Secretory Leukocyte Protease Inhibitor (SLPI)—A Novel Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Study Endpoints
2.3. Biomarkers
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics and Outcomes of Patients
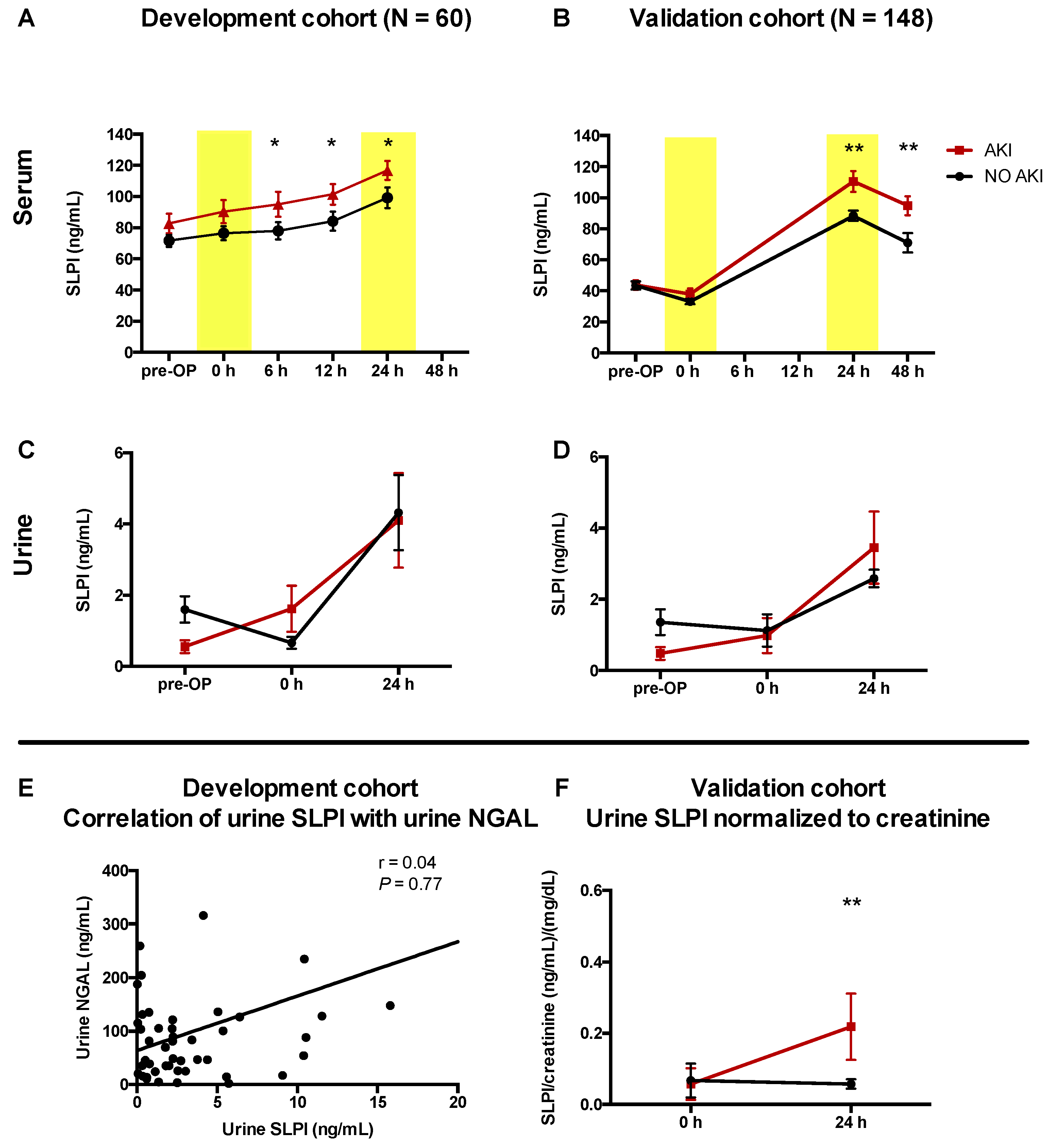
3.2. AKI Was Associated with Higher Serum SLPI in Cardiac Surgery Patients
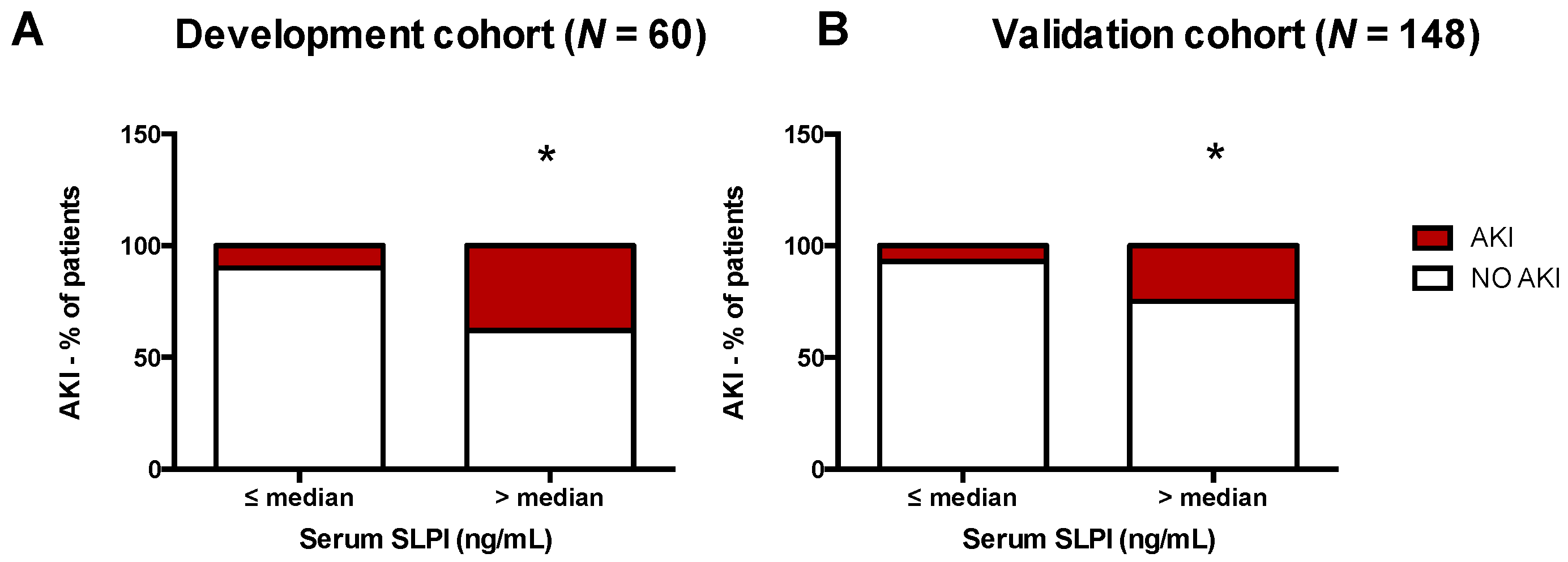
3.3. Accuracy of SLPI for Diagnosis of AKI
3.4. SLPI as a Predictor of AKI in Univariate and Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kheterpal, S.; Tremper, K.K.; Heung, M.; Rosenberg, A.L.; Englesbe, M.; Shanks, A.M.; Campbell, D.A., Jr. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: Results from a national data set. Anesthesiology 2009, 110, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Thiele, R.H.; Isbell, J.M.; Rosner, M.H. AKI associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2015, 10, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Sang, Y.; Coresh, J.; Ballew, S.; Matsushita, K.; Molnar, M.Z.; Szabo, Z.; Kalantar-Zadeh, K.; Kovesdy, C.P. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Yared, J.P.; Worley, S.; Cotman, K.; Paganini, E.P. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003, 64, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Bihorac, A.; Yavas, S.; Subbiah, S.; Hobson, C.E.; Schold, J.D.; Gabrielli, A.; Layon, A.J.; Segal, M.S. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann. Surg. 2009, 249, 851–858. [Google Scholar] [CrossRef]
- Hobson, C.E.; Yavas, S.; Segal, M.S.; Schold, J.D.; Tribble, C.G.; Layon, A.J.; Bihorac, A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009, 119, 2444–2453. [Google Scholar] [CrossRef]
- Meersch, M.; Volmering, S.; Zarbock, A. Prevention of acute kidney injury. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 361–370. [Google Scholar] [CrossRef]
- Zarbock, A.; Kellum, J.A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstadt, H.; Boanta, A.; Gerss, J.; Meersch, M. Effect of Early vs. Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients with Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016, 315, 2190–2199. [Google Scholar] [CrossRef]
