FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function
Abstract
1. Introduction
Flavoprotein Dehydrogenases
2. Materials and Methods
2.1. Protein SequenceSampling and Multiple Sequence Alignment (MSA)
2.2. Crystal Structure Sampling Via Folding Recognition
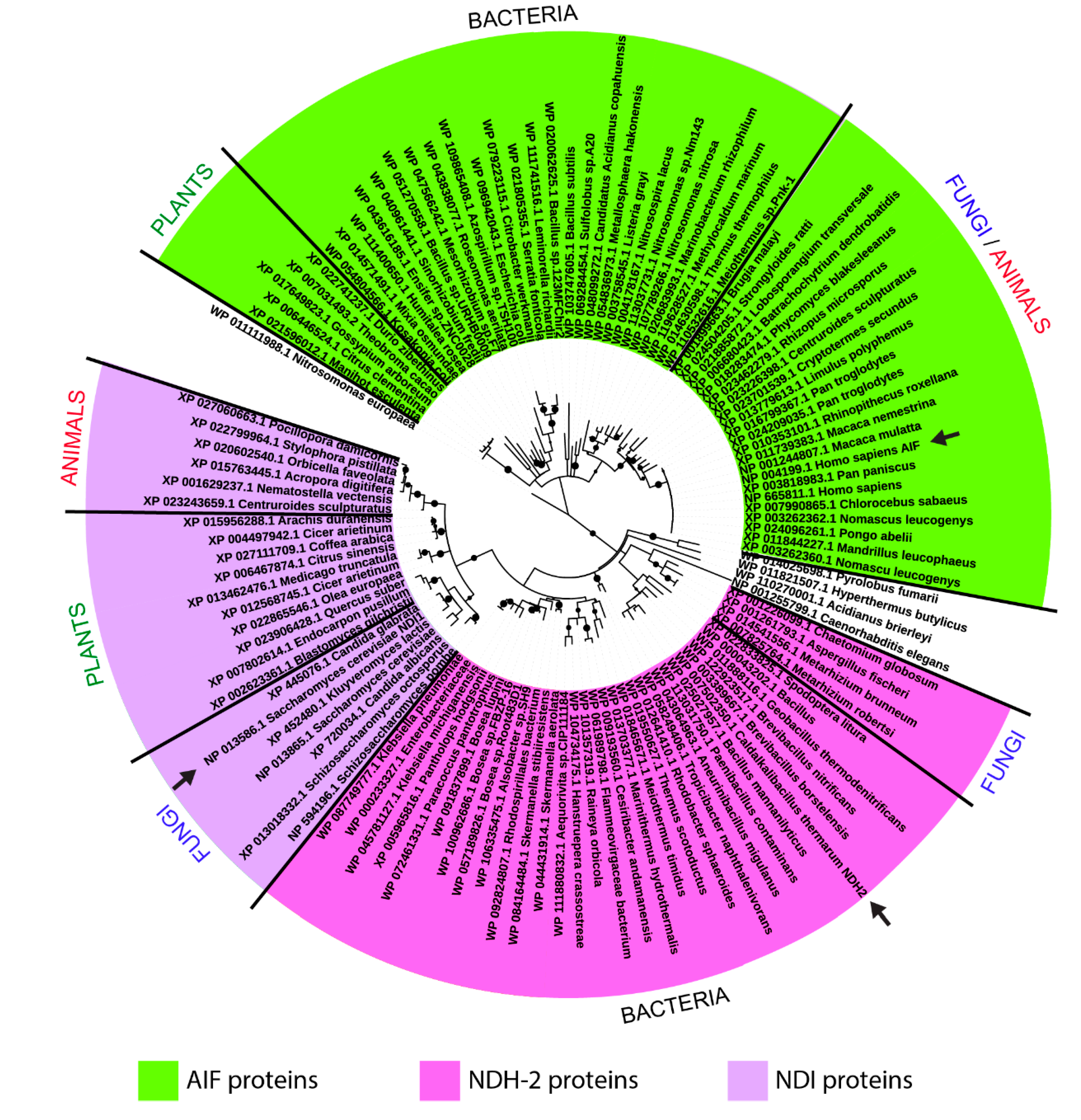
2.3. Phylogenetic Analysis
3. Results
3.1. Evolutionary Relationships among the Sampled AIF/NDH-2/NDI Homologous Sequences
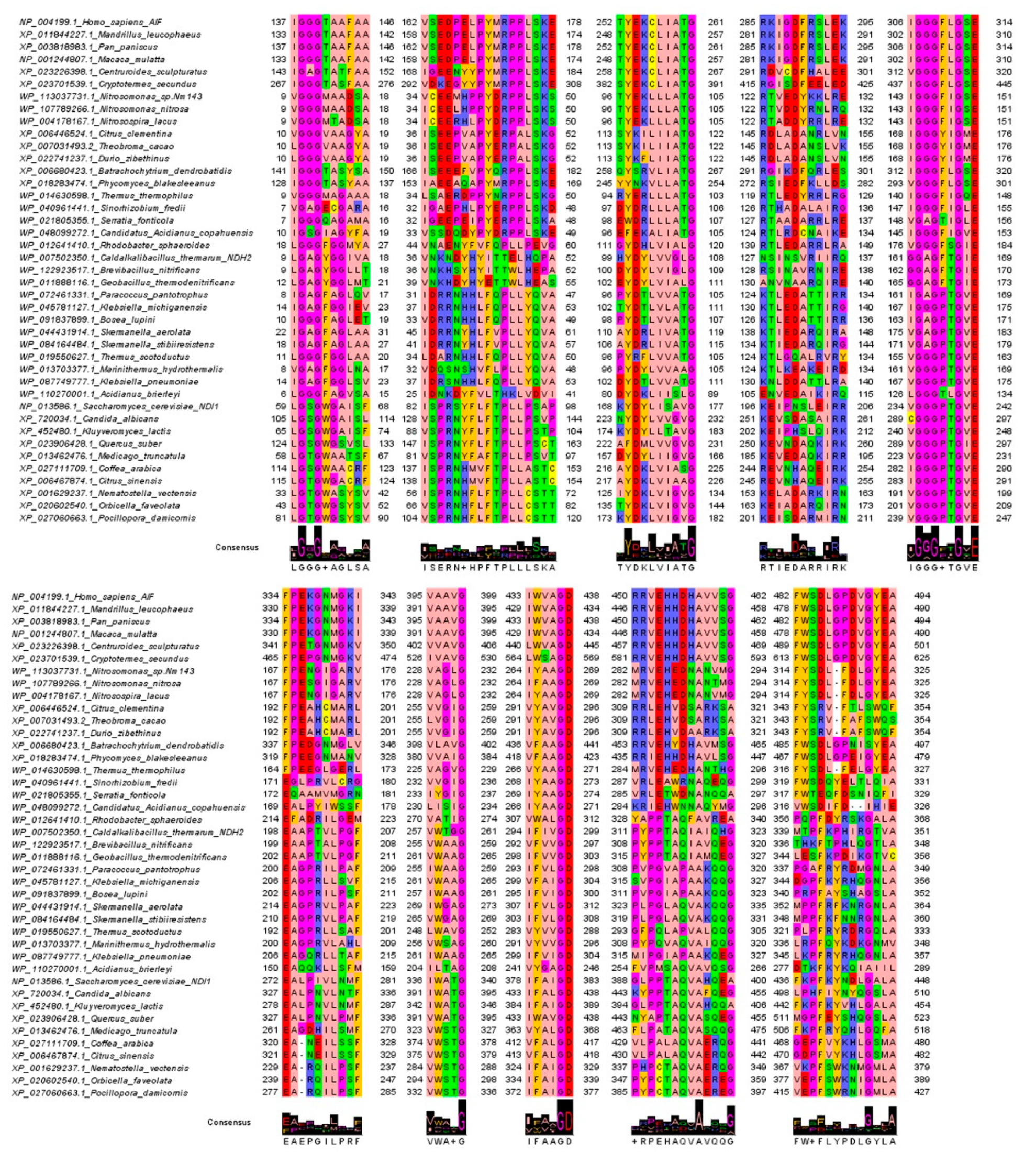
3.2. Sequence Features of the Sampled AIF/NDH-2/NDI Protein Sequences
3.3. Comparative Analysis of AIF, NDH-II, NDI, and the pGenTHREADER-Suggested Template Proteinsfor Comparative Modeling
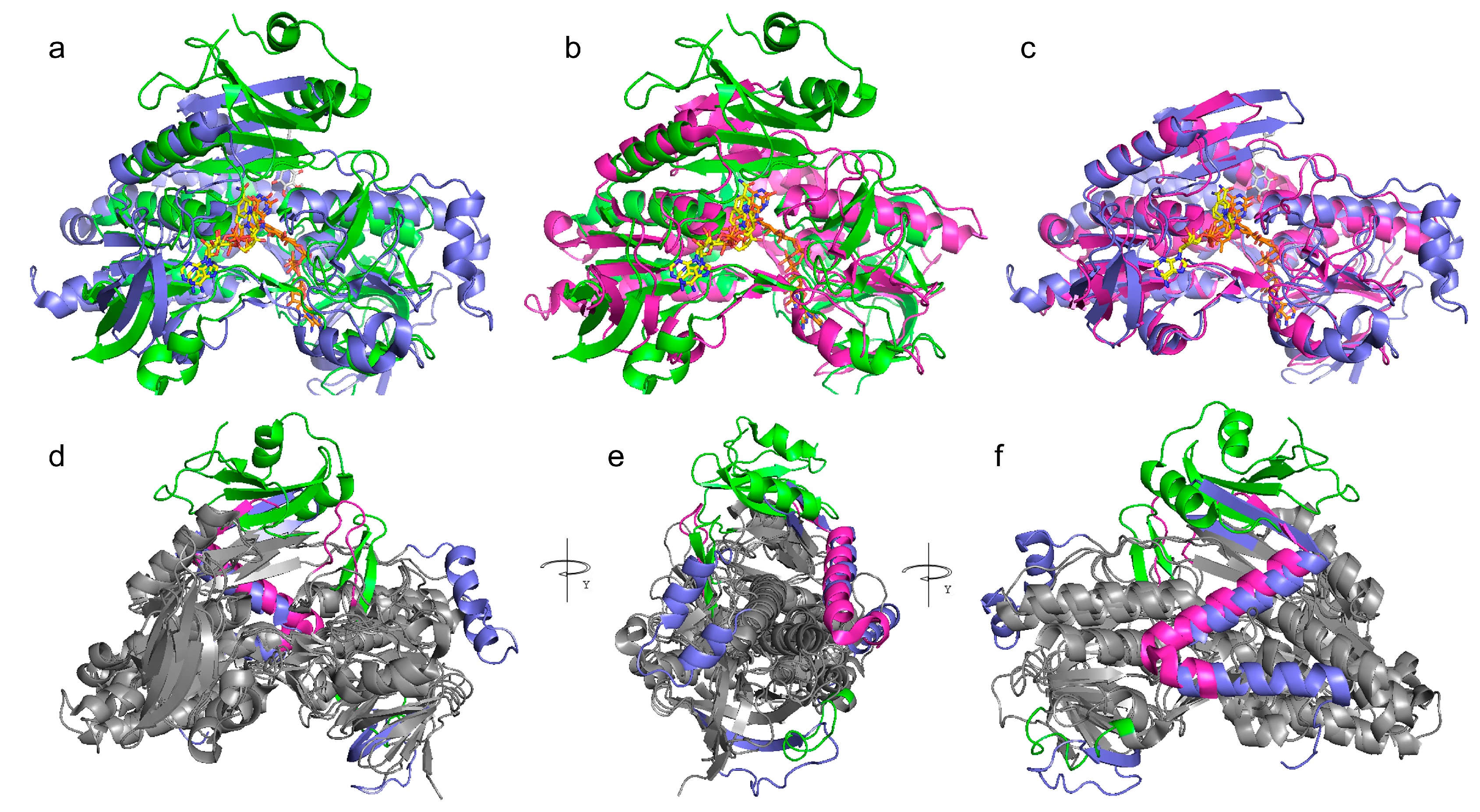
3.3.1. Superimposition of AIF, NDI, and NDH-2
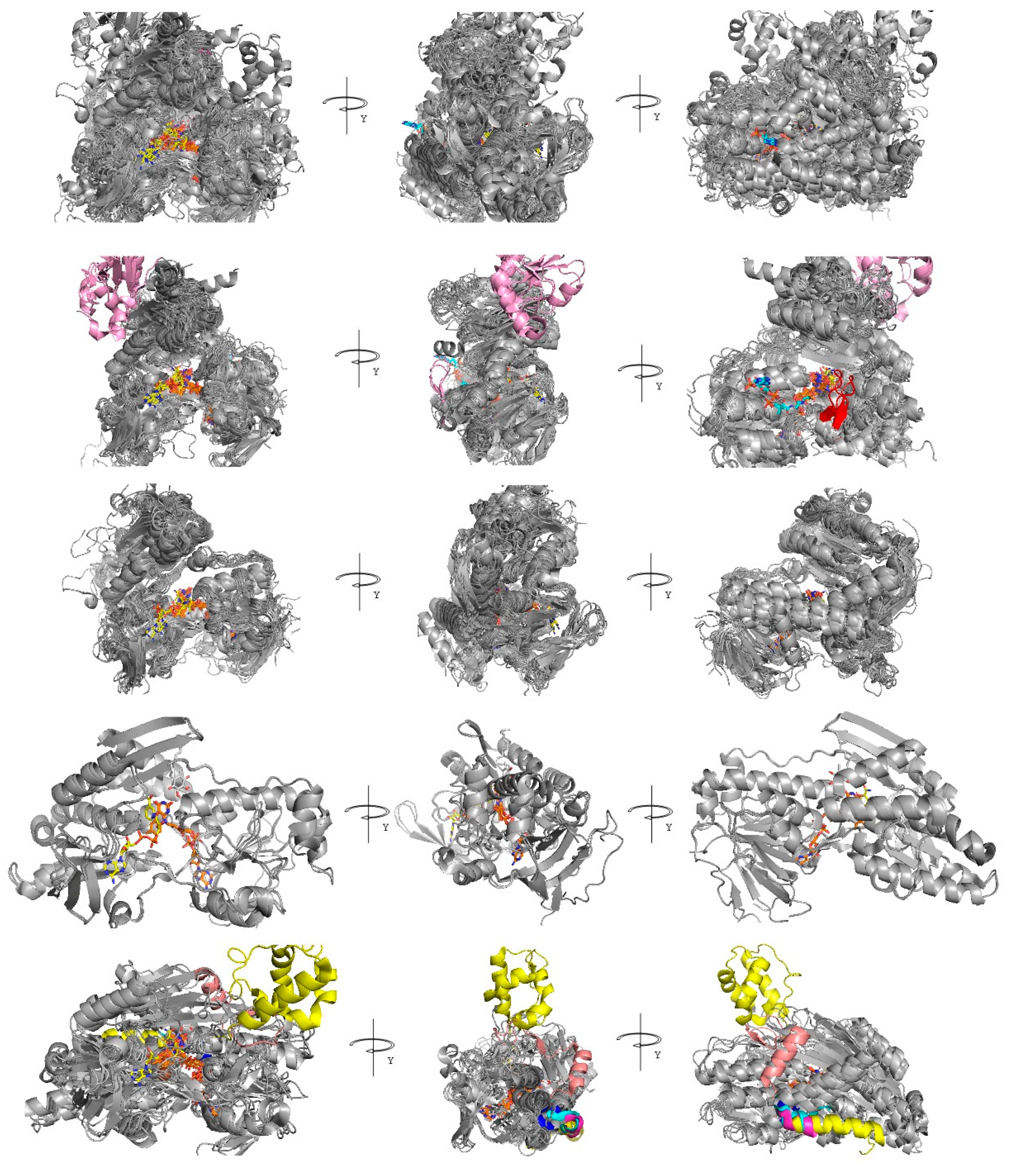
3.3.2. Sampling of Homologous-Crystallized Structures by Folding Recognition Methods
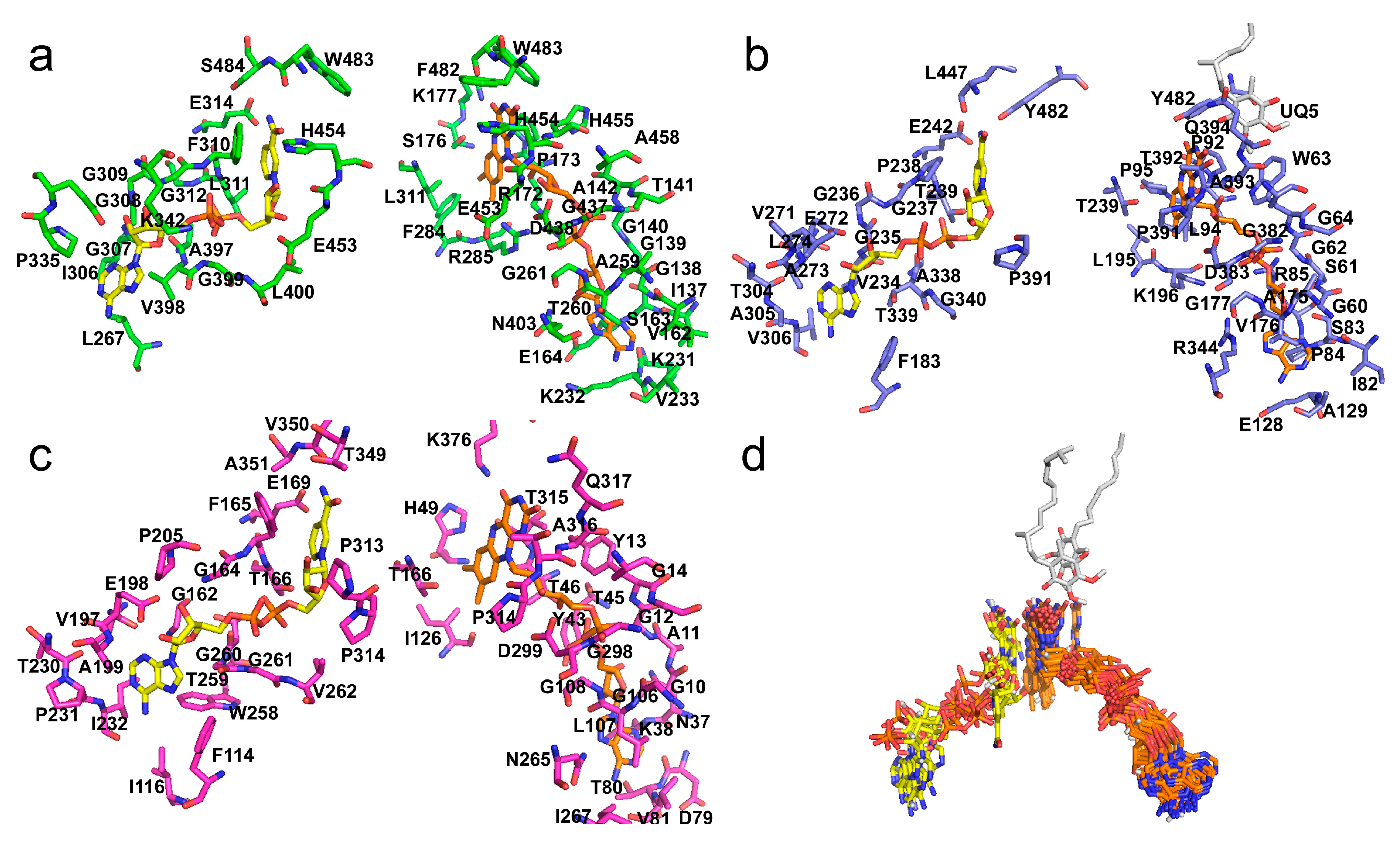
3.4. FAD and NADH Binding Regions
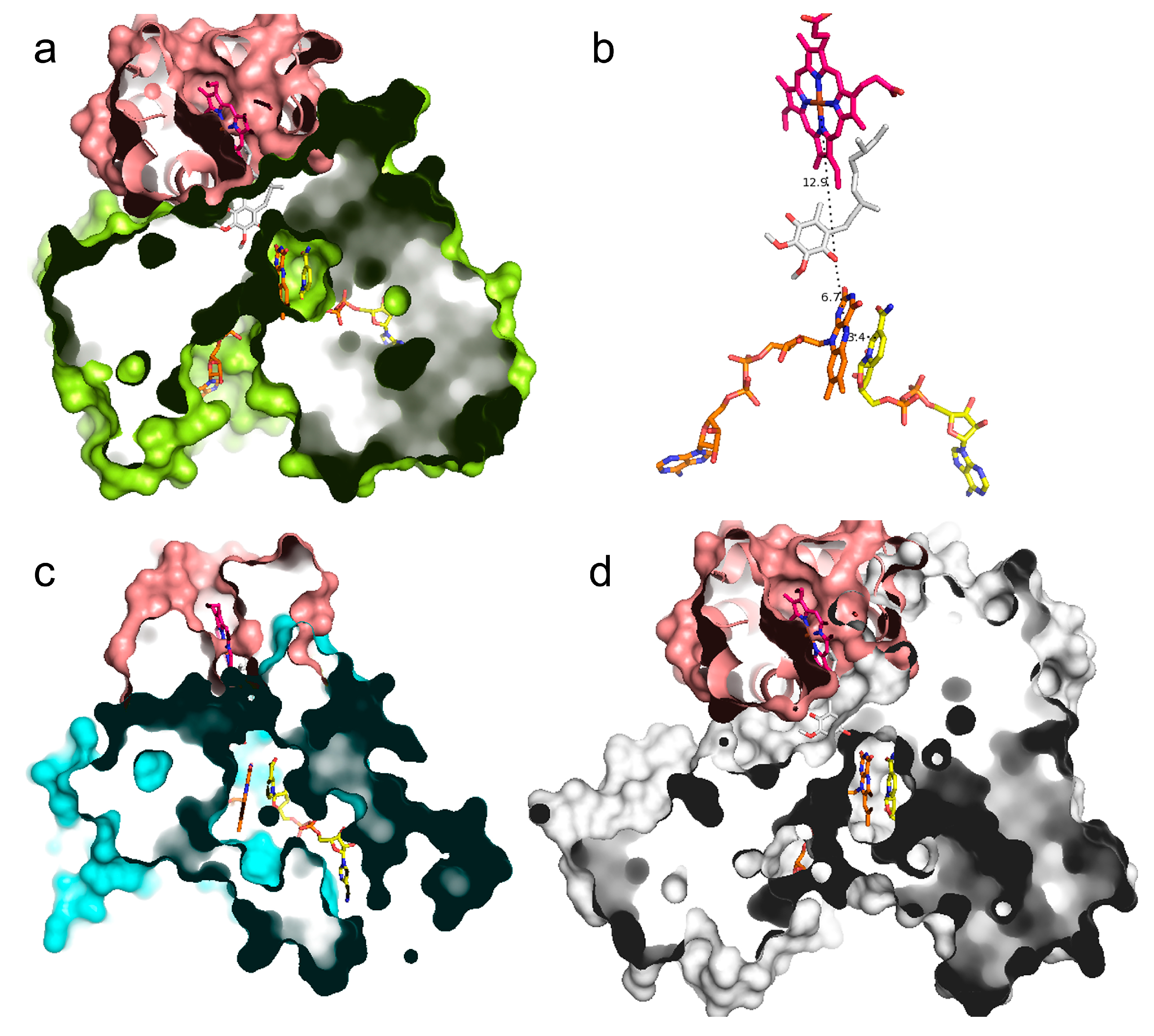
3.5. UQ Binding Site Comparative Analyses between AIF and NDH-2
3.6. Small Molecules and Other Cofactor-Binding Regions
3.7. Small Protein Subunit-Binding Regions
4. Discussion
4.1. A Similarly Located FAD/NADH-Binding Region for All the Investigated Flavoprotein Oxidoreductases and New Clues about a Putative UQ-Binding Region
4.2. Concerns about the Opportunity to Draw New Inhibitors to be Used as Antibiotic/Antiparasitic Drugs, Directed Against the Investigated FAD/NADH Dehydrogenases
4.3. New Clues in Support of AIF Participation in Mitochondrial Respiration
4.4. Pieces of Evidence about the Possible Targeting of AIF for the Development of New Treatments for Mitochondrial Dysfunction in Rare Diseases
4.5. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AIF | Apoptosis-inducing factor | DH | dehydrogenase |
| LD | lipoamide dehydrogenase | DLD | dihydrolipoyl dehydrogenase or dihydrolipoamide dehydrogenase |
| OX | oxidase | RED | reductase |
| GLR1 | glutathione disulfide reductase | Trx | Thioredoxin |
| mt | mitochondrial | TRR1 | Trx-disulfide reductase 1 |
| MSA | multiple sequence alignment | RMSD | root-mean-square deviation |
| MIF | migration inhibitory factor | RYL-552 | 5n.a.fluoron.a.3n.a.methyln.a.2n.a.{4n.a.(4n.a.(trifluoromethoxy)benzyl)phenyl}quinolinn.a.4(1H)n.a.one) |
| SL827 | N~2~-((2-amino-5-bromopyridin-3-yl)sulfonyl)-N-(4-methoxyphenyl)-N~2~-methylglycinamide | KPC | Ketopropylthioethanesulphonate |
| CytC | cytochrome c | NAD+ | nicotinamide adenine dinucleotide |
| FAD | flavin adenine dinucleotide | UQ | ubiquinone |
| CoA | Coenzyme A |
References
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Bogachev, A.V.; Baykov, A.A.; Bertsova, Y.V. Flavin transferase: The maturation factor of flavin-containing oxidoreductases. Biochem. Soc. Trans. 2018, 46, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Harold, L.K.; Antoney, J.; Ahmed, F.H.; Hards, K.; Carr, P.D.; Rapson, T.; Greening, C.; Jackson, C.J.; Cook, G.M. FAD-sequestering proteins protect mycobacteria against hypoxic and oxidative stress. J. Biol. Chem. 2019, 294, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Eggink, G.; Engel, H.; Vriend, G.; Terpstra, P.; Witholt, B. Rubredoxin reductase of Pseudomonas oleovorans. Structural relationship to other flavoprotein oxidoreductases based on one NAD and two FAD fingerprints. J. Mol. Biol. 1990, 212, 135–142. [Google Scholar] [CrossRef]
- Ross, R.P.; Claiborne, A. Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. Comparison with NADH peroxidase and the flavoprotein disulfide reductases. J. Mol. Biol. 1992, 227, 658–671. [Google Scholar] [CrossRef]
- Kuriyan, J.; Krishna, T.S.; Wong, L.; Guenther, B.; Pahler, A.; Williams, C.H.; Model, P. Convergent evolution of similar function in two structurally divergent enzymes. Nature 1991, 352, 172–174. [Google Scholar] [CrossRef]
- Heikal, A.; Nakatani, Y.; Dunn, E.; Weimar, M.R.; Day, C.L.; Baker, E.N.; Lott, J.S.; Sazanov, L.A.; Cook, G.M. Structure of the bacterial type II NADH dehydrogenase: A monotopic membrane protein with an essential role in energy generation. Mol. Microbiol. 2014, 91, 950–964. [Google Scholar] [CrossRef]
- Vinogradov, A.D.; Grivennikova, V.G. Oxidation of NADH and ROS production by respiratory complex I. Biochim. Biophys. Acta 2016, 1857, 863–871. [Google Scholar] [CrossRef]
- Titov, D.V.; Cracan, V.; Goodman, R.P.; Peng, J.; Grabarek, Z.; Mootha, V.K. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 2016, 352, 231–235. [Google Scholar] [CrossRef]
- Blaza, J.N.; Bridges, H.R.; Aragão, D.; Dunn, E.A.; Heikal, A.; Cook, G.M.; Nakatani, Y.; Hirst, J. The mechanism of catalysis by type-II NADH:quinone oxidoreductases. Sci. Rep. 2017, 7, 40165. [Google Scholar] [CrossRef]
