Renal Tubular TRPA1 as a Risk Factor for Recovery of Renal Function from Acute Tubular Necrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Data
2.3. Immunohistochemistry (IHC)
2.4. Histopathology
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patients
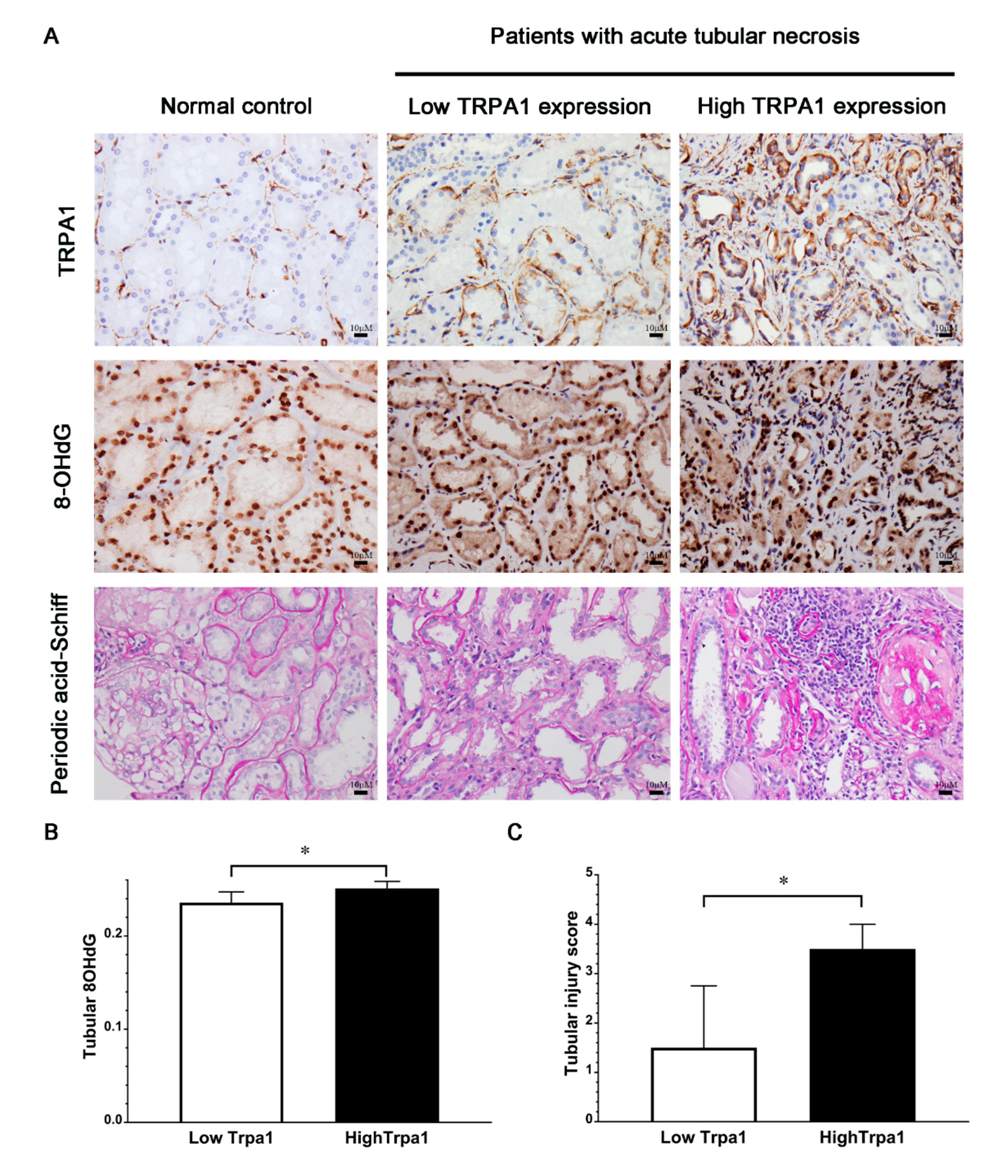
3.2. Association of Tubular Expression of TRPA1 with Expression of 8-OHdG or Tubular Injury Score Among Patients with ATN and Normal Subjects
3.3. Association of Tubular TRPA1 Expression with Complete Recovery of Renal Function
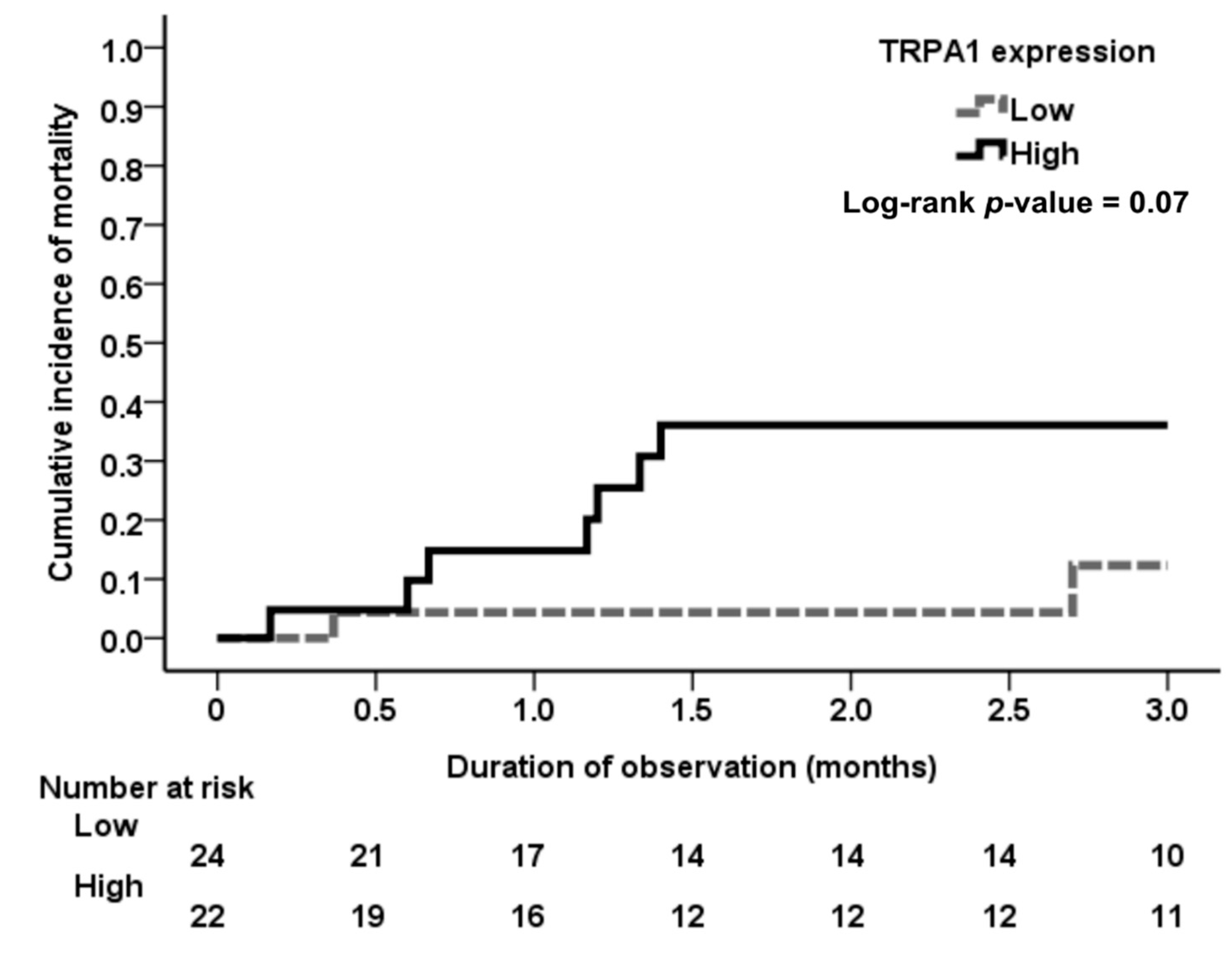
3.4. Association of Tubular TRPA1 Expression with Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gameiro, J.; Agapito Fonseca, J.; Jorge, S.; Lopes, J.A. Acute kidney injury definition and diagnosis: A narrative review. J. Clin. Med. 2018, 7, E307. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Wald, R. Strategies for the optimal timing to start renal replacement therapy in critically ill patients with acute kidney injury. Kidney Int. 2017, 91, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, S.; Fraser, S.D. Epidemiology of aki: Utilizing large databases to determine the burden of aki. Adv. Chronic Kidney Dis. 2017, 24, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, D.; Sud, K. Acute kidney injury and disease: Long-term consequences and management. Nephrology (Carlton) 2018, 23, 969–980. [Google Scholar] [CrossRef]
- Shiao, C.C.; Wu, P.C.; Wu, V.C.; Lin, J.H.; Pan, H.C.; Yang, Y.F.; Lai, T.S.; Huang, T.M.; Wu, C.H.; Yang, W.S.; et al. Nationwide epidemiology and prognosis of dialysis-requiring acute kidney injury (nep-aki-d) study: Design and methods. Nephrology (Carlton) 2016, 21, 758–764. [Google Scholar] [CrossRef]
- Negi, S.; Koreeda, D.; Kobayashi, S.; Yano, T.; Tatsuta, K.; Mima, T.; Shigematsu, T.; Ohya, M. Acute kidney injury: Epidemiology, outcomes, complications, and therapeutic strategies. Semin. Dial. 2018, 31, 519–527. [Google Scholar] [CrossRef]