- Moran, S.M.; Myers, B.D. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985, 27, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Prowle, J.R. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 2018, 14, 217–230. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Bouchard, J.; Ho, J.; Klarenbach, S.; LaFrance, J.P.; Rigatto, C.; Wald, R.; Zappitelli, M.; Pannu, N. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013, 61, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Wilflingseder, J.; Sunzenauer, J.; Toronyi, E.; Heinzel, A.; Kainz, A.; Mayer, B.; Perco, P.; Telkes, G.; Langer, R.M.; Oberbauer, R. Molecular pathogenesis of post-transplant acute kidney injury: Assessment of whole-genome mRNA and miRNA profiles. PLoS ONE 2014, 9, e104164. [Google Scholar] [CrossRef]
- Palevsky, P.M.; Liu, K.D.; Brophy, P.D.; Chawla, L.S.; Parikh, C.R.; Thakar, C.V.; Tolwani, A.J.; Waikar, S.S.; Weisbord, S.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013, 61, 649–672. [Google Scholar] [CrossRef]
- Thakar, C.V.; Arrigain, S.; Worley, S.; Yared, J.P.; Paganini, E.P. A clinical score to predict acute renal failure after cardiac surgery. J. Am. Soc. Nephrol. 2005, 16, 162–168. [Google Scholar] [CrossRef]
- Stoppe, C.; Rex, S.; Goetzenich, A.; Kraemer, S.; Emontzpohl, C.; Soppert, J.; Averdunk, L.; Sun, Y.; Rossaint, R.; Lue, H.; et al. Interaction of MIF family proteins in myocardial ischemia/reperfusion damage and their influence on clinical outcome of cardiac surgery patients. Antioxid. Redox Signal. 2015, 23, 865–879. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A.; NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef]
- Ho, J.; Tangri, N.; Komenda, P.; Kaushal, A.; Sood, M.; Brar, R.; Gill, K.; Walker, S.; MacDonald, K.; Hiebert, B.M.; et al. Urinary, Plasma, and Serum Biomarkers’ Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. Am. J. Kidney Dis. 2015, 66, 993–1005. [Google Scholar] [CrossRef]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): Explanation and elaboration. BMC Med. 2012, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Critchley, H.O.; Kelly, R.W. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol. Hum. Reprod. 2000, 6, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, K.; Bjartell, A.; Lilja, H. Secretory leucocyte protease inhibitor in the male genital tract: PSA-induced proteolytic processing in human semen and tissue localization. J. Androl. 1995, 16, 64–74. [Google Scholar] [PubMed]