- Elguindy, M.M.; Nakamaru-Ogiso, E. Apoptosis-inducing Factor (AIF) and Its Family Member Protein, AMID, Are Rotenone-sensitive NADH:Ubiquinone Oxidoreductases (NDH-2). J. Biol. Chem. 2015, 290, 20815–20826. [Google Scholar] [CrossRef] [PubMed]
- Misevičien, L.; Anusevičius, Ž.; Šarlauskas, J.; Sevrioukova, I.F.; Čnas, N. Redox reactions of the FAD-containing apoptosis-inducing factor (AIF) with quinoidal xenobiotics: A mechanistic study. Arch. Biochem. Biophys. 2011, 512, 183–189. [Google Scholar] [CrossRef]
- Iwata, M.; Lee, Y.; Yamashita, T.; Yagi, T.; Iwata, S.; Cameron, A.D.; Maher, M.J. The structure of the yeast NADH dehydrogenase (Ndi1) reveals overlapping binding sites for water- and lipid-soluble substrates. Proc. Natl. Acad. Sci. USA 2012, 109, 15247–15252. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.M.; Sena, F.V.; Batista, A.P.; Athayde, D.; Brito, J.A.; Archer, M.; Oliveira, A.S.F.; Soares, C.M.; Catarino, T.; Pereira, M.M. The key role of glutamate 172 in the mechanism of type II NADH:quinone oxidoreductase of Staphylococcus aureus. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Amoedo, N.D.; Punzi, G.; Obre, E.; Lacombe, D.; De Grassi, A.; Pierri, C.L.; Rossignol, R. AGC1/2, the mitochondrial aspartate-glutamate carriers. Biochim. Biophys. Acta 2016, 1863, 2394–2412. [Google Scholar] [CrossRef]
- Le Bras, M.; Clément, M.V.; Pervaiz, S.; Brenner, C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol. Histopathol. 2005, 20, 205–219. [Google Scholar]
- Todd, A.E.; Orengo, C.A.; Thornton, J.M. Evolution of protein function, from a structural perspective. Curr. Opin. Chem. Biol. 1999, 3, 548–556. [Google Scholar] [CrossRef]
- Todd, A.E.; Orengo, C.A.; Thornton, J.M. Evolution of function in protein superfamilies, from a structural perspective. J. Mol. Biol. 2001, 307, 1113–1143. [Google Scholar] [CrossRef]
- Zamzami, N.; Kroemer, G. The mitochondrion in apoptosis: How Pandora’s box opens. Nat. Rev. Mol. Cell Biol. 2001, 2, 67–71. [Google Scholar] [CrossRef]
- Modjtahedi, N.; Giordanetto, F.; Madeo, F.; Kroemer, G. Apoptosis-inducing factor: Vital and lethal. Trends Cell Biol. 2006, 16, 264–272. [Google Scholar] [CrossRef]
- Joza, N.; Pospisilik, J.A.; Hangen, E.; Hanada, T.; Modjtahedi, N.; Penninger, J.M.; Kroemer, G. AIF: Not just an apoptosis-inducing factor. Ann. N.Y. Acad. Sci. 2009, 1171, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Prehn, J.H.M. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, L.; Roumier, T.; Kroemer, G. Mitochondria, the killer organelles and their weapons. J. Cell. Physiol. 2002, 192, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 2016, 354, aad6872. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, W.; Li, J.; Wang, J.; Ge, J.; Xu, D.; Liu, Y.; Wu, K.; Zeng, Q.; Wu, J.W.; et al. Structural insight into the type-II mitochondrial NADH dehydrogenases. Nature 2012, 491, 478–482. [Google Scholar] [CrossRef]
- Ferreira, P.; Villanueva, R.; Martínez-Júlvez, M.; Herguedas, B.; Marcuello, C.; Fernandez-Silva, P.; Cabon, L.; Hermoso, J.A.; Lostao, A.; Susin, S.A.; et al. Structural insights into the coenzyme mediated monomer-dimer transition of the pro-apoptotic apoptosis inducing factor. Biochemistry 2014, 53, 4204–4215. [Google Scholar] [CrossRef]
- Pierri, C.L.; Parisi, G.; Porcelli, V. Computational approaches for protein function prediction: A combined strategy from multiple sequence alignment to molecular docking-based virtual screening. Biochim. Biophys. Acta 2010, 1804, 1695–1712. [Google Scholar] [CrossRef]
- Han, X.; Sit, A.; Christoffer, C.; Chen, S.; Kihara, D. A global map of the protein shape universe. PLoS Comput. Biol. 2019. [Google Scholar] [CrossRef]
- Knoverek, C.R.; Amarasinghe, G.K.; Bowman, G.R. Advanced Methods for Accessing Protein Shape-Shifting Present New Therapeutic Opportunities. Trends Biochem. Sci. 2019, 44, 351–364. [Google Scholar] [CrossRef]
- Bossis, F.; De Grassi, A.; Palese, L.L.; Pierri, C.L. Prediction of high- and low-affinity quinol-analogue-binding sites in the aa3 and bo3 terminal oxidases from Bacillus subtilis and Escherichia coli1. Biochem. J. 2014, 461, 305–314. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Lobley, A.; Sadowski, M.I.; Jones, D.T. pGenTHREADER and pDomTHREADER: New methods for improved protein fold recognition and superfamily discrimination. Bioinformatics 2009, 25, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Ordog, R. PyDeT, a PyMOL plug-in for visualizing geometric concepts around proteins. Bioinformation 2008, 2, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Tavani, C.; Bianchi, L.; De Palma, A.; Passeri, G.I.; Punzi, G.; Pierri, C.L.; Lovece, A.; Cavalluzzi, M.M.; Franchini, C.; Lentini, G.; et al. Nitro-substituted tetrahydroindolizines and homologs: Design, kinetics, and mechanism of α-glucosidase inhibition. Bioorg. Med. Chem. Lett. 2017, 27, 3980–3986. [Google Scholar] [CrossRef]