- Hanif, M.O.; Ramphul, K. Acute renal tubular necrosis. In Statpearls; StatePearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative stress and acute kidney injury in critical illness: Pathophysiologic mechanisms-biomarkers-interventions, and future perspectives. Oxid. Med. Cell. Longev. 2017, 2017, 6193694. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Gorin, Y. The kidney: An organ in the front line of oxidative stress-associated pathologies. Antioxid. Redox Signal. 2016, 25, 639–641. [Google Scholar] [CrossRef]
- de Caestecker, M.; Harris, R. Translating knowledge into therapy for acute kidney injury. Semin. Nephrol. 2018, 38, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Viana, F. Trpa1 channels: Molecular sentinels of cellular stress and tissue damage. J. Physiol. 2016, 594, 4151–4169. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. Trpa1: A gatekeeper for inflammation. Ann. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Dembla, S.; Hasan, N.; Becker, A.; Beck, A.; Philipp, S.E. Transient receptor potential a1 channels regulate epithelial cell barriers formed by mdck cells. FEBS Lett. 2016, 590, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Yamasaki, Y.; Sasaki-Yamaguchi, Y.; Ida-Koga, N.; Kamisuki, S.; Sugawara, F.; Nagumo, Y.; Usui, T. Trpa1-dependent reversible opening of tight junction by natural compounds with an alpha,beta-unsaturated moiety and capsaicin. Sci. Rep. 2018, 8, 2251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, S.; Geng, Y.; Song, Y. Transient receptor potential ankyrin 1 protects against sepsis-induced kidney injury by modulating mitochondrial biogenesis and mitophagy. Am. J. Transl. Res. 2018, 10, 4163–4172. [Google Scholar]
- Ma, S.; Zhang, Y.; He, K.; Wang, P.; Wang, D.H. Knockout of trpa1 exacerbates angiotensin ii-induced kidney injury. Am. J. Physiol. Renal. Physiol. 2019, 317, F623–F631. [Google Scholar] [CrossRef]
- Tseng, W.C.; Yang, W.C.; Yang, A.H.; Hsieh, S.L.; Tarng, D.C. Expression of tnfrsf6b in kidneys is a novel predictor for progression of chronic kidney disease. Mod. Pathol. 2013, 26, 984–994. [Google Scholar] [CrossRef][Green Version]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel trpa1: From gene to pathophysiology. Pflugers Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef]