- Bergenfeldt, M.; Nystrom, M.; Bohe, M.; Lindstrom, C.; Polling, A.; Ohlsson, K. Localization of immunoreactive secretory leukocyte protease inhibitor (SLPI) in intestinal mucosa. J. Gastroenterol. 1996, 31, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Ohlsson, K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc. Natl. Acad. Sci. USA 1986, 83, 6692–6696. [Google Scholar] [CrossRef]
- Hannila, S.S. Secretory Leukocyte Protease Inhibitor (SLPI): Emerging Roles in CNS Trauma and Repair. Neuroscientist 2015, 21, 630–636. [Google Scholar] [CrossRef]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Group, A.X.W. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Mahajan, R.; Gupta, K. Food and drug administration’s critical path initiative and innovations in drug development paradigm: Challenges, progress, and controversies. J. Pharm. Bioall. Sci. 2010, 2, 307–313. [Google Scholar] [CrossRef]
- Ohlsson, S.; Ljungkrantz, I.; Ohlsson, K.; Segelmark, M.; Wieslander, J. Novel distribution of the secretory leucocyte proteinase inhibitor in kidney. Mediat. Inflamm. 2001, 10, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Bergenfeldt, M.; Bjork, P.; Ohlsson, K. The elimination of secretory leukocyte protease inhibitor (SLPI) after intravenous injection in dog and man. Scand. J. Clin. Lab. Investig. 1990, 50, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, S.; Hautz, T.; Wahl, S.M.; Brandacher, G.; Sucher, R.; Steinmassl, O.; Steinmassl, P.; Wright, C.D.; Obrist, P.; Werner, E.R.; et al. The effect of secretory leukocyte protease inhibitor (SLPI) on ischemia/reperfusion injury in cardiac transplantation. Am. J. Transplant. 2008, 8, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Lentsch, A.B.; Yoshidome, H.; Warner, R.L.; Ward, P.A.; Edwards, M.J. Secretory leukocyte protease inhibitor in mice regulates local and remote organ inflammatory injury induced by hepatic ischemia/reperfusion. Gastroenterology 1999, 117, 953–961. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Barie, P.S.; Nathan, C.F.; Fuortes, M.; Lin, E.; Lowry, S.F.; Wright, C.D.; Weyant, M.J.; Hydo, L.; Reeves, F.; et al. Secretory leukocyte protease inhibitor, an inhibitor of neutrophil activation, is elevated in serum in human sepsis and experimental endotoxemia. Crit. Care Med. 2000, 28, 1276–1282. [Google Scholar] [CrossRef]
- Ochi, A.; Chen, D.; Schulte, W.; Leng, L.; Moeckel, N.; Piecychna, M.; Averdunk, L.; Stoppe, C.; Bucala, R.; Moeckel, G. MIF-2/D-DT enhances proximal tubular cell regeneration through SLPI- and ATF4-dependent mechanisms. Am. J. Physiol. 2017, 313, F767–F780. [Google Scholar] [CrossRef]