- Pierri, C.L.; Palmieri, F.; De Grassi, A. Single-nucleotide evolution quantifies the importance of each site along the structure of mitochondrial carriers. Cell. Mol. Life Sci. 2014, 71, 349–364. [Google Scholar] [CrossRef]
- Infantino, V.; Pierri, C.L.; Iacobazzi, V. Metabolic routes in inflammation: The citrate pathway and its potential as therapeutic target. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Pierri, C.L.; Bossis, F.; Punzi, G.; De Grassi, A.; Cetrone, M.; Parisi, G.; Tricarico, D. Molecular modeling of antibodies for the treatment of TNFα-related immunological diseases. Pharmacol. Res. Perspect. 2016, 4, e00197. [Google Scholar] [CrossRef]
- Coccaro, N.; Brunetti, C.; Tota, G.; Pierri, C.L.; Anelli, L.; Zagaria, A.; Casieri, P.; Impera, L.; Minervini, C.F.; Minervini, A.; et al. A novel t(3;9)(q21.2; p24.3) associated with SMARCA2 and ZNF148 genes rearrangement in myelodysplastic syndrome. Leuk. Lymphoma 2018, 59, 996–999. [Google Scholar] [CrossRef]
- Itkis, Y.; Krylova, T.; Pechatnikova, N.L.; De Grassi, A.; Tabakov, V.Y.; Pierri, C.L.; Aleshin, V.; Boyko, A.; Bunik, V.I.; Zakharova, E.Y. A novel variant m.641A>T in the mitochondrial MT-TF gene is associated with epileptic encephalopathy in adolescent. Mitochondrion 2019, 47, 10–17. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Harnvoravongchai, P.; Kobori, H.; Orita, I.; Nakamura, S.; Imanaka, T.; Fukui, T. Characterization and gene deletion analysis of four homologues of group 3 pyridine nucleotide disulfide oxidoreductases from Thermococcus kodakarensis. Extremophiles 2014, 18, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Okabe, S.; Yanase, M.; Kataoka, K.; Sakurai, T. Studies of interaction of homo-dimeric ferredoxin-NAD(P)+ oxidoreductases of Bacillus subtilis and Rhodopseudomonas palustris, that are closely related to thioredoxin reductases in amino acid sequence, with ferredoxins and pyridine nucleotide coenzymes. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 594–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, A.K.; Kim, I.S.; Do, H.; Jeon, B.W.; Lee, C.W.; Roh, S.J.; Shin, S.C.; Park, H.; Kim, Y.S.; Kim, Y.H.; et al. Structure and catalytic mechanism of monodehydroascorbate reductase, MDHAR, from Oryza sativa L. japonica. Sci. Rep. 2016, 6, 33903. [Google Scholar] [CrossRef] [PubMed]
- Osipov, E.M.; Lilina, A.V.; Tsallagov, S.I.; Safonova, T.N.; Sorokin, D.Y.; Tikhonova, T.V.; Popova, V.O. Structure of the flavocytochrome C sulfide dehydrogenase associated with the copper-binding protein CopC from the haloalkaliphilic sulfuroxidizing bacterium thioalkalivibrio paradoxus ArH 1. Acta Crystallogr. D Struct. Biol. 2018, 74, 632–642. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Li, X.; Li, J.; Wu, Y.; Yu, J.; Ge, J.; Huang, Z.; Jiang, L.; Rao, Y.; et al. Target Elucidation by Cocrystal Structures of NADH-Ubiquinone Oxidoreductase of Plasmodium falciparum (PfNDH2) with Small Molecule To Eliminate Drug-Resistant Malaria. J. Med. Chem. 2017, 60, 1994–2005. [Google Scholar] [CrossRef]
- Yamashita, T.; Inaoka, D.K.; Shiba, T.; Oohashi, T.; Iwata, S.; Yagi, T.; Kosaka, H.; Miyoshi, H.; Harada, S.; Kita, K.; et al. Ubiquinone binding site of yeast NADH dehydrogenase revealed by structures binding novel competitive- and mixed-type inhibitors. Sci. Rep. 2018, 8, 2427. [Google Scholar] [CrossRef]
- Degli Esposti, M. Inhibitors of NADH-ubiquinone reductase: An overview. Biochim. Biophys. Acta Bioenerg. 1998, 1364, 222–235. [Google Scholar] [CrossRef]
- Bryk, R.; Arango, N.; Maksymiuk, C.; Balakrishnan, A.; Wu, Y.T.; Wong, C.H.; Masquelin, T.; Hipskind, P.; Lima, C.D.; Nathan, C. Lipoamide channel-binding sulfonamides selectively inhibit mycobacterial lipoamide dehydrogenase. Biochemistry 2013, 52, 9375–9384. [Google Scholar] [CrossRef]
- Nocek, B.; Jang, S.B.; Jeong, M.S.; Clark, D.D.; Ensign, S.A.; Peters, J.W. Structural basis for CO 2 fixation by a novel member of the disulfide oxidoreductase family of enzymes, 2-ketopropyl-coenzyme M oxidoreductase/carboxylase. Biochemistry 2002, 41, 12907–12913. [Google Scholar] [CrossRef]
- Xia, L.; Björnstedt, M.; Nordman, T.; Eriksson, L.C.; Olsson, J.M. Reduction of ubiquinone by lipoamide dehydrogenase: An antioxidant regenerating pathway. Eur. J. Biochem. 2001, 268, 1486–1490. [Google Scholar] [CrossRef]
- Nilsen, A.; LaCrue, A.N.; White, K.L.; Forquer, I.P.; Cross, R.M.; Marfurt, J.; Mather, M.W.; Delves, M.J.; Shackleford, D.M.; Saenz, F.E.; et al. Quinolone-3-diarylethers: A new class of antimalarial drug. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef]