- Yokozawa, T.; Fujioka, K.; Oura, H. Increase in kidney 8-hydroxydeoxyguanosine level with the progression of renal failure. Nephron 1992, 61, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Kasuno, K.; Shirakawa, K.; Yoshida, H.; Mori, K.; Kimura, H.; Takahashi, N.; Nobukawa, Y.; Shigemi, K.; Tanabe, S.; Yamada, N.; et al. Renal redox dysregulation in aki: Application for oxidative stress marker of aki. Am. J. Physiol. Renal. Physiol. 2014, 307, F1342–F1351. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Hosokawa, H.; Matsumura, K.; Kobayashi, S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur. J. Neurosci. 2008, 27, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Batista-Schepman, P.A.; Barabas, M.E.; Harris, E.Q.; Dinsmore, T.B.; Kossyreva, E.A.; Foshage, A.M.; Wang, M.H.; Schwab, M.J.; Wang, V.M.; et al. Prostaglandin metabolite induces inhibition of trpa1 and channel-dependent nociception. Mol. Pain 2012, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of trpa1 and trpv1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. Trpa1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory trp channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Lin, A.H.; Liu, M.H.; Ko, H.K.; Perng, D.W.; Lee, T.S.; Kou, Y.R. Lung epithelial trpa1 transduces the extracellular ros into transcriptional regulation of lung inflammation induced by cigarette smoke: The role of influxed ca(2)(+). Mediators Inflamm. 2015, 2015, 148367. [Google Scholar] [CrossRef]
- Kun, J.; Szitter, I.; Kemeny, A.; Perkecz, A.; Kereskai, L.; Pohoczky, K.; Vincze, A.; Godi, S.; Szabo, I.; Szolcsanyi, J.; et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS ONE 2014, 9, e108164. [Google Scholar] [CrossRef]
- Lowin, T.; Apitz, M.; Anders, S.; Straub, R.H. Anti-inflammatory effects of n-acylethanolamines in rheumatoid arthritis synovial cells are mediated by trpv1 and trpa1 in a cox-2 dependent manner. Arthritis Res. Ther. 2015, 17, 321. [Google Scholar] [CrossRef]