- Billings, F.T.t.; Pretorius, M.; Schildcrout, J.S.; Mercaldo, N.D.; Byrne, J.G.; Ikizler, T.A.; Brown, N.J. Obesity and oxidative stress predict AKI after cardiac surgery. J. Am. Soc. Nephrol. 2012, 23, 1221–1228. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef]
- Lopez-Bermejo, A.; Ortega, F.J.; Castro, A.; Ricart, W.; Fernandez-Real, J.M. The alarm secretory leukocyte protease inhibitor increases with progressive metabolic dysfunction. Clin. Chim. Acta 2011, 412, 1122–1126. [Google Scholar] [CrossRef]
- Taggart, C.C.; Cryan, S.A.; Weldon, S.; Gibbons, A.; Greene, C.M.; Kelly, E.; Low, T.B.; O’Neill, S.J.; McElvaney, N.G. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J. Exp. Med. 2005, 202, 1659–1668. [Google Scholar] [CrossRef]
- Taggart, C.C.; Greene, C.M.; McElvaney, N.G.; O’Neill, S. Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IkappaBalpha degradation without affecting phosphorylation or ubiquitination. J. Biol. Chem. 2002, 277, 33648–33653. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, M.; Bergenfeldt, M.; Ohlsson, K. The elimination of secretory leukocyte protease inhibitor (SLPI) from the gastrointestinal tract in man. Scand. J. Clin. Lab. Investig. 1997, 57, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Waldmann, T.A. The role of the kidney in the metabolism of plasma proteins. Nephron 1974, 13, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Smertka, M.; Chudek, J. Using NGAL as an early diagnostic test of acute kidney injury. Ren. Fail. 2012, 34, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Luo, Q.; Wang, L.; Han, L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: A meta-analysis. Eur. J. Cardiothorac. Surg. 2016, 49, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.S.; Mitchell, E.D.; Smith, A.F.; Cairns, D.A.; Messenger, M.; Hutchinson, M.; Wright, J.; Vinall-Collier, K.; Corps, C.; Hamilton, P.; et al. The future for diagnostic tests of acute kidney injury in critical care: Evidence synthesis, care pathway analysis and research prioritisation. Health Technol. Assess. 2018, 22, 1–274. [Google Scholar] [CrossRef]