- Doggett, J.S.; Nilsen, A.; Forquer, I.; Wegmann, K.W.; Jones-Brando, L.; Yolken, R.H.; Bordón, C.; Charman, S.A.; Katneni, K.; Schultz, T.; et al. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15936–15941. [Google Scholar] [CrossRef]
- Stickles, A.M.; Smilkstein, M.J.; Morrisey, J.M.; Li, Y.; Forquer, I.P.; Kelly, J.X.; Pou, S.; Winter, R.W.; Nilsen, A.; Vaidya, A.B.; et al. Atovaquone and ELQ-300 combination therapy as a novel dual-site cytochrome bc1 inhibition strategy for malaria. Antimicrob. Agents Chemother. 2016, 60, 4853–4859. [Google Scholar] [CrossRef] [PubMed]
- Heikal, A.; Nakatani, Y.; Jiao, W.; Wilson, C.; Rennison, D.; Weimar, M.R.; Parker, E.J.; Brimble, M.A.; Cook, G.M. ‘Tethering’ fragment-based drug discovery to identify inhibitors of the essential respiratory membrane protein type II NADH dehydrogenase. Bioorg. Med. Chem. Lett. 2018, 28, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, S.; Singh, M.; Kumar, A.; Singh, S.K. Type-II NADH Dehydrogenase (NDH-2): A promising therapeutic target for antitubercular and antibacterial drug discovery. Expert Opin. Ther. Targets 2017, 21, 559–570. [Google Scholar] [CrossRef]
- Harbut, M.B.; Yang, B.; Liu, R.; Yano, T.; Vilchèze, C.; Cheng, B.; Lockner, J.; Guo, H.; Yu, C.; Franzblau, S.G.; et al. Small Molecules Targeting Mycobacterium tuberculosis Type II NADH Dehydrogenase Exhibit Antimycobacterial Activity. Angew. Chem.Int. Ed. Engl. 2018, 57, 3478–3482. [Google Scholar] [CrossRef]
- Murugesan, D.; Ray, P.C.; Bayliss, T.; Prosser, G.A.; Harrison, J.R.; Green, K.; Soares De Melo, C.; Feng, T.S.; Street, L.J.; Chibale, K.; et al. 2-Mercapto-Quinazolinones as Inhibitors of Type II NADH Dehydrogenase and Mycobacterium tuberculosis: Structure-Activity Relationships, Mechanism of Action and Absorption, Distribution, Metabolism, and Excretion Characterization. ACS Infect. Dis. 2018, 4, 954–969. [Google Scholar] [CrossRef]
- Miller, J.O.; Taylor, J.; Goldman, J.L. Severe acute respiratory failure in healthy adolescents exposed to trimethoprim-sulfamethoxazole. Pediatrics 2019, 143, e20183242. [Google Scholar] [CrossRef]
- Inesi, G. Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life 2017, 69, 211–217. [Google Scholar] [CrossRef]
- Terziyska, N.; Lutz, T.; Kozany, C.; Mokranjac, D.; Mesecke, N.; Neupert, W.; Herrmann, J.M.; Hell, K. Mia40, a novel factor for protein import into the intermembrane space of mitochondria is able to bind metal ions. FEBS Lett. 2005, 579, 179–184. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cefaro, C.; Ciofi-Baffoni, S.; Gallo, A.; Martinelli, M.; Sideris, D.P.; Katrakili, N.; Tokatlidis, K. MIA40 is an oxidoreductase that catalyzes oxidative protein folding in mitochondria. Nat. Struct. Mol. Biol. 2009, 16, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J. Open questions: Respiratory chain supercomplexes-why are they there and what do they do? BMC Biol. 2018, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Saari, S.; Garcia, G.S.; Bremer, K.; Chioda, M.M.; Andjelković, A.; Debes, P.V.; Nikinmaa, M.; Szibor, M.; Dufour, E.; Rustin, P.; et al. Alternative respiratory chain enzymes: Therapeutic potential and possible pitfalls. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.S.; Calisto, F.; Langer, J.D.; Mills, D.J.; Refojo, P.N.; Teixeira, M.; Kühlbrandt, W.; Vonck, J.; Pereira, M.M. Structural basis for energy transduction by respiratory alternative complex III. Nat. Commun. 2018, 9, 1728. [Google Scholar] [CrossRef]
- Miramar, M.D.; Costantini, P.; Ravagnan, L.; Saraiva, L.M.; Haouzi, D.; Brothers, G.; Penninger, J.M.; Peleato, M.L.; Kroemer, G.; Susin, S.A. NADH Oxidase Activity of Mitochondrial Apoptosis-inducing Factor. J. Biol. Chem. 2001, 276, 16391–16398. [Google Scholar] [CrossRef]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Wilkinson, J.C.; Wilkinson, A.S.; Galban, S.; Csomos, R.A.; Duckett, C.S. Apoptosis-Inducing Factor Is a Target for Ubiquitination through Interaction with XIAP. Mol. Cell. Biol. 2008, 28, 237–247. [Google Scholar] [CrossRef]
- Vahsen, N.; Candé, C.; Brière, J.J.; Bénit, P.; Joza, N.; Larochette, N.; Mastroberardino, P.G.; Pequignot, M.O.; Casares, N.; Lazar, V.; et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004, 23, 4679–4689. [Google Scholar] [CrossRef]
- Zheng, Y.; Qu, H.; Xiong, X.; Wang, Y.; Liu, X.; Zhang, L.; Liao, X.; Liao, Q.; Sun, Z.; Ouyang, Q.; et al. Deficiency of Mitochondrial Glycerol 3-Phosphate Dehydrogenase Contributes to Hepatic Steatosis. Hepatology 2019, 70, 84–97. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Lin, C.T.; Ryan, T.E.; Reese, L.R.; Gilliam, L.A.A.; Cathey, B.L.; Lark, D.S.; Smith, C.D.; Muoio, D.M.; Neufer, P.D. Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem. J. 2015, 467, 271–280. [Google Scholar] [CrossRef]