- An, J.N.; Hwang, J.H.; Kim, D.K.; Lee, H.; Ahn, S.Y.; Kim, S.; Park, J.T.; Kang, S.W.; Oh, Y.K.; Kim, Y.S.; et al. Chronic kidney disease after acute kidney injury requiring continuous renal replacement therapy and its impact on long-term outcomes: A multicenter retrospective cohort study in korea. Crit. Care Med. 2017, 45, 47–57. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total Recovery (n = 12) | Nonrecovery or Death a (n = 34) | pb |
|---|---|---|---|
| Demographics | |||
| Age (years) | 46.2 ± 21.7 | 56.8 ± 17.8 | 0.15 b |
| Male (n (%)) | 8 (66.7%) | 21 (61.8%) | 0.76 c |
| Diabetes mellitus (n (%)) | 1 (8.3%) | 13 (38.2%) | 0.05 d |
| Hypertension (n (%)) | 2 (16.7%) | 10 (29.4%) | 0.33 d |
| Heart failure (n (%)) | 0 (0%) | 3 (8.8%) | 0.39 d |
| Severity of AKI | 3 (25%) | 8 (23.5%) | 0.60 d |
| AKIN stage I (n (%)) | 9 (75%) | 26 (76.5%) | |
| AKIN stage II or III (n (%)) | 46.2 ± 21.7 | 56.8 ± 17.8 | |
| Laboratory data | |||
| Baseline serum creatinine (mg/dL) | 1.0 (0.8–1.2) | 1.5 (0.9–2.7) | 0.03 b |
| Baseline eGFR (CKD-EPI) (mL/min/1.73m2) | 88.7 (64.7–113.5) | 47.7 (20.7–87.5) | 0.004 b |
| Urinary PCR (mg/g) | 96.4 (30.0–976.0) | 661.5 (100.0–5432.0) | 0.05 b |
| Hemoglobin (g/dL) | 11.7 (9.1–13.4) | 9.6 (8.7–10.8) | 0.03 b |
| Serum albumin (g/dL) | 2.6 (1.8–3.2) | 2.8 (2.2–3.3) | 0.57 b |
| Serum cholesterol (mg/dL) | 131 (119.0–260) | 193 (157–252) | 0.33 b |
| Serum triglyceride (mg/dL) | 197.4 ± 132.6 | 165.6 ± 94.6 | 0.53 b |
| Serum uric acid (mg/dL) | 8.6 (7.7–13.4) | 8.4 (6.6–9.6) | 0.44 b |
| Serum sodium (mmol/L) | 137 (133.5–140.0) | 133.5 (131–140) | 0.51 b |
| Serum potassium (mmol/L) | 4.4 (3.5–4.9) | 3.9 (3.4–4.1) | 0.15 b |
| Histopathology | |||
| Tubular injury score | 2 (1–3) | 2 (1–4) | 0.18 b |
| Tubular atrophy (%) | 0 (0–1.5) | 6 (3–10) | <0.001 b |
| Interstitial inflammation score | 1 (0–1) | 1 (1–1) | 0.06 b |
| Interstitial fibrosis (%) | 7.0 ± 4.9 | 10.4 ± 8.4 | 0.37 b |
| Medications | |||
| ACEI or ARB (n (%)) | 2 (16.7%) | 7 (20.6%) | 0.57 d |
| Immunosuppressants (n (%)) | 2 (16.7%) | 13 (38.2%) | 0.16 d |
