using three simple questions. Anesthesiology 2013, 118, 1170–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelewijn, J.M.; Sluijs, A.M.; Vrijkotte, T.G.M. Possible relationship between general and pregnancy-related anxiety during the first half of pregnancy and the birth process: A prospective cohort study. BMJ Open 2017, 7, e013413. [Google Scholar] [CrossRef] [PubMed]


| Characteristics Baseline | Measurements | Primary | Repeat | 95% CI Estimate (Upper, Lower) | p Value |
|---|---|---|---|---|---|
| N | Count (Percentage) | 217 (36.5%) | 377 (63.5%) | ||
| Age (years) | Median (IQR) | 30 (7) | 31 (7) | −1.99 (−3.00, −2.00) | 0.00432 |
| ASA | Median (IQR) | 2 (1) | 2 (1) | 0 (0, 0) | 0.7283 |
| CD (#) | Median (IQR) | 1 (0) | 2 (1) | ||
| CD (#) | |||||
| 1 2 3 4 5 8 | Count (Percentage) | 217 (100%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) | 0 (0%) 223 (59%) 113 (30%) 25 (6.7%) 15 (4%) 1 (0.3%) | ||
| Received Prescription for Opioids at Discharge | Count (Percentage) | 178 (82%) | 286 (76%) |
| Characteristics Baseline | Measurments | Primary | Repeat | 95% CI Estimate (Upper, Lower) | p Value |
|---|---|---|---|---|---|
| Opioids in Morphine Equivalents(mg) | |||||
| 0–24 h 24–48 h 48–72 h Total Equivalency(mg) | Median (IQR) | 8 (20) 30 (40) 20 (40) 58 (74) | 0 (15) 19 (40) 0 (30) 35 (80) | 2× 10−5 (−3× 10−5, 3× 10−5) 4 (8× 10-6, 10) 4 (4× 10−5, 10) 15 (6× 10−5, 30) | 0.0704 0.0005 0.0005 0.0005 |
| Non-Opioids-PO Acetaminophen(mg) | |||||
| 0–24 h 24–48 h 48–72 h | Median (IQR) | 1300 (650) 650 (650) 650 (975) | 1300 (650) 650 (650) 1300 (1300) | −5× 10−5 (−3× 10−5, 1× 10−5) −5× 10-7 (−4× 10-6, 6× 10−5) -- | 0.9778 0.7659 0.8625 |
| Non-Opioids-IV Acetaminophen (mg) | |||||
| 0–24 h 24–48 h 48–72 h | Median (IQR) | 1000 (0) -- -- | 1000 (0) -- -- | −8× 10−5 (−8× 10-6, 5× 10−5) -- -- | 0.9778 -- -- |
| Non-Opioids-PO Ibuprofen (mg) | |||||
| 0–24 h 24–48 h 48–72 h | Median (IQR) | 600 (0) 1800 (600) 1800 (600) | 600 (600) 1800 (600) 1800 (1200) | −6× 10-6 (−3× 10−5, 6× 10−5) −5× 10−5 (−3× 10-6, 2× 10−5) 3e× 10−5 (−2× 10−5, 3× 10-6) | 0.9778 0.9928 0.2682 |
| Non-Opioids-IV Toradol (mg) | |||||
| 0–24 h 24–48 h 48–72 h | Median (IQR) | 90 (30) 30 (45) -- | 90 (60) 30 (7.5) -- | 5× 10−5 (−6× 10-6, 3× 10−5) 3× 10−5 (−7× 10−5, 2× 10−5) -- | 0.3748 0.3748 -- |
| Pain Score | |||||
| 0–24 h 24–48 h 48–72 h | Median (IQR) | 1.8 (1.7) 3.0 (2.3) 2.7 (2.0) | 1.8 (1.7) 3.0 (1.8) 2.9 (1.8) | −0.165 (−0.469, 0.154) 0.0226 (−0.467, 0.529) −8e^−5 (−0.419, 0.403) | 0.9778 0.7288 0.7659 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, A.; Pasca, I.; Alschuler, M.; Lee, J.; Woodfin, M.; Pugh, J.; Austin, B.; Ringer, M.; Ramsingh, D. Comparison of Postoperative Opioid Consumption and Pain Scores in Primary Versus Repeat Cesarean Delivery in Opioid Naïve Patients. J. Clin. Med. 2019, 8, 2221. https://doi.org/10.3390/jcm8122221
Chao A, Pasca I, Alschuler M, Lee J, Woodfin M, Pugh J, Austin B, Ringer M, Ramsingh D. Comparison of Postoperative Opioid Consumption and Pain Scores in Primary Versus Repeat Cesarean Delivery in Opioid Naïve Patients. Journal of Clinical Medicine. 2019; 8(12):2221. https://doi.org/10.3390/jcm8122221
Chicago/Turabian StyleChao, Amanda, Ioana Pasca, Matthew Alschuler, Jay Lee, Michelle Woodfin, Justin Pugh, Briahnna Austin, Mark Ringer, and Davinder Ramsingh. 2019. "Comparison of Postoperative Opioid Consumption and Pain Scores in Primary Versus Repeat Cesarean Delivery in Opioid Naïve Patients" Journal of Clinical Medicine 8, no. 12: 2221. https://doi.org/10.3390/jcm8122221
APA StyleChao, A., Pasca, I., Alschuler, M., Lee, J., Woodfin, M., Pugh, J., Austin, B., Ringer, M., & Ramsingh, D. (2019). Comparison of Postoperative Opioid Consumption and Pain Scores in Primary Versus Repeat Cesarean Delivery in Opioid Naïve Patients. Journal of Clinical Medicine, 8(12), 2221. https://doi.org/10.3390/jcm8122221