- Atlante, A.; Calissano, P.; Bobba, A.; Azzariti, A.; Marra, E.; Passarella, S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J. Biol. Chem. 2000, 275, 37159–37166. [Google Scholar] [CrossRef] [PubMed]
- Marzulli, D.; La Piana, G.; Cafagno, L.; Fransvea, E.; Lofrumento, N.E. Proton translocation linked to the activity of the bi-trans-membrane electron transport chain. Arch. Biochem. Biophys. 1995, 319, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Punzi, G.; Porcelli, V.; Ruggiu, M.; Hossain, M.F.; Menga, A.; Scarcia, P.; Castegna, A.; Gorgoglione, R.; Pierri, C.L.; Laera, L.; et al. SLC25A10 biallelic mutations in intractable epileptic encephalopathy with complex I deficiency. Hum. Mol. Genet. 2018, 27, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Brock, S.; Gray, J.J.; Linseman, D.A. Stable over-expression of the 2-oxoglutarate carrier enhances neuronal cell resistance to oxidative stress via Bcl-2-dependent mitochondrial GSH transport. J. Neurochem. 2014, 130, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Craven, L.; Alston, C.L.; Taylor, R.W.; Turnbull, D.M. Recent Advances in Mitochondrial Disease. Annu. Rev. Genomics Hum. Genet. 2017, 18, 257–275. [Google Scholar] [CrossRef]
- Zeviani, M.; Carelli, V. Mitochondrial disorders. Curr. Opin. Neurol. 2007, 20, 564–571. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Billiard, J.; Dennison, J.B.; Briand, J.; Annan, R.S.; Chai, D.; Colón, M.; Dodson, C.S.; Gilbert, S.A.; Greshock, J.; Jing, J.; et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013, 1, 19. [Google Scholar] [CrossRef]
- Abramson, J.; Riistama, S.; Larsson, G.; Jasaitis, A.; Svensson-Ek, M.; Laakkonen, L.; Puustinen, A.; Iwata, S.; Wikström, M. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat. Struct. Biol. 2000, 7, 910–917. [Google Scholar]
- Otera, H.; Ohsakaya, S.; Nagaura, Z.I.; Ishihara, N.; Mihara, K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005, 24, 1375–1386. [Google Scholar] [CrossRef]
- Fedor, J.G.; Jones, A.J.Y.; Di Luca, A.; Kaila, V.R.I.; Hirst, J. Correlating kinetic and structural data on ubiquinone binding and reduction by respiratory complex I. Proc. Natl. Acad. Sci. USA 2017, 114, 12737–12742. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Emmanuele, V.; Quinzii, C.M. Emerging therapies for mitochondrial diseases. Essays Biochem. 2018, 62, 467–481. [Google Scholar] [PubMed]
- Beyrath, J.; Pellegrini, M.; Renkema, H.; Houben, L.; Pecheritsyna, S.; Van Zandvoort, P.; Van Den Broek, P.; Bekel, A.; Eftekhari, P.; Smeitink, J.A.M. KH176 Safeguards Mitochondrial Diseased Cells from Redox Stress-Induced Cell Death by Interacting with the Thioredoxin System/Peroxiredoxin Enzyme Machinery. Sci. Rep. 2018, 8, 6577. [Google Scholar] [CrossRef] [PubMed]
- Fiedorczuk, K.; Letts, J.A.; Degliesposti, G.; Kaszuba, K.; Skehel, M.; Sazanov, L.A. Atomic structure of the entire mammalian mitochondrial complex I. Nature 2016, 538, 406–410. [Google Scholar] [CrossRef]
- Hards, K.; Cook, G.M. Targeting bacterial energetics to produce new antimicrobials. Drug Resist. Updat. 2018, 36, 1–12. [Google Scholar] [CrossRef]
- Lu, J.; Vlamis-Gardikas, A.; Kandasamy, K.; Zhao, R.; Gustafsson, T.N.; Engstrand, L.; Hoffner, S.; Engman, L.; Holmgren, A. Inhibition of bacterial thioredoxin reductase: An antibiotic mechanism targeting bacteria lacking glutathione. FASEB J. 2013, 27, 1394–1403. [Google Scholar] [CrossRef]
- Volpicella, M.; Costanza, A.; Palumbo, O.; Italiano, F.; Claudia, L.; Placido, A.; Picardi, E.; Carella, M.; Trotta, M.; Ceci, L.R. Rhodobacter sphaeroides adaptation to high concentrations of cobalt ions requires energetic metabolism changes. FEMS Microbiol. Ecol. 2014, 88, 345–357. [Google Scholar] [CrossRef][Green Version]
- Bullerwell, C.E.; Gray, M.W. Evolution of the mitochondrial genome: Protist connections to animals, fungi and plants. Curr. Opin. Microbiol. 2004, 7, 528–534. [Google Scholar] [CrossRef]
- Schägger, H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim. Biophys. Acta Bioenerg. 2002, 1555, 154–159. [Google Scholar] [CrossRef]
- Archibald, J.M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef]

| Functional Annotation | Res. N. | Organism | Crystallized cofactors | Crystallized Inhibitors | AIF (4bur) | NDH2-(5kmr) | NDI (4g73) | H. sapiens Blast Hits | C. thermarum Blast Best Hit | S. cerevisiae S288C Blast Best Hit | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSD (Å) | Protein Name | Accession | Query cover | E-val | %ID | Protein Name | Accession | Query cover | E-val | %ID | Protein Name | Accession | Query cover | E-val | %ID | ||||||||
