Dose-Ranging Study of Ramosetron for the Prevention of Nausea and Vomiting after Laparoscopic Gynecological Surgery: A Prospective Randomized Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
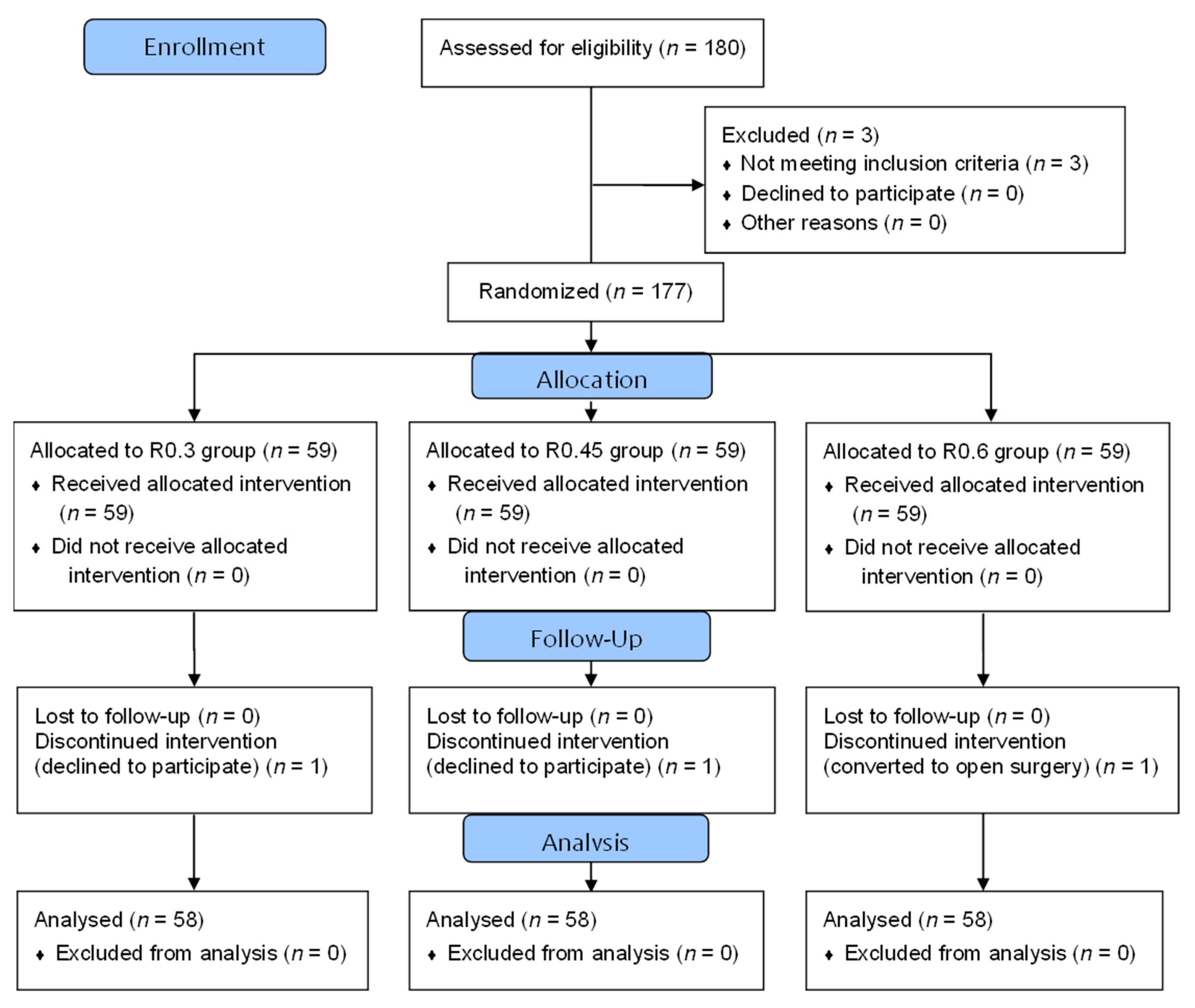
2.2. Randomization and Blinding
2.3. Patient Management
2.4. Assessments and Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Postoperative Nausea and Vomiting
3.3. Postoperative Pain
3.4. Adverse Events, Premature Discontinuation of IV-PCA, and Patient Satisfaction
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef]
- Weilbach, C.; Rahe-meyer, N.; Raymondos, K.; Weissig, A.; Scheinichen, D.; Piepenbrock, S. Postoperative nausea and vomiting (ponv): Usefulness of the apfel-score for identification of high risk patients for ponv. Acta Anaesthesiol. Belg. 2006, 57, 361–363. [Google Scholar]
- Apfel, C.C.; Laara, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Diemunsch, P.; Habib, A.S.; Kovac, A.; Kranke, P.; Meyer, T.A.; Watcha, M.; Chung, F.; Angus, S.; Apfel, C.C.; et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 2014, 118, 85–113. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Meyer, T.; Apfel, C.C.; Chung, F.; Davis, P.J.; Eubanks, S.; Kovac, A.; Philip, B.K.; Sessler, D.I.; Temo, J.; et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth. Analg. 2003, 97, 62–71, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Rabasseda, X. Ramosetron, a 5-ht3 receptor antagonist for the control of nausea and vomiting. Drugs Today 2002, 38, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; So, Y.M.; Hwang, J.; Do, S.H. Ramosetron versus ondansetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg. Endosc. 2010, 24, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kim, S.C.; Baek, Y.H.; Ok, S.Y.; Kim, S.H. Comparison of ramosetron with ondansetron for prevention of postoperative nausea and vomiting in patients undergoing gynaecological surgery. Br. J. Anaesth. 2009, 103, 549–553. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.; Kim, J.; Jeong, S. Effective dose of ramosetron for prophylaxis of postoperative nausea and vomiting in high-risk patients. Biomed. Res. Int. 2015, 2015, 951474. [Google Scholar] [CrossRef][Green Version]
- Lee, B.; Kim, K.; Suh, D.H.; Shin, H.J.; No, J.H.; Lee, J.R.; Jee, B.C.; Hwang, J.W.; Do, S.H.; Kim, Y.B. Efficacy of single-dose and 2-dose intravenous administration of ramosetron in preventing postoperative nausea and vomiting after laparoscopic gynecologic operation: A randomized, double-blind, placebo-controlled, phase 2 trial. Surg. Laparosc. Endosc. Percutaneous Tech. 2017, 27, 183–188. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, H. Double-blind, placebo-controlled, dose-ranging study of ramosetron for the prevention of nausea and vomiting after thyroidectomy. Clin. Ther. 2002, 24, 1148–1153. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, H. Randomized, double-blind, placebo-controlled, dosed-finding study of the antiemetic effects and tolerability of ramosetron in adults undergoing middle ear surgery. Clin. Ther. 2003, 25, 3100–3108. [Google Scholar] [CrossRef]