- Nisula, S.; Yang, R.; Kaukonen, K.M.; Vaara, S.T.; Kuitunen, A.; Tenhunen, J.; Pettila, V.; Korhonen, A.M.; Group, F.S. The urine protein NGAL predicts renal replacement therapy, but not acute kidney injury or 90-day mortality in critically ill adult patients. Anesth. Analg. 2014, 119, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bihorac, A.; Chawla, L.S.; Shaw, A.D.; Al-Khafaji, A.; Davison, D.L.; Demuth, G.E.; Fitzgerald, R.; Gong, M.N.; Graham, D.D.; Gunnerson, K.; et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am. J. Respir. Crit. Care Med. 2014, 189, 932–939. [Google Scholar] [CrossRef]
- Honore, P.M.; Nguyen, H.B.; Gong, M.; Chawla, L.S.; Bagshaw, S.M.; Artigas, A.; Shi, J.; Joannes-Boyau, O.; Vincent, J.L.; Kellum, J.A.; et al. Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Risk Stratification of Acute Kidney Injury in Patients With Sepsis. Crit. Care Med. 2016, 44, 1851–1860. [Google Scholar] [CrossRef]


| Acute Kidney Injury within 72 h after Cardiac Surgery | Development Cohort (n = 60) | Persistent AKI > 48 h | Validation Cohort (n = 148) | Persistent AKI > 48 h | |||
|---|---|---|---|---|---|---|---|
| AKI according to KDIGO diagnostic criteria | 14 | (25%) | 6 (43%) | 22 | (15%) | 9 (41%) | |
| KDIGO Stage 1 | 8 | (57%) | 12 | (54%) | |||
| KDIGO Stage 2 | 5 | (36%) | 8 | (36%) | |||
| KDIGO Stage 3 | 1 | (7%) | 2 | (9%) | |||
| Diagnostic criteria met | |||||||
| Increased creatinine | 14 | (100%) | 22 | (100%) | |||
| Oliguria (<0.5 mL/kg/h for ≥6 h) | 3 | (21%) | 5 | (23%) | |||
| Time point of diagnosis | |||||||
| 24 h after surgery | 3 | (21%) | 1 (33%) | 6 | (27%) | 2 (33%) | |
| 48 h after surgery | 7 | (50%) | 3 (42%) | 9 | (41%) | 6 (67%) | |
| 72 h after surgery | 4 | (29%) | 2 (50%) | 7 | (32%) | 1 (14%) | |
| Characteristic | Development Cohort | Validation Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No AKI | AKI | p-Value | No AKI | AKI | p-Value | ||||||
| (n = 46) | (n = 14) | (n = 126) | (n = 22) | ||||||||
| Demographics | |||||||||||
| Age (years) | 67 | (59–75) | 69 | (68–78) | 0.047 | 67 | (59–75) | 69 | (68–78) | 0.171 | |
| Sex (female) | 11 | (24) | 4 | (29) | 0.678 | 33 | (26) | 6 | (27) | 0.869 | |
| BMI (kg/m2) | 27.4 | (25.0–29.9) | 26.5 | (23.8–33.2) | 0.767 | 27.1 | (24.8–30.3) | 28.5 | (22.9–30.4) | 0.876 | |
| Medication, No (%) | |||||||||||
| Beta blockers | 40 | (87) | 9 | (75) | 0.292 | 91 | (73) | 17 | (77) | 0.733 | |
| ACE Inhibitors | 35 | (76) | 8 | (67) | 0.478 | 69 | (55) | 12 | (55) | 0.943 | |
| Sartans | 6 | (13) | 1 | (8) | 0.840 | 27 | (22) | 7 | (32) | 0.271 | |
| Calcium channel blockers | 6 | (13) | 5 | (42) | 0.037 | 35 | (28) | 6 | (27) | 0.993 | |