| AIF-like structures | |||||||||||||||||||||||
| 4bur | AIF | 511 | H.sapines | FAD/NADH | n.a. | 0 | 3.12 | 2.22 | mt isoform AIF-exB | NP_665811.1 | 100% | 0.0 | 100% | n.a. | n.a. | n.a. | n.a. | n.a. | Irc15p | NP_015308 | 25% | 0.98 | 25.55% |
| 5fs8 | AIF | 474 | H.sapines | FAD | n.a. | 1.06 | 2.71 | 1.82 | mt isoform AIF | NP_004199.1 | 99% | 0 | 99.61% | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 5vn0 | NADH OX | 449 | L.brevis | FAD/NADH/O2 | n.a. | 2.28 | 2.03 | 2.18 | protein transport protein Sec23A | NP_006355.2 | 100% | 0.0 | 100% | CoA-disulfide reductase | WP_007505374.1 | 2% | 3 × 10−86 | 50.00% | GTPase-activating protein SEC23 | NP_015507.1 | 98% | 0.0 | 49.80% |
| 1xhc | NADH OX /nitrile RED | 555 | P. furiosus | FAD | n.a. | 1.799 | 2.443 | 2.458 | AIF 3 isoform 2 | NP_001018070.1 | 73% | 9 × 10−20 | 28.47% | CoA-disulfide RED | WP_007505374.1 | 98% | 9 × 10−92 | 35.83% | Aif1p | NP_014472.1 | 54% | 9 × 10−11 | 26.36% |
| 2bc0 | NADH OX | 473 | S. pyrogens | FAD | n.a. | 1.53 | 1.859 | 1.658 | AIF 3 isoform 1 | NP_653305.1 | 67% | 2 × 10−18 | 25.75% | CoA-disulfide RED | WP_007505374.1 | 92% | 2 × 10−74 | 31.29% | GLR1 | NP_015234.1 | 40% | 8 × 10−10 | 27.09% |
| 2cdu | NAD(P)H OX | 452 | L. sanfrancensis | FAD/ADP | n.a. | 2.226 | 2.2 | 2.095 | mt isoform AIF | NP_004199.1 | 56% | 2 × 10−11 | 25.46% | CoA-disulfide RED | WP_007505374.1 | 96% | 3 × 10−74 | 31.52% | GLR1 | NP_015234.1 | 39% | 3 × 10−8 | 27.66% |
| 1nhs | NADH PerOX | 447 | E. fecalis | FAD | n.a. | 1.738 | 1.932 | 2.041 | AIF 3 isoform 1 | NP_653305.1 | 55% | 7 × 10−22 | 27.17% | CoA-disulfide RED | WP_007505374.1 | 98% | 9 × 10−92 | 35.83% | GLR1 | NP_015234.1 | 39% | 8 × 10−4 | 22.40% |
| 3lxd | Ferredoxin RED | 409 | E. coli | n.a | n.a. | 1.949 | 2.842 | 2.606 | AIF 3 isoform 1 | NP_653305.1 | 89% | 8 × 10−51 | 30.24% | CoA-disulfide RED | WP_007505374.1 | 80% | 6 × 10−26 | 29.48% | GLR1 | NP_015234.1 | 35% | 2 × 10−3 | 24.03% |
| 3fg2 | Ferredoxin RED | 404 | R. palustris | FAD | n.a. | 1.633 | 2.087 | 2.340 | AIF 3 isoform 1 | NP_653305.1 | 91% | 6 × 10−47 | 29.22% | CoA-disulfide RED | WP_007505374.1 | 77% | 7 × 10−22 | 24.15% | GLR1 | NP_015234.1 | 32% | 4 × 10−3 | 25.90% |
| 2gqw | Ferredoxin RED | 401 | Pseudomonas sp. KKS102 | FAD | n.a. | 1.986 | 2.362 | 2.365 | AIF 3 isoform 2 | NP_001018070.1 | 91% | 5 × 10−27 | 28.39% | CoA-disulfide RED | WP_007505374.1 | 52% | 3 × 10−14 | 29.78% | Irc15p | NP_015308.1 | 24% | 6 × 10−3 | 28.85% |
| 2v3a | Rubredoxin RED | 381 | P. aeruginosa | FAD | n.a. | 2.278 | 3.067 | 6.698 | AIF 2 | NP_001185625.1 | 61% | 3 × 10−3 | 24% | CoA-disulfide RED | WP_007505374.1 | 71% | 2 × 10−23 | 27.02% | n.a. | n.a. | n.a. | n.a. | n.a. |
| 3klj | NADH:rubredoxinoxidoRED | 378 | C. acetobutylicum | FAD | n.a. | 2.498 | 2.745 | 2.335 | AIF 3 isoform 2 | NP_001018070.1 | 95% | 5 × 10−27 | 23.41% | CoA-disulfide RED | WP_007505374.1 | 89% | 1 × 10−21 | 23.69% | mRNA-binding ubiquitin-specific protease UBP3 | NP_011078.3 | 7% | 0.77 | 53.57% |
| 3ef6 | Toluene 2,3-Dioxygenase RED | 400 | P. putida | FAD | n.a. | 1.914 | 1.979 | 2.146 | AIF 3 isoform 1 | NP_653305.1 | 83% | 7 × 10−44 | 33.14% | CoA-disulfide RED | WP_007505374.1 | 43% | 9 × 10−15 | 33.33% | Aif1p | NP_014472.1 | 48% | 6 × 10−4 | 25.35% |
| 1q1r | Putidaredoxin RED | 421 | P. putida | FAD | n.a. | 1.581 | 2.85 | 3.069 | AIF 3 isoform 3 | NP_001139760.1 | 87% | 5 × 10−40 | 28.95% | CoA-disulfide RED | WP_007505374.1 | 72% | 9 × 10−25 | 27.74% | GLR1 | NP_015234.1 | 38% | 2 × 10−4 | 21.64% |
| 3oc4 | Pyridine nucleotide-disulfide oxidoRED | 422 | E. faecalis | FAD | n.a. | 3.143 | 2.451 | 3.207 | AIF 3 isoform 1 | NP_653305.1 | 62% | 4 × 10−13 | 25.26% | CoA-disulfide RED | WP_007505374.1 | 95% | 4 × 10−45 | 26.53% | GLR1 | NP_015234.1 | 51% | 1 × 10−5 | 23.36% |
| 3iwa | Pyridine nucleotide-disulphideoxidoRED | 397 | D. vulgaris | n.a. | n.a. | 2.243 | 3.06 | 2.664 | AIF 3 isoform 2 | NP_001018070.1 | 68% | 8 × 10−24 | 28.66% | CoA-disulfide RED | WP_007505374.1 | 95% | 5 × 10−82 | 33.55% | GLR1 | NP_015234.1 | 59% | 3 × 10−9 | 22.49% |
| 3cgb | Pyridine Nucleotide Coenzyme A-Disulfide RED | 444 | B.anthracis | FAD/CoA | n.a. | 2.17 | 2.37 | 2.341 | glycerol-3-phosphate DH, mt | NP_000399.3 | 10% | 0.8 | 39.58% | CoA-disulfide RED | WP_007505374.1 | 91% | 5 × 10−137 | 47.05% | Irc15p | NP_015308.1 | 60% | 1 × 10−5 | 23.51% |
| 4fx9 | CoA disulfide RED | 453 | P. horikoshii | FAD/CoA | n.a. | 2.148 | 2.223 | 2.488 | AIF 3 isoform 1 [Homo sapiens] | NP_653305.1 | 70% | 1 × 10−22 | 27.55% | CoA-disulfide RED | WP_007505374.1 | 96% | 3 × 10−105 | 39.28% | GLR1 | NP_015234.1 | 64% | 2 × 10−12 | 24.36% |