- Oh, D.S.; Lee, J.H.; Lee, S.E.; Kim, Y.H.; Lim, S.H.; Lee, K.M.; Cheong, S.H.; Choe, Y.K.; Kim, Y.J.; Shin, C.M. Comparison of ramosetron plus dexamethasone with ramosetron alone in the prevention of nausea and vomiting after gynecologic laparoscopic surgery. Korean J. Anesthesiol. 2009, 56, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Cho, E.J. A randomized, double-blind trial of palonosetron compared with ondansetron in preventing postoperative nausea and vomiting after gynaecological laparoscopic surgery. J. Int. Med. Res. 2011, 39, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Lee, K.B.; Lim, S.; Chang, Y.G. Comparison of palonosetron, granisetron, and ramosetron for the prevention of postoperative nausea and vomiting after laparoscopic gynecologic surgery: A prospective randomized trial. BMC Anesthesiol. 2015, 15, 121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinsornsak, P.; Teeyaphudit, M.; Ruetiwarangkoon, C.; Chaiwuttisak, A. Comparison of ramosetron with ondansetron for prevention of intrathecal morphine-induced nausea and vomiting after primary total knee arthroplasty: A randomized control trial. Br. J. Arthroplasty 2017, 32, 1040–1043. [Google Scholar] [CrossRef]
- Agarkar, S.; Chatterjee, A.S. Comparison of ramosetron with ondansetron for the prevention of post-operative nausea and vomiting in high-risk patients. Indian J. Anaesth. 2015, 59, 222–227. [Google Scholar]
- Kim, W.O.; Koo, B.N.; Kim, Y.K.; Kil, H.K. Ramosetron for the prevention of postoperative nausea and vomiting (ponv): A meta-analysis. Korean J. Anesthesiol. 2011, 61, 405–412. [Google Scholar] [CrossRef]
- Lerman, J. Surgical and patient factors involved in postoperative nausea and vomiting. Br. J. Anaesth. 1992, 69, 24S–32S. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, H. Results of a prospective, randomized, double-blind, placebo-controlled, dose-ranging trial to determine the effective dose of ramosetron for the prevention of vomiting after tonsillectomy in children. Clin. Ther. 2003, 25, 3135–3142. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, H.; Ito, M. A randomized clinical trial of a single dose of ramosetron for the prevention of vomiting after strabismus surgery in children: A dose-ranging study. Arch. Ophthalmol. 2005, 123, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Kranke, P.; Katz, M.H.; Goepfert, C.; Papenfuss, T.; Rauch, S.; Heineck, R.; Greim, C.A.; Roewer, N. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: A randomized controlled trial of factorial design. Br. J. Anaesth. 2002, 88, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Philip, B.K.; Cakmakkaya, O.S.; Shilling, A.; Shi, Y.Y.; Leslie, J.B.; Allard, M.; Turan, A.; Windle, P.; Odom-Forren, J.; et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? J. Am. Anesthesiol. 