| Diuretics | 36 | (78) | 11 | (92) | 0.456 | 52 | (42) | 13 | (59) | 0.138 | |
| Statins | 45 | (98) | 12 | (100) | 0.929 | 106 | (85) | 19 | (86) | 0.975 | |
| Acetylsalicylic acid | 44 | (96) | 12 | (100) | 0.860 | 103 | (82) | 18 | (82) | 0.848 | |
| Comorbidities, No (%) | |||||||||||
| Arterial hypertension | 28 | (61) | 11 | (85) | 0.159 | 89 | (71) | 18 | (82) | 0.362 | |
| Pulmonary hypertension | 3 | (7) | 1 | (8) | 0.741 | 6 | (5) | 2 | (9) | 0.328 | |
| Congestive heart disease | 7 | (15) | 4 | (29) | 0.255 | 16 | (13) | 0 | (0) | 0.201 | |
| LVEF < 35% | 10 | (22) | 2 | (14) | 0.651 | 6 | (5) | 2 | (9) | 0.328 | |
| Chronic kidney disease | 3 | (7) | 2 | (14) | 0.345 | 9 | (7) | 4 | (18) | 0.090 | |
| COPD | 3 | (7) | 2 | (14) | 0.345 | 15 | (12) | 3 | (14) | 0.707 | |
| Diabetes, insulin | 3 | (7) | 5 | (38) | 0.012 | 13 | (10) | 3 | (14) | 0.545 | |
| Previous cardiac surgery | 3 | (7) | 0 | (0) | 0.632 | 8 | (6) | 1 | (5) | 0.970 | |
| Serum creatinine at baseline (mg/dL) | 0.93 | (0.78–1.04) | 1.22 | (0.83–1.36) | 0.011 | 0.99 | (0.80–1.10) | 1.08 | (0.94–1.28) | 0.018 | |
| Type of Surgery | |||||||||||
| Isolated CABG | 24 | (52) | 3 | (21) | 0.064 | 78 | (62) | 11 | (50) | 0.274 | |
| Isolated valvular surgery | 8 | (17) | 4 | (29) | 0.344 | 16 | (13) | 4 | (18) | 0.425 | |
| Combined procedure | 14 | (30) | 7 | (50) | 0.191 | 30 | (24) | 7 | (32) | 0.403 | |
| other | 5 | (4) | 1 | (5) | |||||||
| Risk of AKI | |||||||||||
| Cleveland Clinic Foundation Score | 3 | (2–3) | 4 | (3–5) | 0.005 | 3 | (2–4) | 3 | (2–4) | 0.636 | |
| Duration of Surgery | |||||||||||
| Aortic cross clamp | 74.5 | (57.5–99) | 78.5 | (47–105) | 0.934 | 73 | (55–89) | 78 | (60–101) | 0.232 | |
| Cardiopulmonary bypass | 115 | (91–144) | 118.5 | (89.5–148.5) | 0.769 | 109 | (87–133) | 139 | (97–150) | 0.046 | |
| SOFA on POD 1 | 10 | (7.5–12) | 9 | (7–10) | 0.674 | 8 | (6–9) | 9 | (7–12) | 0.044 | |
| Serum SLPI | ||||||||||
| SLPI (ng/mL) | Development Cohort (n = 60) | Validation Cohort (n = 148) | ||||||||
| No AKI | AKI | p-Value | No AKI | AKI | p-Value | |||||
| (n = 46) | (n = 14) | (n = 226) | (n = 22) | |||||||
| Pre-OP | 67.3 | (57.2–82.1) | 87.6 | (65.3–98.5) | 0.14 | 40.1 | (31.6 –48.5) | 43.7 | (36.6–52.4) | 0.280 |
| 0 h after surgery | 66.3 | (52.8–81.15) | 102.7 | (83.2–128.2) | 0.06 | 29.7 | (22.4–39.9) | 37.9 | (25.4–45.3) | 0.127 |
| 6 h after surgery | 64.9 | (53.9–84.7) | 102.1 | (93.2–131.5) | <0.001 | |||||