| 3ics | CoA disulfide RED | 555 | B. anthracis | ADP/FAD/CoA | n.a. | 1.845 | 1.958 | 1.98 | DLD. mt isoform 4 | NP_001276681.1 | 51% | 2 × 10−9 | 26.46% | CoA-disulfide RED | WP_007505374.1 | 75% | 3 × 10−83 | 32.13% | thiosulfate sulfurtransferase RDL2 | NP_014929.3 | 10% | 1 × 10−3 | 29.73% |
| 3ntd | NADH-dependent persulfide RED | 565 | S. ioihica | FAD/CoA | n.a. | 1.92 | 1.95 | 1.95 | AIF mt isoform AIF-exB | NP_665811.1 | 47% | 6 × 10−10 | 28.01% | CoA-disulfide RED | WP_007505374.1 | 82% | 1 × 10−85 | 31.57% | DLD | NP_116635.1 | 45% | 2 × 10−7 | 25.87% |
| Type II NADH DH-like structures | |||||||||||||||||||||||
| 5kmr | Type II NADH DH | 405 | C. thermarum | FAD/NAD | n.a. | 3.12 | 0 | 1.31 | AIF 2 | NP_001185625.1 | 72% | 2 × 10−10 | 25.34% | NAD(P)/FAD-dependent oxidoRED | WP_007502350.1 | 98% | 0.0 | 100.00% | NADH-ubiquinone RED (H(+)-translocating) NDE1 | NP_013865.1 | 80% | 7 × 10−29 | 28.29% |
| 5n1t | FlavoCytC sulfide DH | 393 | T. paradoxus | CytC, COPC, FAD | n.a. | 2.82 | 3.33 | 2.96 | n.a. | n.a. | n.a. | n.a. | n.a. | NAD(P)/FAD-dependent oxidoRED | WP_007505419.1 | 74% | 5 × 10−22 | 26.33% | n.a. | n.a. | n.a. | n.a. | n.a. |
| 5na1 | NADH:quinoneoxidoRED | 398 | S. aureus | FAD | n.a. | 2.63 | 0.82 | 1.39 | n.a. | n.a. | n.a. | n.a. | n.a. | NAD(P)/FAD-dependent oxidoRED | WP_007502350.1 | 97% | 3 × 10−130 | 46.48% | nucleoside triphosphate pyrophosphohydrolase HAM1 | NP_012603.1 | 12% | 0.59 | 34.69% |
| 5jwc | Type II NADH DH | 495 | P. falciparum | FAD | RYL-552 | 3.88 | 1.68 | 0.85 | AIF 2 | NP_001185625.1 | 6% | 1.7 | 44.44% | NAD(P)/FAD-dependent oxidoRED | WP_042685058.1 | 49% | 1 × 10−11 | 22.68% | NADH-UQ RED (H(+)-translocating) NDE1 | NP_013865.1 | 94% | 8 × 10−60 | 30.32% |
| 3hyw | Sulfide:quinoneoxidoRED | 429 | aeolicus | FAD/DCQ/H2S | n.a. | 3.52 | 2.75 | 2.86 | sulfide:quinoneoxidoRED. mt (Homo sapiens) | NP_001258142.1 | 68% | 3 × 10−12 | 23.70% | NAD(P)/FAD-dependent oxidoRED | WP_007505419.1 | 75% | 4 × 10−15 | 22.82% | NADH-UQ RED (H(+)-translocating) NDE2 | NP_010198.1 | 55% | 1 × 10−9 | 27.27% |
| Ndi1 - NADH DH like structures | |||||||||||||||||||||||
| 4g73 | Ndi1 - NADH DH | 502 | S. cerevisiae | FAD/NAD/UQ5 | n.a. | 2.22 | 1.31 | 0 | n.a. | n.a. | n.a. | n.a. | n.a. | NAD(P)/FAD-dependent oxidoRED | WP_007502350.1 | 86% | 1 × 10−30 | 25.93% | NADH-UQ RED (H(+)-translocating) NDI1 | NP_013586.1 | 97% | 0.0 | 99.80% |
| 5yjw | Ndi1 - NADH DH. | 454 | S. cerevisiae | FAD | Stigmatellin | 2.12 | 1.32 | 0.49 | n.a. | n.a. | n.a. | n.a. | n.a. | NAD(P)/FAD-dependent oxidoRED | WP_007502350.1 | 90% | 7 × 10−31 | 25.93% | NADH-UQ RED (H(+)-translocating) NDI1 | NP_013586.1 | 100% | 0.0 | 100.00% |
| Other DH | |||||||||||||||||||||||
| 4m52 | LD. | 465 | M. tubercolosis | FAD | SL827 | 4.28 | 4.341 | 1.974 | DLD. mt isoform 1 | NP_000099.2 | 98% | 8 × 10−87 | 35.82% | DLD | WP_007503768.1 | 96% | 2 × 10−120 | 44.81% | DLD | NP_116635.1 | 96% | 3 × 10−86 | 37.63% |
| 6aon | DLD | 473 | B. pertussis | n.a. | n.a. | 4.84 | 2.03 | 3.76 | DLD. mt isoform 1 | NP_000099.2 | 98% | 7 × 10−141 | 46.74% | DLD | WP_007503768.1 | 98% | 1 × 10−106 | 40.46% | DLD | NP_116635.1 | 97% | 3 × 10−143 | 47.81% |
| 4jq9 | DLD | 471 | E. coli | FAD | n.a. | 3.61 | 2.168 | 2.238 | DLD. mt isoform 1 | NP_000099.2 | 94% | 2 × 10−113 | 43.61% | DLD | WP_007505013.1 | 95% | 3 × 10−121 | 43.74% | DLD | NP_116635.1 | 93% | 3 × 10−100 | 40.79% |
| 6awa | DLD | 475 | P. putida | FAD/AMP | n.a. | 3.47 | 2.6 | 2.67 | DLD. mt isoform 1 | NP_000099.2 | 96% | 1 × 10−153 | 50.43% | DLD | WP_007505013.1 | 97% | 6 × 10−121 | 43.19% | DLD | NP_116635.1 | 97% | 1 × 10−140 | 46.47% |
| 5j5z | DLD | 477 | H. sapiens | FAD | n.a. | 3.23 | 2.77 | 3.13 | DLD. mt isoform 1 | NP_000099.2 | 95% | 0.0 | 99.97% | DLD | WP_007505013.1 | 92% | 2 × 10−108 | 42.30% | DLD | NP_116635.1 | 93% | 0.0 | 57.17% |
| 1zmd | DLD | 474 | H. sapiens | FAD/NAD | n..a. | 3.27 | 2.83 | 2.77 | DLD. mt isoform 1 (Homo sapiens) | NP_000099.2 | 100% | 0.0 | 99.79% | DLD | WP_007505013.1 | 96% | 2 × 10−110 | 42.52% | DLD | NP_116635.1 | 98% | 0.0 | 57.59% |
| 5u25 | DLD | 478 | N. gonorrhoeae | FAD | n.a. | 3.68 | 2.44 | 2.47 | DLD. mt isoform 1 | NP_000099.2 | 75% | 7 × 10−98 | 39.48% | DLD | WP_007505013.1 | 77% | 2 × 10−112 | 41.76% | DLD | NP_116635.1 | 76% | 2 × 10−96 | 40.17% |
| 3urh | DLD | 491 | R. meliloti | FAD | n.a. | 3.376 | 2.29 | 1.732 | DLD. mt isoform 1 | NP_000099.2 | 95% | 1 × 10−179 | 55.08% | DLD | WP_007505013.1 | 94% | 3 × 10−117 | 42.58% | DLD | NP_116635.1 | 94% | 2 × 10−163 | 51.37% |
| 1lvl | LD | 458 | P. putida | FAD/NADH | n.a. | 3.664 | 2.976 | 4.089 | DLD. mt isoform 1 | NP_000099.2 | 98% | 5 × 10−90 | 38.09% | DLD | WP_007505013.1 | 99% | 2 × 10−132 | 43.94% | DLD | NP_116635.1 | 98% | 1 × 10−81 | 36.86% |