2012, 117, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Visser, K.; Hassink, E.A.; Bonsel, G.J.; Moen, J.; Kalkman, C.J. Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with isoflurane-nitrous oxide: Postoperative nausea with vomiting and economic analysis. J. Am. Soc. Anesthesiol. 2001, 95, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.C.; Bai, S.J.; Lee, K.Y.; Shin, S.; Choi, E.K.; Lee, J.W. Total intravenous anesthesia with propofol reduces postoperative nausea and vomiting in patients undergoing robot-assisted laparoscopic radical prostatectomy: A prospective randomized trial. Yonsei Med. J. 2012, 53, 1197–1202. [Google Scholar] [CrossRef]
- Walder, B.; Schafer, M.; Henzi, I.; Tramer, M.R. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol. Scand. 2001, 45, 795–804. [Google Scholar] [CrossRef]
- Tramer, M.R. A rational approach to the control of postoperative nausea and vomiting: Evidence from systematic reviews. Part i. Efficacy and harm of antiemetic interventions, and methodological issues. Acta Anaesthesiol. Scand. 2001, 45, 4–13. [Google Scholar] [CrossRef]
| R0.3 Group (n = 58) | R0.45 Group (n = 58) | R0.6 Group (n = 58) | p-Value | |
|---|---|---|---|---|
| Age (years) | 42.2 (22–64) | 41.0 (21–62) | 41.2 (20–70) | 0.809 |
| Body mass index (kg/m2) | 23.9 ± 4.2 | 22.9 ± 3.5 | 22.8 ± 3.4 | 0.178 |
| ASA classification (I/II/III) | 52/4/2 | 49/6/3 | 50/8/2 | 0.774 |
| Motion sickness (n) | 14 (24%) | 15 (26%) | 14 (24%) | 0.970 |
| Previous PONV (n) | 2 (3%) | 3 (5%) | 0 | 0.237 |
| Non-smoker (n) | 56 (97%) | 56 (97%) | 56 (97%) | >0.999 |
| Apfel’s score | ||||
| 2 (n) | 2 (3%) | 2 (3%) | 2 (3%) | 0.996 |
| 3 (n) | 41 (71%) | 40 (69%) | 42 (72%) | |
| 4 (n) | 15 (26%) | 16 (28%) | 14 (25%) | |
| Operation duration (min) | 69.0 (52.0, 100.5) | 69.0 (40.0, 104.8) | 77.0 (58.5, 111.80) | 0.221 |
| Anesthesia duration (min) | 95.0 (75.0, 131.3) | 95.0 (69.8, 132.5) | 100.0 (80.0, 140.0) | 0.239 |
| Type of surgery | ||||
| Salpingo-oophorectomy | 18 | 15 | 10 | 0.585 |
| Ovarian cyst enuclation | 15 | 22 | 20 | |
| Myomectomy | 9 | 7 | 10 | |
| Hysterectomy | 16 | 14 | 18 |
| R0.3 Group (n = 58) | R0.45 Group (n = 58) | R0.6 Group (n = 58) | p-Value | |
|---|---|---|---|---|
| Incidence of nausea | ||||
| Early period (the first 6 h after surgery) | 12 (21%) | 11 (19%) | 12 (21%) | 0.965 |
| Late period (6 to 48 h after surgery) | 16 (28%) | 15 (26%) | 14 (24%) | 0.914 |
| Overall | 20 (35%) | 22 (38%) | 20 (35%) | 0.905 |
| Severity of nausea | ||||
| Early period (the first 6 h after surgery) (none/mild/moderate/severe/intractable) | 46/0/9/2/1 | 47/1/8/2/0 | 46/4/7/1/0 | 0.447 |
| Late period (6 to 48 h after surgery) (none/mild/moderate/severe/intractable) | 42/4/4/8/0 | 43/0/8/7/0 | 44/1/10/3/0 | 0.119 |