| 12 h after surgery | 74.7 | (52.0–88.1) | 114.5 | (95.0–134.5) | <0.001 | |||||
| 24 h after surgery | 86.1 | (69.0–113.5) | 117.9 | (105.6–145.2) | 0.001 | 80.4 | (64.7–111.7) | 106.6 | (83.0–135.3) | 0.008 |
| 48 h after surgery | 58.5 | (58.5–90.0) | 98.8 | (76.0–110.4) | 0.000 | |||||
| Urinary SLPI | ||||||||||
| SLPI (ng/mL) | Development Cohort (n = 60) | Validation Cohort (n = 148) | ||||||||
| No AKI | AKI | p-Value | No AKI | AKI | p-Value | |||||
| (n = 46) | (n = 14) | (n = 226) | (n = 22) | |||||||
| Pre-OP | 1.10 | (0.40–2.09) | 0.40 | (0.17–0.96) | 0.022 | 0.51 | (0.15–1.53) | 0.8 | (0.20–1.36) | 0.520 |
| 0 h after surgery | 0.23 | (0.07–1.09) | 0.58 | (0.31–2.02) | 0.056 | 0.13 | (0.025–0.35) | 0.98 | (0.98–1.40) | 0.073 |
| 24 h after surgery | 2.20 | (0.74–5.05) | 2.38 | (0.33–9.23) | 0.942 | 1.15 | (0.71–1.92) | 1.08 | (0.90–1.62) | 0.575 |
| Time Point after Surgery | Optimal Cut-off (ng/mL) | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | Likelihood Ratio | Youden Index |
|---|---|---|---|---|---|---|---|
| Development cohort, Serum SLPI | |||||||
| 6 h | >85.20 | 64.3 | 35.1–87.2 | 68.29 | 51.9–81.9 | 2.027 | 0.32 |
| 12 h | >92.72 | 66.7 | 34.9–90.1 | 73.17 | 57.1–85.8 | 2.485 | 0.39 |
| 24 h | >87.93 | 100.0 | 75.3–100.0 | 54.55 | 38.9–69.6 | 2.200 | 0.54 |
| Validation cohort, Serum SLPI | |||||||
| 24 h | >101.8 | 70.0 | 45.7–88.1 | 67.6 | 57.8–76.4 | 2.162 | 0.38 |
| 48 h | >78.45 | 77.8 | 52.4–93.6 | 71.2 | 61.4–79.9 | 2.709 | 0.49 |
| (A) AKI, Time Point not Considered | |||||||||
| Univariable Logistic Regression (Median) | Multivariable Logistic Regression (Median) | ||||||||
| Time Point after Surgery | Median | OR | 95% CI | p-value | OR | adj. 95% CI | p-value | ||
| Development Cohort | |||||||||
| Pre-OP | 71.3 | 1.37 | 0.42 | 4.57 | 0.601 | 1.12 | 0.30 | 4.16 | 0.868 |
| 0 h after surgery | 77.2 | 2.06 | 0.63 | 7.28 | 0.230 | 1.69 | 0.46 | 6.61 | 0.431 |
| 6 h after surgery | 69.6 | 2.19 | 0.67 | 7.82 | 0.197 | 1.74 | 1.18 | 2.84 | 0.004 |
| 12 h after surgery | 79.9 | 3.80 | 1.03 | 17.09 | 0.045 | 1.72 | 1.15 | 2.83 | 0.008 |
| 24 h after surgery | 95 | 3.92 | 1.10 | 17.31 | 0.035 | 1.76 | 1.16 | 2.98 | 0.007 |
| Validation Cohort | |||||||||
| Pre-OP | 41.00 | 1.46 | 0.58 | 3.75 | 0.417 | 1.47 | 0.59 | 3.76 | 0.412 |
| 0 h after surgery | 13.00 | 1.029 | 0.41 | 2.59 | 0.945 | 1.01 | 0.38 | 2.66 | 0.19 |