| 1ebd | DLD | 455 | G. stearothermophilus | FAD/Dihydrolipoamide acetyltransferase | n.a. | 3.22 | 2.19 | 3.69 | DLD.mt isoform 1 | NP_000099.2 | 98% | 3 × 10−119 | 43.89% | DLD | WP_007505013.1 | 99% | 0.0 | 68.65% | DLD | NP_116635.1 | 98% | 3 × 10−110 | 43.29% |
| 2yqu | LD | 455 | T. thermophilus | FAD | n.a. | 3.884 | 3.548 | 2.97 | DLD. mt isoform 1 | NP_000099.2 | 98% | 4 × 10−140 | 46.41% | DLD | WP_007505013.1 | 99% | 1 × 10−115 | 43.41% | DLD | NP_116635.1 | 99% | 2 × 10−143 | 48.00% |
| 3lad | Lipoamide deydrogenase | 476 | A. vinelandii | FAD | n.a. | 4.442 | 2.221 | 2.995 | DLD. mt isoform 1 | NP_000099.2 | 96% | 3 × 10−150 | 49.36% | DLD | WP_007505013.1 | 98% | 3 × 10−121 | 42.55% | DLD | NP_116635.1 | 98% | 3 × 10−135 | 44.61% |
| 2r9z | Glutathione amide RED | 463 | C. gracile | FAD | n.a. | 2.813 | 2.396 | 2.046 | glutathione RED. mt isoform 1 | NP_000628.2 | 95% | 9 × 10−147 | 48.80% | DLD | WP_007505013.1 | 94% | 2 × 10−64 | 30.25% | GLR1 | NP_015234.1 | 96% | 1 × 10−141 | 46.12% |
| 6n7f | Glutathione RED | 451 | S. pyogenes | Riboflavin/FAD | n.a. | 3.7 | 2.74 | 1.98 | glutathione RED. mt isoform 1 | NP_000628.2 | 99% | 9 × 10−160 | 52.48% | NAD(P)/FAD-dependent oxidoRED | WP_042684715.1 | 88% | 1 × 10−53 | 29.70% | GLR1 | NP_015234.1 | 98% | 9 × 10−149 | 48.70% |
| 5vdn | Glutathione RED | 449 | Y. pestis | FAD | n.a. | 3.73 | 2.41 | 2.66 | glutathione RED. mt isoform 1 | NP_000628.2 | 96% | 1 × 10−161 | 53.90% | DLD | WP_007505013.1 | 95% | 5 × 10−61 | 32.09% | GLR1 | NP_015234.1 | 96% | 9 × 10−149 | 50.75% |
| 4j56 | Trx RED | 504 | P. falciparum | FAD/Trx | n.a. | 3.45 | 3.33 | 2.01 | Trx RED 2. mt isoform 2 | NP_001339229.1 | 91% | 6 × 10−146 | 45.13% | DLD | WP_007505013.1 | 89% | 3 × 10−31 | 26.23% | GLR1 | NP_015234.1 | 89% | 3 × 10−74 | 33.47% |
| 1xdi | Flavoprotein Disulfide RED | 499 | M. tuberculosis | FAD | n.a. | 4.29 | 2.975 | 3.65 | DLD. mt isoform 1 | NP_000099.2 | 91% | 2 × 10−35 | 25.49% | DLD | WP_007503768.1 | 91% | 2 × 10−47 | 29.12% | DLD | NP_116635.1 | 92% | 8 × 10−35 | 26.10% |
| 1mo9 | NADPH:2-ketopropyl-coenzyme M oxidoRED/carboxylase (2-KPCC) | 523 | X. autotrophicus | FAD | KPC | 4.724 | 2.596 | 3.091 | DLD. mt isoform 1 | NP_000099.2 | 87% | 4 × 10−24 | 22.88% | NAD(P)/FAD-dependent oxidoRED | WP_042684715.1 | 69% | 2 × 10−30 | 28.65% | DLD | NP_116635.1 | 86% | 2 × 10−22 | 22.13% |
| 4k7z | Mercuric RED | 467 | P. aeruginosa | FAD/NADP | n.a. | 3.807 | 3.341 | 3.498 | DLD. mt isoform 1 | NP_000099.2 | 97% | 2 × 10−54 | 29.32% | DLD | WP_007505013.1 | 94% | 3 × 10−71 | 35.68% | DLD | NP_116635.1 | 95% | 3× 10−51 | 29.64% |
| Outliers | |||||||||||||||||||||||
| 4up3 | Trx RED | 312 | E. histolytica | FAD/NADPH | n.a. | 3.59 | 3.76 | 3.76 | (F-actin)-monooxygenase MICAL2 isoform f | NP_001269597.1 | 13% | 0.2 | 30.23% | Trx-disulfide RED | WP_007502507.1 | 99% | 8 × 10−68 | 37.50% | TRR1 | NP_010640.1 | 98% | 4 × 10−138 | 60.83% |
| 5u63 | Trx RED | 315 | H. influenzae | FAD/NADP | n.a. | 2.01 | 3.06 | 4.393 | DLD. mt isoform 1 | NP_000099.2 | 9% | 2.2 | 48.28% | Trx-disulfide RED | WP_007502507.1 | 97% | 3 × 10−76 | 39.43% | TRR1 | NP_011974.1 | 99% | 7 × 10−101 | 49.85% |
| 1ps9 | 2,4-dienoyl-CoA RED | 671 | E. coli | FMN/FAD/NADP | n.a. | 2.02 | 3.93 | 2.16 | L-amino-acid OX isoform 2 | NP_001244946.1 | 6% | 3.1 | 40.48% | NADPH DH NamA | WP_007504681.1 | 49% | 1 × 10−30 | 30.00% | NADPH DH | NP_012049.1 | 34% | 3 × 10−19 | 26.98% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trisolini, L.; Gambacorta, N.; Gorgoglione, R.; Montaruli, M.; Laera, L.; Colella, F.; Volpicella, M.; De Grassi, A.; Pierri, C.L. FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function. J. Clin. Med. 2019, 8, 2117. https://doi.org/10.3390/jcm8122117
Trisolini L, Gambacorta N, Gorgoglione R, Montaruli M, Laera L, Colella F, Volpicella M, De Grassi A, Pierri CL. FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function. Journal of Clinical Medicine. 2019; 8(12):2117. https://doi.org/10.3390/jcm8122117
Chicago/Turabian StyleTrisolini, Lucia, Nicola Gambacorta, Ruggiero Gorgoglione, Michele Montaruli, Luna Laera, Francesco Colella, Mariateresa Volpicella, Anna De Grassi, and Ciro Leonardo Pierri. 2019. "FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function" Journal of Clinical Medicine 8, no. 12: 2117. https://doi.org/10.3390/jcm8122117
APA StyleTrisolini, L., Gambacorta, N., Gorgoglione, R., Montaruli, M., Laera, L., Colella, F., Volpicella, M., De Grassi, A., & Pierri, C. L. (2019). FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function. Journal of Clinical Medicine, 8(12), 2117. https://doi.org/10.3390/jcm8122117