| Variables | Univariable | Model 1 (Adjusted for Age and Sex) | ||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| High tubular TRPA1 expression | 7.14 (1.35–37.75) | 0.02 | 6.86 (1.26–37.27) | 0.03 |
| Hypertension | 2.08 (0.39–11.27) | 0.39 | 1.84 (0.33–10.28) | 0.49 |
| Diabetes mellitus | 6.81 (0.78–59.10) | 0.08 | 5.34 (0.58–49.25) | 0.14 |
| Tubular atrophy (%) | 1.96 (1.16–3.32) | 0.01 | 2.01 (1.14–3.55) | 0.02 |
| Interstitial fibrosis (%) | 1.08 (0.96–1.21) | 0.19 | 1.06 (0.95–1.19) | 0.29 |
| Baseline eGFR (mL/min/1.73 m2) | 0.97 (0.94–0.99) | 0.01 | 0.97 (0.95–0.99) | 0.02 |
| Urinary protein-to-creatinine ratio (10 mg/mg) | 1.00 (1.00–1.01) | 0.14 | 1.00 (1.00–1.01) | 0.14 |
| Hemoglobin (g/dL) | 0.65 (0.45–0.93) | 0.02 | 0.68 (0.47–0.99) | 0.04 |
| Concomitant use of ACEIs or ARBs | 1.30 (0.23–7.32) | 0.77 | 1.30 (0.22–7.80) | 0.78 |
| Concomitant use of immunosuppressants | 3.10 (0.58–16.41) | 0.18 | 4.41 (0.74–26.29) | 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-K.; Wu, C.-L.; Su, T.-C.; Kou, Y.R.; Kor, C.-T.; Lee, T.-S.; Tarng, D.-C. Renal Tubular TRPA1 as a Risk Factor for Recovery of Renal Function from Acute Tubular Necrosis. J. Clin. Med. 2019, 8, 2187. https://doi.org/10.3390/jcm8122187
Wu C-K, Wu C-L, Su T-C, Kou YR, Kor C-T, Lee T-S, Tarng D-C. Renal Tubular TRPA1 as a Risk Factor for Recovery of Renal Function from Acute Tubular Necrosis. Journal of Clinical Medicine. 2019; 8(12):2187. https://doi.org/10.3390/jcm8122187
Chicago/Turabian StyleWu, Chung-Kuan, Chia-Lin Wu, Tzu-Cheng Su, Yu Ru Kou, Chew-Teng Kor, Tzong-Shyuan Lee, and Der-Cherng Tarng. 2019. "Renal Tubular TRPA1 as a Risk Factor for Recovery of Renal Function from Acute Tubular Necrosis" Journal of Clinical Medicine 8, no. 12: 2187. https://doi.org/10.3390/jcm8122187
APA StyleWu, C.-K., Wu, C.-L., Su, T.-C., Kou, Y. R., Kor, C.-T., Lee, T.-S., & Tarng, D.-C. (2019). Renal Tubular TRPA1 as a Risk Factor for Recovery of Renal Function from Acute Tubular Necrosis. Journal of Clinical Medicine, 8(12), 2187. https://doi.org/10.3390/jcm8122187