| 24 h after surgery | 88.3 | 3.89 | 1.44 | 12.08 | 0.007 | 3.91 | 1.44 | 12.13 | 0.007 |
| 48 h after surgery | 65.3 | 9.24 | 2.69 | 48.30 | <0.001 | 9.45 | 2.74 | 49.55 | <0.001 |
| (B) AKI, Time Point Considered | |||||||||
| Univariable Logistic Regression (Median) | Multivariable Logistic Regression (Median) | ||||||||
| Time Point | Median | OR | 95% CI | p-value | OR | adj. 95% CI | p-value | ||
| Development Cohort | |||||||||
| SLPI measured at 24 h for AKI diagnosed later: 48 or 72 h after surgery (11 of 14 cases of AKI) | 95 | 4.45 | 1.07 | 25.61 | 0.039 | 2.48 | 0.50 | 15.35 | 0.268 |
| Validation Cohort | |||||||||
| SLPI measured at 24 h for AKI diagnosed later: 48 or 72 h after surgery (16 of 22 AKI cases) | 88.3 | 4.94 | 1.55 | 20.15 | 0.006 | 4.89 | 1.54 | 19.92 | 0.006 |
| SLPI measured at 48 h for AKI diagnosed later: 72 h after surgery (7 of 22 cases of AKI) | 65.3 | 15.4 | 1.67 | 2042 | 0.011 | 15.24 | 1.63 | 2025.31 | 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Averdunk, L.; Fitzner, C.; Levkovich, T.; Leaf, D.E.; Sobotta, M.; Vieten, J.; Ochi, A.; Moeckel, G.; Marx, G.; Stoppe, C. Secretory Leukocyte Protease Inhibitor (SLPI)—A Novel Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery: A Prospective Observational Study. J. Clin. Med. 2019, 8, 1931. https://doi.org/10.3390/jcm8111931
Averdunk L, Fitzner C, Levkovich T, Leaf DE, Sobotta M, Vieten J, Ochi A, Moeckel G, Marx G, Stoppe C. Secretory Leukocyte Protease Inhibitor (SLPI)—A Novel Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery: A Prospective Observational Study. Journal of Clinical Medicine. 2019; 8(11):1931. https://doi.org/10.3390/jcm8111931
Chicago/Turabian StyleAverdunk, Luisa, Christina Fitzner, Tatjana Levkovich, David E. Leaf, Michael Sobotta, Jil Vieten, Akinobu Ochi, Gilbert Moeckel, Gernot Marx, and Christian Stoppe. 2019. "Secretory Leukocyte Protease Inhibitor (SLPI)—A Novel Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery: A Prospective Observational Study" Journal of Clinical Medicine 8, no. 11: 1931. https://doi.org/10.3390/jcm8111931
APA StyleAverdunk, L., Fitzner, C., Levkovich, T., Leaf, D. E., Sobotta, M., Vieten, J., Ochi, A., Moeckel, G., Marx, G., & Stoppe, C. (2019). Secretory Leukocyte Protease Inhibitor (SLPI)—A Novel Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery: A Prospective Observational Study. Journal of Clinical Medicine, 8(11), 1931. https://doi.org/10.3390/jcm8111931