| Overall | 38/2/9/8/1 | 36/1/12/9/0 | 38/3/13/4/0 | 0.666 |
| Emesis | ||||
| Early period (the first 6 h after surgery) | 1 (2%) | 2 (3.4%) | 3 (5.2%) | 0.596 |
| Late period (6 to 48 h after surgery) | 0 | 1 (2%) | 2 (3.4%) | 0.361 |
| Overall | 1 (1.7%) | 3 (5.2%) | 5 (8.6%) | 0.245 |
| PONV | ||||
| Early period (the first 6 h after surgery) | 12 (21%) | 11 (19%) | 12 (21%) | 0.965 |
| Late period (6 to 48 h after surgery) | 16 (28%) | 15 (26%) | 14 (24%) | 0.914 |
| Overall | 20 (35%) | 22 (38%) | 20 (35%) | 0.905 |
| Patients requiring additional antiemetics | ||||
| Early period (the first 6 h after surgery) | 11 (19%) | 10 (17%) | 8 (14%) | 0.749 |
| Late period (6 to 48 h after surgery) | 6 (10%) | 13 (22%) | 11 (19%) | 0.208 |
| Overall | 16 (28%) | 21 (36%) | 16 (28%) | 0.507 |
| R0.3 Group (n = 58) | R0.45 Group (n = 58) | R0.6 Group (n = 58) | p-Value | |
|---|---|---|---|---|
| Pain score | ||||
| Early period (at 6 h after surgery) | 3 (2, 3) | 3 (3, 3) | 3 (2, 3) | 0.229 |
| Late period (at 48 h after surgery) | 2 (2, 3) | 2 (2, 3) | 2 (2, 2) | 0.135 |
| Worst pain score | ||||
| Early period (the first 6 h after surgery) | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) | 0.879 |
| Late period (6 to 48 h after surgery) | 3 (2, 5) | 3 (2, 4) | 3 (2, 3) | 0.374 |
| Patients requiring additional analgesics | ||||
| Early period (the first 6 h after surgery) | 21 (36%) | 17 (29%) | 17 (29%) | 0.654 |
| Late period (6 to 48 h after surgery) | 15 (26%) | 14 (24%) | 8 (14%) | 0.229 |
| R0.3 Group (n = 58) | R0.45 Group (n = 58) | R0.6 Group (n = 58) | p-Value | |
|---|---|---|---|---|
| Dizziness | 16 (28%) | 14 (24%) | 13 (22%) | 0.806 |
| Headache | 10 (17%) | 6 (10%) | 9 (16%) | 0.545 |
| Drowsiness | 0 | 0 | 0 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.S.; Kim, S.W.; Lee, S.; Yoo, Y.C. Dose-Ranging Study of Ramosetron for the Prevention of Nausea and Vomiting after Laparoscopic Gynecological Surgery: A Prospective Randomized Study. J. Clin. Med. 2019, 8, 2188. https://doi.org/10.3390/jcm8122188
Cho JS, Kim SW, Lee S, Yoo YC. Dose-Ranging Study of Ramosetron for the Prevention of Nausea and Vomiting after Laparoscopic Gynecological Surgery: A Prospective Randomized Study. Journal of Clinical Medicine. 2019; 8(12):2188. https://doi.org/10.3390/jcm8122188
Chicago/Turabian StyleCho, Jin Sun, Sang Wun Kim, Sugeun Lee, and Young Chul Yoo. 2019. "Dose-Ranging Study of Ramosetron for the Prevention of Nausea and Vomiting after Laparoscopic Gynecological Surgery: A Prospective Randomized Study" Journal of Clinical Medicine 8, no. 12: 2188. https://doi.org/10.3390/jcm8122188
APA StyleCho, J. S., Kim, S. W., Lee, S., & Yoo, Y. C. (2019). Dose-Ranging Study of Ramosetron for the Prevention of Nausea and Vomiting after Laparoscopic Gynecological Surgery: A Prospective Randomized Study. Journal of Clinical Medicine, 8(12), 2188. https://doi.org/10.3390/jcm8122188





